Characterisation of genes involved in galactolipids and sulfolipids metabolism in maize and Arabidopsis and their differential responses to phosphate deficiency
Feng Wang A , Dong Ding A , Jiaxin Li A , Lin He A , Xiaoxuan Xu A , Ying Zhao A , Bowei Yan A , Zuotong Li A B and Jingyu Xu A B
A B
A Key Lab of Modern Agricultural Cultivation and Crop Germplasm Improvement of Heilongjiang Province, Heilongjiang Engineering Technology Research Centre for Crop Straw Utilisation, College of Agriculture, Heilongjiang Bayi Agricultural University, Daqing, 163319, China.
B Corresponding authors. Email: xujingyu2003@hotmail.com; lxg6401999@163.com
Functional Plant Biology 47(4) 279-292 https://doi.org/10.1071/FP19082
Submitted: 26 March 2019 Accepted: 24 October 2019 Published: 5 March 2020
Journal Compilation © CSIRO 2020 Open Access CC BY-NC-ND
Abstract
Galactolipids (MGDG and DGDG) and sulfolipids (SQDG) are key components of plastidic membranes, and play important roles in plant development and photosynthesis. In this study, the whole families of MGD, DGD and SQD were identified in maize genome, and were designated as ZmMGD1-3, ZmDGD1-5 and ZmSQD1-5 respectively. Based on the phylogenetic analyses, maize and Arabidopsis MGDs, DGDs and SQDs were clearly divided into two major categories (Type A and Type B) along with their orthologous genes from other plant species. Under low-phosphorus condition, the expression of Type B MGD, DGD and SQD genes of maize and Arabidopsis were significantly elevated in both leaf and root tissues. The lipid analysis was also conducted, and an overall increase in non-phosphorus lipids (MGDG, DGDG and SQDG), and a decrease in phosphorus lipids (PC, PE and PA) were observed in maize leaves and roots under phosphate deficiency. Several maize MGD and SQD genes were found involved in various abiotic stress responses. These findings will help for better understanding the specific functions of MGDs, DGDs and SQDs in 18:3 plants and for the generation of improved crops adapted to phosphate starvation and other abiotic stresses.
Additional keywords: abiotic stress, DGD, digalactosyldiacylglycerol synthase, low-phosphate, maize, MGD, monogalactosyldiacylglycerol synthase, SQD, sulfoquinovosyldiacylglycerol synthase, Zea mays.
Introduction
The photosynthetic membranes surrounding the chloroplast and thylakoid of higher plants are lipid bilayers with embedded proteins, which provide a platform for photosynthesis (Kobayashi et al. 2007; Rocha et al. 2018). The four main types of glycerolipids that make up the photosynthetic membrane are monogalactosyldiacylglycerol (MGDG), digalactosyldiacylglycerol (DGDG), sulfoquinovosyldiacylglycerol (SQDG), and phosphatidylglycerol (PG), and the outer envelope of chloroplast membrane includes a higher scale of typical eukaryotic lipids for instance the phospholipid phosphatidylcholine (PC) (Denev and Minkov 2001; Narayanan et al. 2018). Studies have shown that glycerolipid synthesis in chloroplast inner envelope is of critical importance for proper thylakoid biogenesis and photosynthesis (Kelly et al. 2016; Li et al. 2018).
The galactolipids MGDG and DGDG are synthesised in the chloroplast membrane with synthesis catalysed by two galactosyltransferases – MGDG synthase (MGD) and DGDG synthase (DGD) respectively (Denev and Minkov 2001; Rocha et al. 2018). MGD catalyses the galactose conversion from UDP-galactose to the diacylglycerol (DAG) backbone to form MGDG, and then the second galactose is transferred from UDP-galactose to MGDG by DGD for the final formation of DGDG (Li et al. 2016a). In Arabidopsis, MGDG synthase is divided into two subtypes: Type A (AtMGD1) and Type B (AtMGD2 and AtMGD3) (Awai et al. 2001). AtMGD1 maintains high expression during the growth and development of Arabidopsis, but no significant expression of AtMGD2 has been detected (Kobayashi et al. 2006). Studies of AtMGD1 mutant(mgd1-2) showed that the photosynthetic membrane was disrupted, resulting in complete loss of photosynthetic capacity and halting photosynthetic autotrophic growth (Dörmann et al. 1995; Fujii et al. 2018). Furthermore, deletion of AtDGD1 showed abnormal chloroplast phenotypes, which suggested that MGD and DGD has important effects on the function of photosynthetic membranes (Dörmann et al. 1995; Fujii et al. 2018).
A phosphate-saving mechanism involving the enhanced accumulation of DGDGs has been discovered in plants under phosphate deficient conditions. This mechanism could produce extra-plastidial membranes where they substitute phospholipids (Kobayashi et al. 2006; Rocha et al. 2018). The proportion of non-phospholipids to phospholipids in Arabidopsis increases sharply under phosphate stress (Härtel et al. 2000). Under normal conditions, the enzymes MGD1 and DGD1 produce large amount of galactolipids for the expansion of the thylakoid membrane; however, under phosphate stress, the activities of AtMGD2 and AtMGD3 are strongly induced (Härtel et al. 2000; Kobayashi et al. 2006). When AtMGD2 and AtMGD3 were activated, two DGDG synthase genes, DGD1 and DGD2, were also triggered, resulting in an increase of DGDG content (Nussaume et al. 2011). The same mechanism has also been reported in marine heterotrophic bacteria (Sebastián et al. 2015).
The sulfur-containing anionic glycerolipid SQDG is situated only in thylakoid membranes, and is generated by sulfoquinovosyldiacylglycerol synthase (SQD) catalysing the conversion of the sulfoquinovose moiety to diacylglycerol (DAG) (Yu et al. 2002; Zhan et al. 2017). In Arabidopsis, SQD1 is situated to the chloroplast matrix and is soluble, whereas SQD2 is localised to the inner envelope of the chloroplast (Essigmann et al. 1998; Yu et al. 2002). Arabidopsis plants that have mutations in SQD1 or SQD2 genes exhibit delayed growth under phosphate stress condition, although significant growth defects and a lack of the novel anionic glycolipid glucuronide diacylglycerol (GlcADG) was detected only in the sqd2 mutant (Okazaki et al. 2013; Zhan et al. 2017). In bacteria, sulfolipid-deficient null mutants stop growing earlier than wild types under phosphate stress (Benning et al. 1993; Güler et al. 1996).
In plant cells, the synthesis of glycerolipids could be processed via plastid/chloroplast compartmentalised prokaryotic pathway, and endoplasmic reticulum localised eukaryotic pathway respectively (Ohlrogge et al. 1991). Some plants, such as Arabidopsis, whose major chloroplast lipids (MGDG and DGDG) are generated jointly by both pathways, are typically characterised by the high levels of 16:3 fatty acids, are termed 16:3 plants (Heinz and Rougham 1983; Dörmann et al. 1995),whereas the others, such as maize, whose MGDG and DGDG synthesis relies almost entirely on the ER pathway, have products with high levels of 18:3 fatty acids, so they are called 18:3 plants(Wada and Murata 2010; Gu et al. 2017). Previous studies on the metabolism and function of galactolipids have focussed mainly on the 16:3 plant Arabidopsis, but only few studies have been conducted on 18:3 plant species (Chen and Thelen 2013; Gu et al. 2017).
Phosphate deficiency of soil and subsequent abiotic stresses are major factors that limit crop production in many regions of the world. In the present study, the MGD, DGD and SQD families were characterised from maize genome, and bioinformatics analyses were conducted to reveal their evolutionary and structural features. The expression profiles of these genes under low-phosphorus and various abiotic stress conditions were determined and a comparative analysis between maize and Arabidopsis were conducted. This study provides valuable information on deciphering the roles of MGDs, DGDs and SQDs in typical 18:3 plants and important field crop maize.
Materials and methods
Identification and classification of MGD, DGD and SQD genes in maize and Arabidopsis
The protein sequences of Arabidopsis MGD, DGD and SQD family members were obtained from TAIR and NCBI database, and were used as queries to search the MaizeGDB and NCBI database by sequence alignment using the BLASTp program. The screening criteria were E ≤ 1e–10 and protein length ≥200 amino acids (aa). The candidate genes encoding maize MGDs, DGDs and SQDs were retrieved from maize genome, and were confirmed at the online Pfam and SMART websites using the specific gene name and typical functional domains. A total of 13 non-redundant genes were reserved for further analysis.
Phylogenetic analysis, chromosomal location and syntenic analysis of the MGD, DGD and SQD families
The full-length amino acid sequences of MGD, DGD and SQD families from Arabidopsis. Thaliana (L.) Heynh), maize (Zea mays L.), sorghum (Sorghum bicolor (L.) Moench), soybean (Glycine max (L.) Merr., rice (Oryza sativa) L.), Brachypodium sylvaticum (Huds.) Beauv.)) and Medicago truncatula Gaertn. were obtained from online database using NCBI BLASTP tools. The protein sequences from different plant species were aligned by MEGA5.0 software (https://www.megasoftware.net/, accessed 28 January 2020). The phylogenetic trees for each family were constructed via the un-rooted neighbour joining method with the bootstrap values set at 1000 replicates using MEGA5.0. The chromosomal location of those genes was depicted using the MapInspect software. The syntenic blocks among Arabidopsis, maize, sorghum, soybean and rice homologous genes were generated according to the PGD Database, and only those had a linear relationship were shown (Xu et al. 2018).
Analyses of gene structure, functional domains and cis-elements in the promoters of the MGD, DGD and SQD families
The exon-intron structures of MGD, DGD and SQD genes in maize and Arabidopsis were acquired from the Gene Structure Display Server. The information on conserved functional domains was derived from Phytozome and SMART. The 1.5-kb upstream regions of MGD, DGD and SQD genes were derived from the transcription start site based on the maize and Arabidopsis genome. They were then searched against the PlantCARE database to determine the putative cis-elements.
Expression profiles of the maize MGD, DGD and SQD genes in diverse tissues and under different stress treatments
The expression data of MGD, DGD and SQD in maize sampled at different developing stages or various tissues (60 different tissues, including 11 major maize organ systems) were acquired from the online Maize eFP database. The expression data of MGD, DGD and SQD genes in 40 different Arabidopsis tissues were collected from TAIR database. The heat-map representing the gene expression intensities were generated and cluster analysis was completed by Tree View ver. 1.6 software (see Table S1, available as Supplementary Material to this paper). For expression analysis of maize MGD, DGD and SQD genes under abiotic stresses, the publicly available maize RNA-seq transcriptome datasets deposited in the NCBI SRA database were used. The transcriptome data includes cold (SRX2672484) (Gu et al. 2017), heat (SRR1238715, SRR1819196 and SRR1819198), salt (SRR1238719) and UV (SRR1238720) (Table S2) (Makarevitch et al. 2015). For cold stress, seeds of maize (He 344) were grown at 22°C and 16 h/8 h (light/dark) daily photoperiodic cycle. The 2-week-old seedlings were removed to another growth chamber set at 5°C for cold treatment (other conditions remained the same), and the seedlings grown at 22°C were used as controls. Samples of maize leaf were collected at 3-day time point under cold treatment (Gu et al. 2017). Heat, salt and UV stresses were applied to maize variety B73: maize seeds were grown at 24°C in 1 : 1 mix of autoclaved field soil and MetroMix, and the light conditions under all conditions are natural light conditions For heat stress, seedlings were placed at 50°C for 4 h. For high salt stress, plants were watered with 300 mmol NaCl 20 h before collecting. UV stress was applied in the growth room using UV-B lamps for 2 h (Makarevitch et al. 2015).
Quantitative real-time RT-PCR analyses of maize and Arabidopsis MGD, DGD and SQD genes in response to phosphate deficiency
The maize seedlings (variety He 344) were cultured in the nutrient solution supplied with sufficient phosphate (+P, 1000 μmol KH2PO4) 3 days after germination. The basic nutrient solution contains: 2 mmol Ca(NO3)2•4H2O, 1.25 mmol NH4NO3, 0.1 mmol KCl, 0.65 mmol K2SO4, 0.65 mmol MgSO4, 10 mmol H3BO3, 0.5 mmol (NH4)6Mo7O24, 1 mmol MnSO4, 0.1 mmol CuSO4•5H2O, 1 mmol ZnSO4•7H2O and 0.1 mmol Fe-EDTA. After 2 weeks, some maize seedlings (with 2–3 leaves) were transferred to nutrient solution containing very low concentration of phosphate (–P, 5 μmol KH2PO4, and 1 mmol KCl was added to complement the concentration of K+), and maize seedlings remained in sufficient phosphate nutrient solution(+P, 1000 μmol KH2PO4) were used as control. The greenhouse condition was set as: temperature 28°C (day) and 25°C (night), regime at a photon flux density (PFD) of 1000 μmol m–2 s–1with a 16/8 h light/dark cycle and ~65% RH. Leaf and root samples were collected at 0, 24 and 72 h under both +P and –P treatment. Arabidopsis (wild type Colombia) seeds were germinated on MS medium and transplanted into nutrient solution after 5 days containing 1000 μmol KH2PO4 (+P). After 15 days of growing, some of the plants were transferred into low phosphate culture (–P, 5 μmol KH2PO4), and Arabidopsis kept growing in +P solution were used as control. All samples had at least three replicates and the collected samples were quickly wrapped in tin foil, and frozen in liquid nitrogen immediately and stored at –80°C.
The maize GAPDH (glyceraldehyde-3-phosphate dehydrogenase) and Actin genes were used as endogenous controls. Each 10 μL PCR reaction included 3.4 μL ddH2O, 1.0 μL cDNA template and 0.3 μL each primer, and 5.0 μL buffer Mix. The PCR reaction started with a 95°C/3 min initial step; followed by 35 cycles of 94°C/60 s, 94°C/15 s and 55°C/30 s; and terminated at 72°C for 60 s. Quantitative real-time RT-PCR was implemented in an optical 96-well plate using the SYBR Select Master Mix RT-PCR system. Primers used for quantitative real-time PCR are shown in Table S3.Three independent biological replicates were performed and the results of quantitative real-time RT-PCR were analysed using 2–ΔΔCT method.
Maize lipid extraction and analysis
The total lipids were extracted from leaves and roots that collected at 72 h under both +P and –P treatment. The total lipid extraction method could refer to the previous study (Narayanan et al. 2016). Remove ~200 mg of sample and quickly place it in a Teflon-lined 50 mL glass tubes (with screw-cap) containing 3.0 mL 75°C is opropanol (with 0.01% BHT) for 15 min. After cooling to 25°C, 1.5 mL chloroform and 0.6 mL water were added and vortexed rapidly, followed by shaking and mixing for 1 h, and then the lipid extract was transferred to a new glass tube. The extraction step was repeated with chloroform/methanol (2 : 1), and the combined lipid extracts were cleaned with 1.0 mL 1 mol KCl. The lipid extracts were then dried with nitrogen and stored at –80°C. Mass spectrometry and lipid analysis were carried out by the Kansas Lipidomics Research Centre (KLRC, Kansas State University, Manhattan, KS, USA).
Statistical analysis
The statistical analysis was conducted by SPSS statistics 22.0, and the Student’s t-test was used to determine the significance levels. The significant level was P < 0.05. Data are presented as means ± s.d. The bars without error lines were control, and different lower case letters means there are significant differences among the bars.
Results
Genome-wide identification of MGD, DGD and SQD gene family members from maize
The protein sequences of the Arabidopsis MGD, DGD and SQD families were used as queries for the BLASTp searching against the maize genome, phytozome, and NCBI databases. A total of three MGD genes, five DGD genes, and five SQD genes were identified in maize genome, and were designated as ZmMGD1-3, ZmDGD1-5 and ZmSQD1-5, respectively, after their corresponding Arabidopsis orthologs (Table S4). As shown in Table S4, the Arabidopsis MGD family members have a coding region between 1398 and 1602 bp, and the encoded proteins have 465 to 533 amino acids with a molecular weight ranging from 52.73 to 58.54 kDa, and the predicted isoelectric point (pI) values are 6.30 to 9.30. In maize, the coding region of ZmMGDs is between 1434 to 1590 bp, encoding 477 to 529 amino acids, and the molecular weight is between 53.01 to 56.90 kDa with a predicted isoelectric point ranging from 6.84 to 9.55. Two DGD genes in Arabidopsis are quite different in size, AtDGD1 has a coding span of 2427 bp encoding a protein of 808 amino acids; whereas AtDGD2 has a coding span of 1422 bp encoding a protein of 473 amino acids. Similarly, the five DGD members in maize showed large size difference. ZmDGD1-3 have a coding region over 2200 bp, and the encoded proteins are close to 800 amino acids; whereas ZmDGD4-5 have a coding region around 1400 bp, and the encoded proteins are less than 500 amino acids. The coding regions of the two Arabidopsis SQD genes are 1434 bp (AtSQD1) and 1533 bp (AtSQD2), and encode proteins of 477 and 510 amino acids respectively. The maize SQD family also has five members, having a coding region among 1245 and 1494 bp, encoding 414 to 497 amino acids.
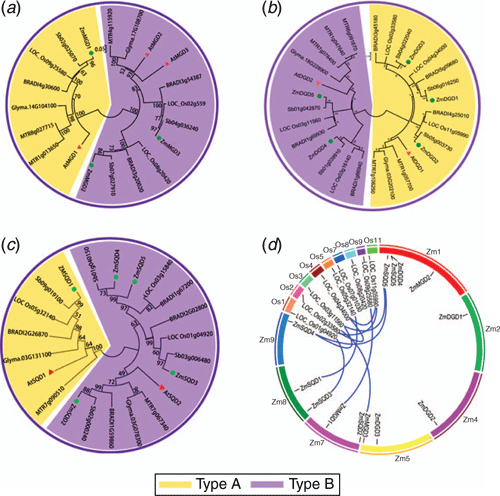
The chromosomal localisation analysis demonstrated that the 13 maize genes of three families located on seven maize chromosomes except chromosomes 3, 6 and 10 (see Fig. S1, available as Supplementary Material to this paper). Chromosome 1 harboured four genes, including ZmDGD4, 5, ZmMGD2 and ZmSQD5. To understand the replication relationship of these genes in maize and other plants genome, the tandem duplicated analysis among maize, rice, soybean, sorghum and Arabidopsis was conducted, and a syntenic block was generated. As shown in Fig. 1d, a total of six repetitive gene pairs (10 repeat genes) were found, including OsDGD1/OsDGD4/OsDGD5, OsMGD1/ZmMGD1, OsMGD3/ZmMGD3, OsSQD1/ZmSQD3, OsSQD3/ZmSQD1 and OsSQD2/ZmSQD4/ZmSQD5. They are primarily segmental repeat events, and no tandem replication events occur. Repetitive genes exemplified their common genomic origin and potential functional similarity.
|
Phylogenetic analyses of the MGD, DGD and SQD gene families
The full-length amino acid sequences of MGD, DGD and SQD families from maize (Z. mays), A. thaliana, rice (O. sativa), sorghum (S. bicolor), soybean (G. max), B. sylvaticum and M. truncatula were obtained from online database using NCBI BLASTP tools (Fig. 1). In previous studies, the Arabidopsis MGD family has been divided into two subtypes, Type A (AtMGD1) and Type B (AtMGD2 and AtMGD3), based on their differential response to Pi-deprived conditions (Mie et al. 2013). In the present study, the phylogenetic analysis of MGD orthologues genes divided them into two distinct subgroups: one subgroup containing the Type A AtMGD1, ZmMGD1, and their orthologs; and another subgroup containing the Type B MGDs form Arabidopsis, maize and other plant species (Fig. 1a). In each subfamily, MGDs from monocotyledons and dicotyledons were further clustered into different branches. AtMGDs displayed high homology with MGDs from other monocotyledons, including soybean and Medicago; whereas ZmMGDs were clustered with MGDs from other dicotyledons, including rice, sorghum and Brachypodium. ZmMGDs and their sorghum orthologs had much closer relationship. Similar phylogenetic relationships were also found in the DGD and SQD families, and two clearly separated subgroups were revealed and classified as Type A and Type B (Fig. 1b, c). The Type A DGDs contained AtDGD1 and ZmDGD1-3 and their orthologs from other plant species, whereas Type B DGDs included AtDGD2, ZmDGD4-5 and their orthologs. Similar to MGDs, in each subgroup, AtDGD displayed high homology with DGDs from other monocotyledons, while ZmDGDs were clustered with DGDs from other dicotyledons (Fig. 1b). SQDs were also divided into Type A and Type B subgroups. AtSQD1 and ZmSQD1 were classed into Type A subgroup, whereas AtSQD2 and ZmSQD2-5 were classed into Type B subgroup. Among them, ZmSQD4 and ZmSQD5 were more closely related to each other. In each cluster, maize SQDs and their sorghum orthologs formed phylogenetic branches at higher values, indicating that they have a high degree of sequence homology.
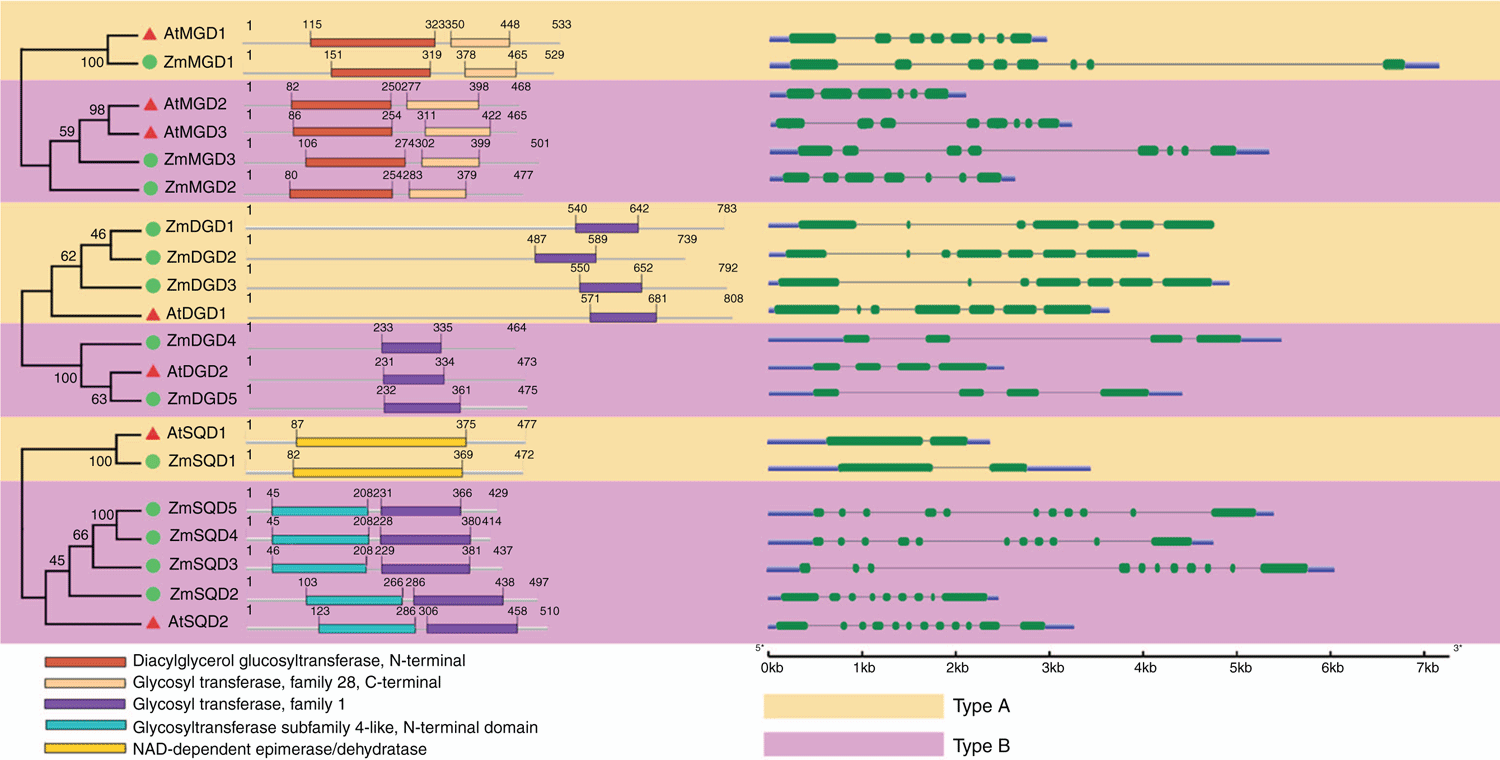
Gene architecture and conserved functional domains in MGD, DGD and SQD families
An exon–intron distribution diagram of 20 MGD, DGD and SQD genes from Arabidopsis and maize was generated according to their genomic and coding sequences, and an un-rooted phylogenetic tree of the 20 protein sequences was constructed to show their evolutionary relationships (Fig. 2). The results showed that the number of introns in the same subgroup was relatively consistent, but the difference between Type A and Type B subgroups was obvious. As can be seen in Fig. 2, configuration analysis showed that the number of exons/introns in Arabidopsis or maize MGD genes differed little, ranging from 5 to 7, and similar gene structure patterns were observed in each of the two subgroups. The similarity of the exon/intron structure of the MGD genes suggested that they had undergone gene duplication during the evolutionary processes. The Arabidopsis and maize DGD genes also showed extremely high structural similarity in each subgroup. Type A DGDs contain six introns, but Type B DGDs have only three introns. In the SQD gene family, there is a big difference in the number of introns between Type A and Type B SQDs. The Type A SQD genes contained only one intron, whereas the Type B SQD genes possessed 6–11 introns. Significant differences between the two types of DGD and SQD genes indicated that the genes might have undergone species specific evolutionary processes. Among the Type B SQD genes, ZmSQD4 and ZmSQD5 have very similar intron distribution, which suggests that these two genes had replicated during evolution.
As shown in Fig. 2, the functional domains of MGD, DGD and SQD proteins have been identified and illustrated. In the MGD protein family, highly conserved diacylglycerol glycosyltransferase (N-terminal) and glycosyltransferase (C-terminal) domains have been revealed. In the DGD protein family, only one glycosyltransferase family1 domain was identified, which was located closer to the C-terminus of the Type A DGDs compared with the smaller Type B DGD proteins. We note that the two types SQD proteins have completely different conserved domains. The Type A AtSQD1 and ZmSQD1 have NAD-dependent dehydratase polypeptides, whereas the Type B SQD proteins have one glycosyltransferase subfamily4-like domain and one glycosyltransferasefamily1 domain. In the SQD protein family, members of each type have a high degree of similarity in the conserved domain construction; however, the Type A and Type B SQDs differ greatly, indicating potential functional differences and differentiation of proteins.
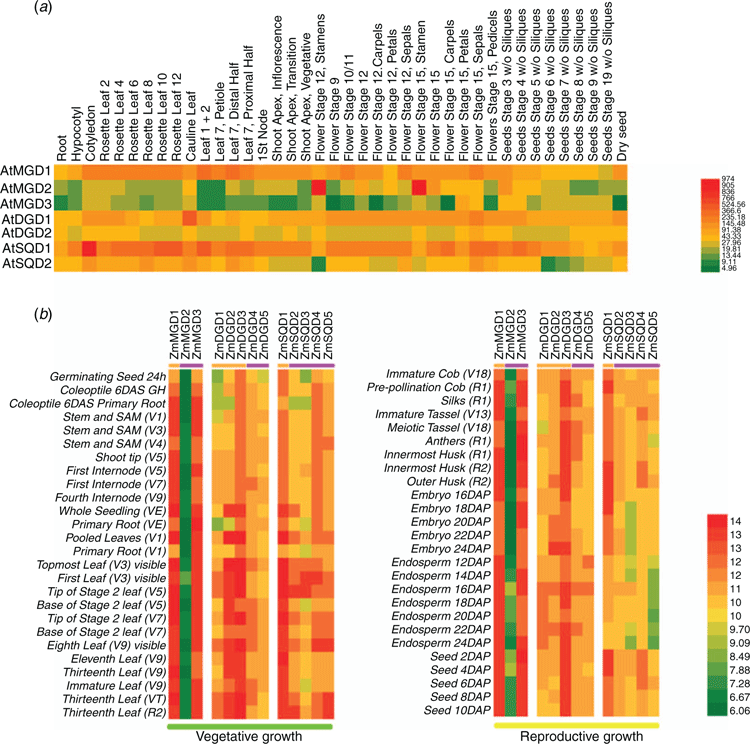
Expression profiles of MGD, DGD and SQD genes in maize and Arabidopsis tissues
To determine the expression profiles of MGD, DGD and SQD genes in different developmental stages of maize and Arabidopsis, we collected data from maize eFP and TAIR database and performed gene expression analysis. As shown in Fig. 3a, Type A Arabidopsis MGD gene (AtMGD1) expressed at a higher level throughout the growth and development stages, but the expression level of Type B AtMGD2 and AtMGD3 was low. Similarly, the expression of Type A DGD (AtDGD1) was higher than that of Type B DGD (AtDGD2) during the whole growth and development stages. The expression of Type A SQD (SQD1) was also significantly higher than that of Type B SQD (SQD2). As shown in Fig. 3b, the expression of ZmMGD1 and ZmMGD3 was at a relative high level throughout both reproductive and vegetative growth stages, whereas the level of ZmMGD2 was extremely low. Among the five ZmDGD genes, the expression of ZmDGD3 was significantly higher than that of other ZmDGD genes. Furthermore, the expression of type A ZmDGDs (ZmDGD1-3) was higher in various leaf developmental stages. In the ZmSQD family, the expression of Type A ZmSQD1 was obviously higher than Type B ZmSQDs, whereas the expression of Type B ZmSQDs was observed at early leaf developmental stages, which could reflect their diverse functions in chloroplast and photosynthesis.
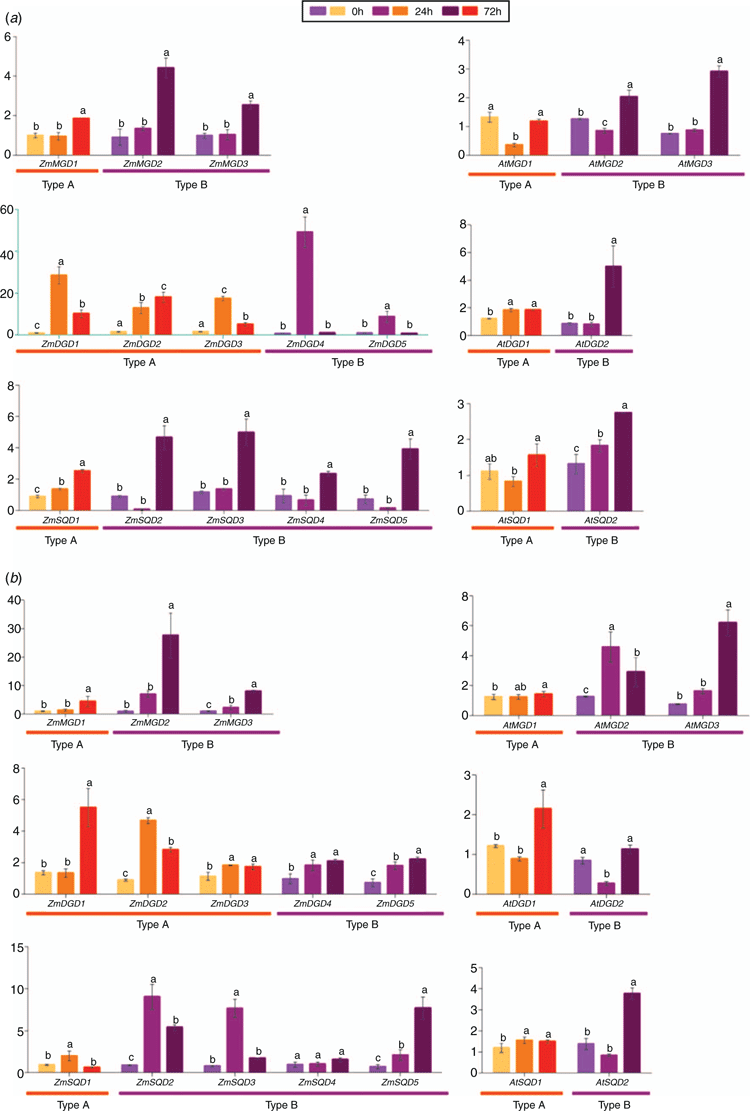
Gene expression analyses of MGD, DGD and SQD genes in maize and Arabidopsis against phosphate starvation
In order to understand the response of MGD, DGD, and SQD genes to phosphorus deficiency, we performed quantitative real-time RT-PCR analysis on the gene expression of Arabidopsis and maize MGD, DGD, and SQD genes. As shown in Fig. 4, under low-phosphorus stress, the expression of ZmMGDs was induced after 72 h under low phosphate treatment, and the induction of Type B ZmMGDs was much significant in both leaf and root tissues. The obvious induction of Type B AtMGDs was also observed (Fig. 4a, b, right panel), and the induction was more pronounced in root tissues compared with the leaves. As for the DGD genes, the expression of Type A and Type B ZmDGDs was significantly upregulated after 24 h under low-phosphorus treatment, and the induction was more evident in maize leaves (Fig. 4a, b, left panel). In Arabidopsis, the Type B AtDGD2 in leaves was apparently induced after 72 h of phosphate starvation, whereas the induction in roots was not significant. In both maize leaf and root tissues under phosphate starvation, a remarkable induced expression of Type B ZmSQDs (ZmSQD2-5) was observed in comparison to Type A ZmSQD (ZmSQD1) (Fig. 4a, b, left panel). In Arabidopsis, the expression of Type B AtSQD2 was also found obviously upregulated under low phosphate, especially in roots(Fig. 4a, b, right panel).
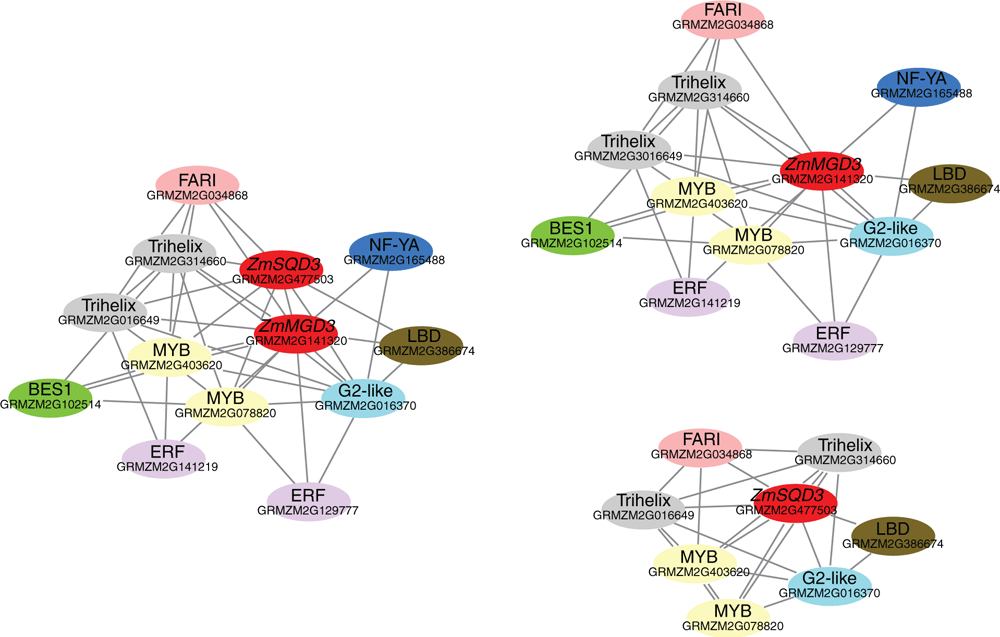
The co-expression analysis of transcription factors and MGD and SQD genes in maize
The significant response of several maize MGD, DGD and SQD genes in response to phosphate starvation prompted us to explore their regulation at the transcriptional level. Co-expression analysis of transcription factors (TFs) and the most low-phosphate responsive genes (ZmMGD3 and ZmSQD3) was performed using Cytoscape ver. 3.4.10. The transcription factors were screened from phosphorus stress transcriptome data using the plant TF database PlanTFDB. The strong low-phosphate responsive genes ZmMGD3 and ZmSQD3 were named as ‘guide genes’ and used as probes to perform co-expression relationship analysis. As shown in Fig. 5, a total of 11 transcription factors involved in the regulation of the guide genes, including MYB (GRMZM2G078820, GRMZM2G403620), ERF (GRMZM2G129777, GRMZM2G141219), Trihelix (GRMZM2G016649, GRMZM2G314660), BES1 (GRMZM2G102514), FAR1 (GRMZM2G034868)), G2 (GRMZM2G016370), LBD (GRMZM2G386674), and NF-YA (GRMZM2G165488). The co-expression network was generated for each individual guide gene, and was also established among both guide genes and the corresponding TFs (Fig. 5). Three transcription factors were found to be significantly associated with ZmMGD3, including MYB (GRMZM2G078820, GRMZM2G403620), and ERF (GRMZM2G129777 and GRMZM2G141219); whereas, Trihelix (GRMZM2G016649, GRMZM2G314660), and MYB(GRMZM2G078820, GRMZM2G403620) were significantly associated with ZmSQD3. Therefore, these transcription factors might be involved in the regulation of low-phosphorus stress response of ZmMGD3 and ZmSQD3.
Metabolism of maize membrane glycerolipids under low phosphorus stress
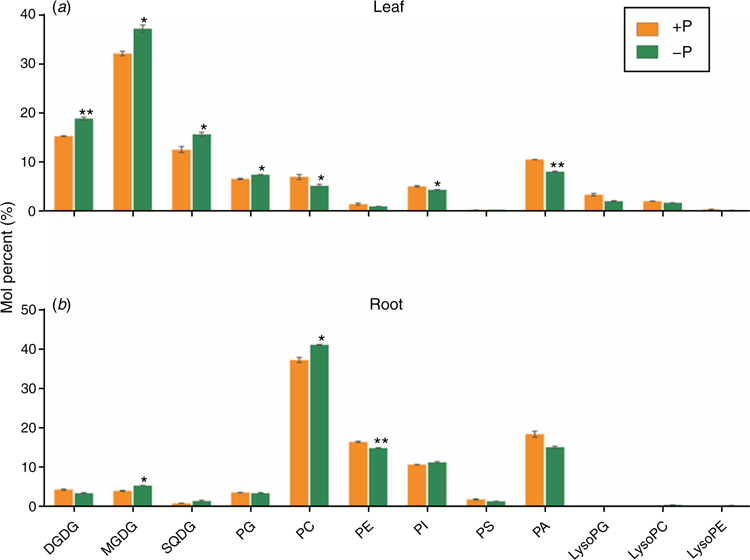
In order to study the membrane lipid metabolism in maize under low-phosphorus stress, the changes of major glycerolipids in maize leaves and roots that under –P treatments for 72 h were measured by lipidomics analysis (Welti et al. 2002). As shown in Fig. 6a, in leaf tissues of the maize seedlings under low phosphorus stress, the molar percentage of the galactolipid (including MGDG and DGDG) and sulfolipid (SQDG) was significantly increased; while the exclusive plastidial phospholipid PG was slightly increased as well. Nevertheless, the content of most of the phospholipids, including PC, PE, PA and PI, was decreased. In root tissues (Fig. 6b), the phospholipids are dominant in proportions. Under low phosphorus condition, the level of PC was elevated; whereas PE and PA were decreased. The changes of the four major chloroplast bilayer lipids were not significant.
Expression profiles of maize MGD, DGD and SQD genes under environmental stresses
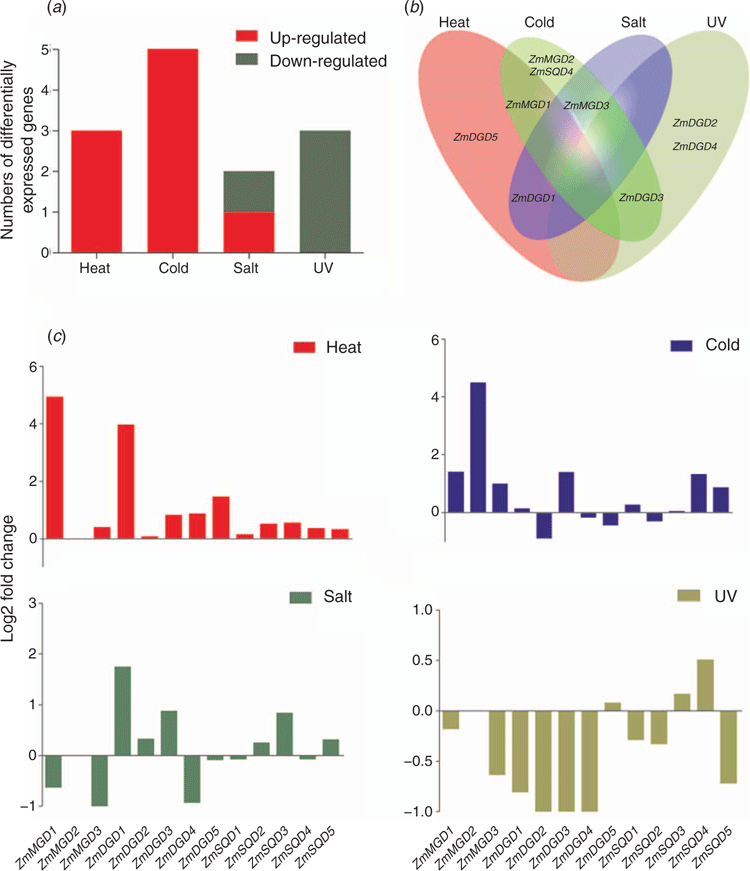
In order to investigate the potential roles of maize MGD, DGD and SQD genes in environmental stress responses, the transcriptional data of these genes under various stress treatment was obtained by searching the available RNA-seq database downloaded from the NCBI sequence-reading archive (SRA). The RNA-seq analysis was conducted on samples from the aerial parts of maize seedlings, and the abiotic stresses treatment includes: cold, heat, salt, and UV. As shown in Fig. 7, under cold stress, ZmMGD1-3, ZmDGD3, and ZmSQD4–5 were upregulated, albeit only ZmMGD2 was significantly differentially expressed (|Log2FC| ≥ 2). Under heat stress, ZmMGD1, ZmDGD1 and ZmDGD3-5 were upregulated, among which ZmMGD1 and ZmDGD1 were differentially expressed. Salt treatment obviously induced the transcripts accumulation of ZmDGD1 and ZmDGD3 and ZmSQD3. However, under UV treatment, most genes were downregulated (Fig. 7). To further understand the regulation patterns, the cis-elements in the promoter regions of these genes were revealed. The results showed that there was large number of cis-elements related to various abiotic stresses and hormone responses identified and could be divided into 12 categories (Fig. S2). The number of cis-elements recognised by transcription factors MYB and WRKY was found relatively high in the promoter region of Type B ZmMGD2 and ZmMGD3 and the Type B SQD genes.
Discussion
In plants, galactolipids(MGDG and DGDG)and sulfolipids (SQDG) account for more than 80% of plastidic membrane lipids, and play a variety of roles on maintaining the structure of cells and organelles, converting light energy to form chemical energy and protecting cells against low-phosphate and adverse environmental conditions (Aronsson et al. 2008; Li et al. 2016b). The enzymes catalysing the formation of MGDG, DGDG and SQDG were termed MGD, DGD and SQD, respectively, and have been identified from various plant species (Awai et al. 2001; Kelly et al. 2003). In previous studies, differential regulation of two types of MGDs under phosphate-limited conditions has been observed in Arabidopsis and several other plants, and the low-phosphate induced MGD isoforms could lead to increased synthesis of non-phosphorus membrane lipid to replace the phosphorus-containing lipid under phosphate deficiency (Nakamura et al. 2009; Koichi et al. 2010).
To better understand the distinct characteristics of different isoforms of MGD, DGD and SQD in plants, we identified their family members in maize and Arabidopsis, and performed comparative bioinformatics and expression analysis. The phylogenetic analysis divided MGD genes into two subgroups: one subgroup containing the AtMGD1 and its orthologs; and another subgroup containing the Arabidopsis AtMGD2 and AtMGD3 and their orthologs from maize and other plant species. In previous studies, the Arabidopsis MGDG synthase is divided into two subtypes: Type A (AtMGD1) and Type B (AtMGD2 and AtMGD3), which function differently in plant development and in their low-phosphate responses (Awai et al. 2001; Kobayashi et al. 2006). In the present study, MGDs from various plant species are also classified with Arabidopsis Type A and Type B MGD orthologs, which indicates that MGDs from other plant species also compliant this classification. Similarly, DGDs and SQDs from different plant species were clustered into two subgroups as well, and were marked as Type A and Type B with reference to the MGDs. Since there are no such classification of DGDs and SQDs reported, this finding suggested that distinct isoforms of these two families of genes might function differently and subjected to differential regulation under certain conditions similar to Type A and Type B MGDs. The functional domain analysis showed that the maize and Arabidopsis MGDs and DGDs shared high similarity in domain structure, whereas members from different groups of SQDs varied significantly, indicating the potential functional divergence. Both AtSQD1 and ZmSQD1 on the Type A branch have a NAD-dependent dehydratase domain, while other SQD proteins have both glycosyltransferase subfamily 4-like and glycosyl transferase family 1 domain. The Arabidopsis SQD2 has been implicated to be relevant to the accumulation a novel plant lipid–glucuronosyldiacylglycerol (GlcADG) under phosphorus depletion condition (Okazaki et al. 2013). This agrees with another study showing that SQD2 participated in flavonoid glycosylation, regulating sugar metabolism and seed setting in rice (Zhan et al. 2017).
In the long-term evolution process, plants gradually formed a protective mechanism of the photosynthetic membrane under phosphorus deficiency, that is, the synthesis of non-phosphorus membrane lipid such as DGDG and SQDG to replace phosphorus-containing membrane lipids, to save phosphate and keep normal photosynthesis under adverse conditions (Andersson and Tjellstrom 2013; Shi et al. 2013). In Arabidopsis, the Type B AtMGD2 and AtMGD3 were strongly activated under phosphate starvation (Mie et al. 2013), and led to the induced MGDG and DGDG synthesis (Kobayashi et al. 2006; Nussaume et al. 2011). In plants, the synthesis of galactolipids MGDG and DGDG involves two different pathways: the plastid/chloroplast localised prokaryotic pathway and the endoplasmic reticulum compartmentalised eukaryotic pathway (Ohlrogge et al. 1991). In Arabidopsis, a typical 16:3 plant, the galactolipids synthesis relies on both eukaryotic pathway and prokaryotic pathway, whereas the 18:3 plant maize has been shown to mainly use the eukaryotic pathway for galactolipids synthesis (Li et al. 2016a; Gu et al. 2017). Since most of the previous studies on the galactolipids metabolism under phosphorous deficiency conditions have focussed on the 16:3 plant Arabidopsis, the information on the 18:3 plants has been lacking.
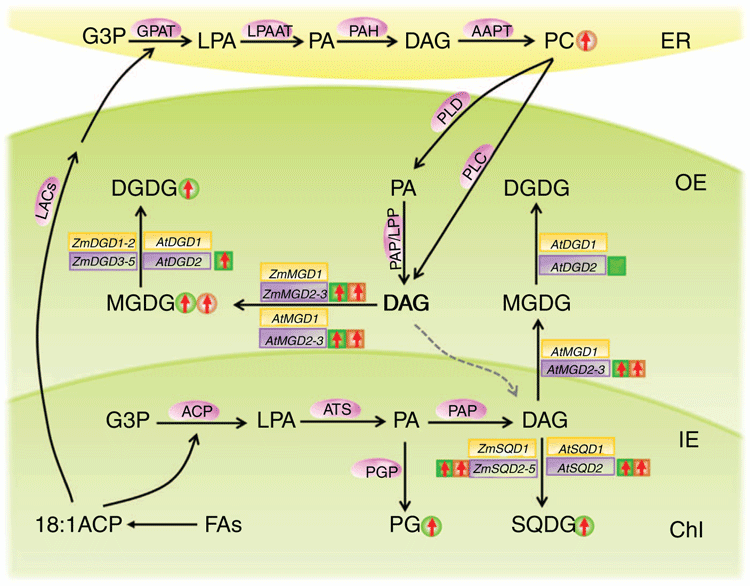
In the present study, the transcriptional response of all maize and Arabidopsis MGD, DGD and SQD genes against phosphate starvation was investigated using quantitative real-time RT-PCR, lipids accumulation was determined, and a comparative analysis was conducted and interpreted (Fig. 8). As shown in Fig. 8, under low phosphorus conditions, the expression of maize and Arabidopsis Type B MGD genes was significantly elevated in both leaf and root tissues, which is in agreement with the previous findings (Shi et al. 2013; Sarkis et al. 2014). The expression of both Type A and Type B DGD genes was induced by phosphorus starvation in maize and Arabidopsis. It has been reported that in Arabidopsis, although both DGD1 and DGD2 were induced by phosphate deprivation, DGD2 seems to be the major enzyme and preferably used MGDG generated by Type B MGD2/MGD3 to produce DGDG in the outer envelope of chloroplast (Härtel et al. 2000; Benning 2009). In comparison of Type A SQDs, the expression of maize and Arabidopsis Type B SQD genes were significantly induced in both leaf and root tissues. In Arabidopsis, AtSQD2 is considered to be directly related to plant response to phosphate stress (Yu et al. 2002). These findings suggested that the Type B MGD, DGD and SQD genes in plants are involved in the synthesis of non-phosphorus lipids under low-phosphate conditions.
In the present study, the lipid analysis was also conducted in maize leaves and roots under low-phosphate conditions. An overall increase in non-phosphorus lipids (MGDG, DGDG and SQDG), and a decrease in phosphorus lipids (PC, PE, PI and PA) were observed, especially in leaf tissues. The results implied that under low-phosphorus stress, the degradation of phospholipids from eukaryotic pathway was enhanced to provide PA and DAG for the synthesis of MGDG, and then DGDG in the 18:3 plant maize. The DAG derived from ER eukaryotic pathway could also be used as substrate for the synthesis of SQDG, leading to increased generation of SQDG (Fig. 8). It has been reported that DGDG is the major bilayer lipid in chloroplast membrane and functions pivotally in maintaining the membrane integrity under stress conditions (Li et al. 2016b). Studies also showed that phospholipid synthesis in leaves is affected by the lack of phosphorus supply, whereas photosynthetic membrane lipids were significantly elevated to maintain membrane lipid stability (Benning 2009; Moellering and Benning 2011).
Phosphate starvation usually combines with other abiotic stresses. Plant MGD, DGD and SQD genes are found play certain roles in various environmental stress responses. The overexpression of rice MGDG synthase gene (OsMGD) in tobacco produces a large amount of galactolipid in leaves, resulting in enhanced salt tolerance of the tobacco plant (Wang et al. 2014). DGD gene could regulate the heat tolerance of Arabidopsis by regulating the ratio of the thylakoid MGDG and DGDG, and improved the heat resistance of the plant at the physiological level (Chen et al. 2006). In addition, the drought response of plants in maize is affected by the upregulation of galactolipid biosynthesis genes (Chen et al. 2018). In our study, differential expression profiles of maize MGD, DGD and SQD genes was observed under the heat, cold, salt and UV stresses. The expression of ZmMGD and ZmDGD genes was significantly affected by temperature stress, especially the Type A ZmMGDs. The Type B ZmSQDs were more responsive to temperature and salt stresses. It is worth noting that most maize MGD, DGD and SQD genes were repressed by UV treatment.
Phosphate deficiency of soil and abiotic stresses are major factors limit crop production in many regions of the world, As a result, plants have developed various biochemical adaptive mechanisms to deal with these stresses, including membrane lipid remodelling (Okazaki et al. 2013; Li et al. 2016a). In the present study, the bioinformatics analysis of maize MGD, DGD and SQD genes and their responses to phosphate depletion and abiotic stresses produced findings that will help for further characterisation of genes against phosphorus depletion and for the generation of improved crops adapted to phosphate starvation and other abiotic stresses.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
We kindly acknowledge Dr Pinghua Li (Shandong Agricultural University, China) and Dr Liying Song (Harbin Botai Bio-tech Co. Ltd, China) for their assistance on the transcriptome data analysis. We would like to thank Dr Ruth Welti and Mary Roth (Kansas Lipidomics Research Centre) for their help with lipidomic analysis. This work was supported by grants from the National Transgenic Science and Technology Program (2019ZX08010-003); National Natural Science Foundation of China (31701328); The National Key Research and Development Program of China (2016YF0101002); Heilongjiang Bayi Agricultural University Scientific Start-up Found for the Returned Overseas Chinese Scholar (2031011047); Heilongjiang Bayi Agricultural University Support Program for San Heng San Zong. The authors declare that the supporting sources had no involvement in the preparation of the data or manuscript or the decision to submit for publication.
References
Andersson MX, Tjellstrom H (2013) Plastid membrane lipids in stress and development. Current Chemical Biology 6, 205–216.| Plastid membrane lipids in stress and development.Crossref | GoogleScholarGoogle Scholar |
Aronsson H, Schottler MA, Kelly AA, Sundqvist C, Dörmann P, Karim S, Jarvis P (2008) Monogalactosyldiacylglycerol deficiency in Arabidopsis affects pigment composition in the prolamellar body and impairs thylakoid membrane energization and photoprotection in leaves. Plant Physiology 148, 580–592.
| Monogalactosyldiacylglycerol deficiency in Arabidopsis affects pigment composition in the prolamellar body and impairs thylakoid membrane energization and photoprotection in leaves.Crossref | GoogleScholarGoogle Scholar | 18641085PubMed |
Awai K, Maréchal E, Block MA, Brun D, Masuda T, Shimada H, Takamiya K, Ohta H, Joyard J (2001) Two types of MGDG synthase genes, found widely in both 16:3 and 18:3 plants, differentially mediate galactolipid syntheses in photosynthetic and nonphotosynthetic tissues in Arabidopsis thaliana. Proceedings of the National Academy of Sciences of the United States of America 98, 10960–10965.
| Two types of MGDG synthase genes, found widely in both 16:3 and 18:3 plants, differentially mediate galactolipid syntheses in photosynthetic and nonphotosynthetic tissues in Arabidopsis thaliana.Crossref | GoogleScholarGoogle Scholar | 11553816PubMed |
Benning C (2009) Mechanisms of lipid transport involved in organelle biogenesis in plant cells. Annual Review of Cell and Developmental Biology 25, 71–91.
| Mechanisms of lipid transport involved in organelle biogenesis in plant cells.Crossref | GoogleScholarGoogle Scholar | 19572810PubMed |
Benning C, Beatty JT, Prince RC, Somerville CR (1993) The sulfolipid sulfoquinovosyldiacylglycerol is not required for photosynthetic electron transport in Rhodobacter sphaeroides but enhances growth under phosphate limitation. Proceedings of the National Academy of Sciences of the United States of America 90, 1561–1565.
| The sulfolipid sulfoquinovosyldiacylglycerol is not required for photosynthetic electron transport in Rhodobacter sphaeroides but enhances growth under phosphate limitation.Crossref | GoogleScholarGoogle Scholar | 8434018PubMed |
Chen M, Thelen JJ (2013) Acyl-lipid desaturase2 is required for chilling and freezing tolerance in Arabidopsis. The Plant Cell 25, 1430–1444.
| Acyl-lipid desaturase2 is required for chilling and freezing tolerance in Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 23585650PubMed |
Chen J, Burke JJ, Xin Z, Xu C, Velten J (2006) Characterization of the Arabidopsis thermosensitive mutant atts02 reveals an important role for galactolipids in thermotolerance. Plant, Cell & Environment 29, 1437–1448.
| Characterization of the Arabidopsis thermosensitive mutant atts02 reveals an important role for galactolipids in thermotolerance.Crossref | GoogleScholarGoogle Scholar |
Chen D, Wang S, Qi L, Yin L, Deng X (2018) Galactolipid remodeling is involved in drought-induced leaf senescence in maize. Environmental and Experimental Botany 150, 57–68.
| Galactolipid remodeling is involved in drought-induced leaf senescence in maize.Crossref | GoogleScholarGoogle Scholar |
Denev I, Minkov I (2001) Structure and function of photosynthetic membranes in higher plants. Proceedings of the National Academy of Sciences of the United States of America 98, 10960–10965.
Dörmann P, Hoffmann-Benning S, Balbo I, Benning C (1995) Isolation and characterization of an Arabidopsis mutant deficient in the thylakoid lipid digalactosyl diacylglycerol. The Plant Cell 7, 1801–1810.
Essigmann B, Güler S, Narang RA, Linke D, Benning C (1998) Phosphate availability affects the thylakoid lipid composition and the expression of SQD1, a gene required for sulfolipid biosynthesis in Arabidopsis thaliana. Proceedings of the National Academy of Sciences of the United States of America 95, 1950–1955.
| Phosphate availability affects the thylakoid lipid composition and the expression of SQD1, a gene required for sulfolipid biosynthesis in Arabidopsis thaliana.Crossref | GoogleScholarGoogle Scholar | 9465123PubMed |
Fujii S, Kobayashi K, Nagata N, Masuda T, Wada H (2018) Digalactosyldiacylglycerol is essential for organization of the membrane structure in etioplasts. Plant Physiology 177, 1487–1497.
| Digalactosyldiacylglycerol is essential for organization of the membrane structure in etioplasts.Crossref | GoogleScholarGoogle Scholar | 29946018PubMed |
Gu Y, He L, Zhao C, Wang F, Yan B, Gao Y (2017) Biochemical and transcriptional regulation of membrane lipid metabolism in maize leaves under low temperature. Frontiers of Plant Science 8, 2053
| Biochemical and transcriptional regulation of membrane lipid metabolism in maize leaves under low temperature.Crossref | GoogleScholarGoogle Scholar |
Güler S, Seeliger A, Härtel H, Renger G, Benning C (1996) A null mutant of Synechococcus sp. PCC7942 deficient in the sulfolipid sulfoquinovosyl diacylglycerol. The Journal of Biological Chemistry 271, 7501
| A null mutant of Synechococcus sp. PCC7942 deficient in the sulfolipid sulfoquinovosyl diacylglycerol.Crossref | GoogleScholarGoogle Scholar | 8631780PubMed |
Härtel H, Dörmann P, Benning C (2000) DGD1-independent biosynthesis of extraplastidic galactolipids after phosphate deprivation in Arabidopsis. Proceedings of the National Academy of Sciences of the United States of America 97, 10649
| DGD1-independent biosynthesis of extraplastidic galactolipids after phosphate deprivation in Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 10973486PubMed |
Heinz E, Roughan PG (1983) Similarities and differences in lipid metabolism of chloroplasts isolated from 18:3 and 16:3 plants. Plant Physiology 72, 273–279.
| Similarities and differences in lipid metabolism of chloroplasts isolated from 18:3 and 16:3 plants.Crossref | GoogleScholarGoogle Scholar | 16662992PubMed |
Kelly AA, Froehlich JE, Dörmann P (2003) Disruption of the two digalactosyldiacylglycerol synthase genes DGD1 and DGD2 in Arabidopsis reveals the existence of an additional enzyme of galactolipid synthesis. The Plant Cell 15, 2694
| Disruption of the two digalactosyldiacylglycerol synthase genes DGD1 and DGD2 in Arabidopsis reveals the existence of an additional enzyme of galactolipid synthesis.Crossref | GoogleScholarGoogle Scholar | 14600212PubMed |
Kelly AA, Kalisch B, Hölzl G, Schulze S, Thiele J, Michael M, Roston RL, Benning C, Doman P (2016) Synthesis and transfer of galactolipids in the chloroplast envelope membranes of Arabidopsis thaliana. Proceedings of the National Academy of Sciences of the United States of America 113, 10714
| Synthesis and transfer of galactolipids in the chloroplast envelope membranes of Arabidopsis thaliana.Crossref | GoogleScholarGoogle Scholar | 27601658PubMed |
Kobayashi K, Masuda T, Takamiya K, Ohta H (2006) Membrane lipid alteration during phosphate starvation is regulated by phosphate signaling and auxin/cytokinin cross-talk. The Plant Journal 47, 238–248.
| Membrane lipid alteration during phosphate starvation is regulated by phosphate signaling and auxin/cytokinin cross-talk.Crossref | GoogleScholarGoogle Scholar | 16762032PubMed |
Kobayashi K, Kondo M, Fukuda H, Nishimura M, Ohta H (2007) Galactolipid synthesis in chloroplast inner envelope is essential for proper thylakoid biogenesis, photosynthesis, and embryogenesis. Proceedings of the National Academy of Sciences of the United States of America 104, 17216–17221.
| Galactolipid synthesis in chloroplast inner envelope is essential for proper thylakoid biogenesis, photosynthesis, and embryogenesis.Crossref | GoogleScholarGoogle Scholar | 17940034PubMed |
Koichi K, Koichiro A, Masanobu N, Akira N, Tatsuru M (2010) Type-B monogalactosyldiacylglycerol synthases are involved in phosphate starvation-induced lipid remodeling, and are crucial for low-phosphate adaptation. The Plant Journal 57, 322–331.
Lei L, Shi J, Chen J, Zhang M, Sun S, Xie S, Li X, Zeng B, Peng L, Hauck A, Zhao H, Song W, Fan Z, Lai J (2015) Ribosome profiling reveals dynamic translational landscape in maize seedlings under drought stress. Plant Journal for Cell & Molecular Biology 84, 1206–1218.
| Ribosome profiling reveals dynamic translational landscape in maize seedlings under drought stress.Crossref | GoogleScholarGoogle Scholar |
Li HM, Yu CW (2018) Chloroplast galactolipids the link between photosynthesis, chloroplast shape, jasmonates, phosphate starvation and freezing tolerance. Plant & Cell Physiology 59, 1128–1134.
| Chloroplast galactolipids the link between photosynthesis, chloroplast shape, jasmonates, phosphate starvation and freezing tolerance.Crossref | GoogleScholarGoogle Scholar |
Li N, Xu C, Li-Beisson Y, Philippar K (2016a) Fatty acid and lipid transport in plant cells. Trends in Plant Science 21, 145
| Fatty acid and lipid transport in plant cells.Crossref | GoogleScholarGoogle Scholar | 26616197PubMed |
Li Q, Shen W, Zheng Q, Fowler DB, Zou J (2016b) Adjustments of lipid pathways in plant adaptation to temperature stress. Plant Signaling & Behavior 11, e1058461
Makarevitch I, Waters AJ, West PT, Stitzer M, Hirsch CN, Ross-Ibarra J, Springer NM (2015) Transposable elements contribute to activation of maize genes in response to abiotic stress. PLOS Genetics 11, e1004915
| Transposable elements contribute to activation of maize genes in response to abiotic stress.Crossref | GoogleScholarGoogle Scholar | 26452261PubMed |
Mie S, Takahide W, Yuka M, Ryota K, Yamamoto MP, Masuda K, Yamada K, Masuda S, Ohta H (2013) Differential regulation of two types of monogalactosyldiacylglycerol synthase in membrane lipid remodeling under phosphate-limited conditions in sesame plants. Frontiers of Plant Science 4, 469
| Differential regulation of two types of monogalactosyldiacylglycerol synthase in membrane lipid remodeling under phosphate-limited conditions in sesame plants.Crossref | GoogleScholarGoogle Scholar |
Moellering ER, Benning C (2011) Galactoglycerolipid metabolism under stress: a time for remodeling. Trends in Plant Science 16, 98–107.
| Galactoglycerolipid metabolism under stress: a time for remodeling.Crossref | GoogleScholarGoogle Scholar | 21145779PubMed |
Nakamura Y, Koizumi R, Shui G, Shimojima M, Wenk MR, Ito T, Ohta H (2009) Arabidopsis lipids mediate eukaryotic pathway of lipid metabolism and cope critically with phosphate starvation. Proceedings of the National Academy of Sciences of the United States of America 106, 20978–20983.
| Arabidopsis lipids mediate eukaryotic pathway of lipid metabolism and cope critically with phosphate starvation.Crossref | GoogleScholarGoogle Scholar | 19923426PubMed |
Narayanan V, Honce MJ, Mehrotra S, Camidge DR (2016) Cystic brain metastases occurring in anaplastic lymphoma kinase gene rearranged non–small-cell lung cancer patients receiving Crizotinib. Clinical Lung Cancer 17, 85–90.
| Cystic brain metastases occurring in anaplastic lymphoma kinase gene rearranged non–small-cell lung cancer patients receiving Crizotinib.Crossref | GoogleScholarGoogle Scholar | 26314227PubMed |
Narayanan S, Pvv P, Welti R (2018) Alterations in wheat pollen lipidome during high day and night temperature stress. Plant, Cell & Environment 41, 1749–1761.
| Alterations in wheat pollen lipidome during high day and night temperature stress.Crossref | GoogleScholarGoogle Scholar |
Nussaume L, Maréchal E, Thibaud MC, Block MA (2011) Plant plasma membrane and phosphate deprivation. Plant Cell Monographs 19, 237–251.
| Plant plasma membrane and phosphate deprivation.Crossref | GoogleScholarGoogle Scholar |
Ohlrogge JB, Browse J, Somerville CR (1991) The genetics of plant lipids. Biochimica et Biophysica Acta 1082, 1
| The genetics of plant lipids.Crossref | GoogleScholarGoogle Scholar | 1901223PubMed |
Okazaki Y, Otsuki H, Narisawa T, Kobayashi M, Sawai S, Kamide Y, Kusano M, Aoki T, Hirai MY, Saito K (2013) A new class of plant lipid is essential for protection against phosphorus depletion. Nature Communications 4, 1510
| A new class of plant lipid is essential for protection against phosphorus depletion.Crossref | GoogleScholarGoogle Scholar | 23443538PubMed |
Rocha J, Nitenberg M, Girard-Egrot A, Jouhet J, Maréchal E, Block MA, Breton C (2018) Do galactolipid synthases play a key role in the biogenesis of chloroplast membranes of higher plants. Frontiers of Plant Science 9, 126
| Do galactolipid synthases play a key role in the biogenesis of chloroplast membranes of higher plants.Crossref | GoogleScholarGoogle Scholar |
Sarkis J, Rocha J, Maniti O, Jouhet J, Vié V, Block MA, Breton C, Maréchal E, Girard-Egrot A (2014) The influence of lipids on MGD1 membrane binding highlights novel mechanisms for galactolipid biosynthesis regulation in chloroplasts. The FASEB Journal 28, 3114
| The influence of lipids on MGD1 membrane binding highlights novel mechanisms for galactolipid biosynthesis regulation in chloroplasts.Crossref | GoogleScholarGoogle Scholar | 24692595PubMed |
Sebastián M, Smith AF, González JM, Fredricks HF, Mooy BV, Koblížek M, Brandsma J, Koster G, Mestre M, Mostajir M, Pitta P, Postle AD, Sánchez P, Gasol JM, Scanlan DJ, Chen Y (2015) Lipid remodelling is a wide spread strategy in marine heterotrophic bacteria upon phosphorus deficiency. The ISME Journal 10, 968–978.
| Lipid remodelling is a wide spread strategy in marine heterotrophic bacteria upon phosphorus deficiency.Crossref | GoogleScholarGoogle Scholar | 26565724PubMed |
Shi ZH, Wang SW, Yin LN, Zhang MJ, Deng XP (2013) Tolerance of tobacco plans with OsMGD gene to low phosphorus stress. Journal of Northwest A & F University 41, 97–104.
Wada H, Murata N (2010) Lipids in thylakoid membranes and photosynthetic cells. Advances in Photosynthesis & Respiration 30, 1–9.
Wang S, Uddin MI, Tanaka K, Yin L, Shi Z, Qi Y, Mano J, Matsui K, Shimomura N, Sakaki T, Deng X, Zhang S (2014) Maintenance of chloroplast structure and function by overexpression of the rice MONOGALACTOSYLDIACYLGLYCEROL SYNTHASE gene leads to enhanced salt tolerance in tobacco. Plant Physiology 165, 1144
| Maintenance of chloroplast structure and function by overexpression of the rice MONOGALACTOSYLDIACYLGLYCEROL SYNTHASE gene leads to enhanced salt tolerance in tobacco.Crossref | GoogleScholarGoogle Scholar | 24843077PubMed |
Welti R, Li W, Li M, Sang Y, Biesiada H, Zhou H-E, Rajashekar CB, Williams TD, Wang X (2002) Profiling membrane lipids in plant stress responses. Role of phospholipase Dalpha in freezing-induced lipid changes in Arabidopsis. Journal of Biological Chemistry 277, 31994–32002.
| Profiling membrane lipids in plant stress responses. Role of phospholipase Dalpha in freezing-induced lipid changes in Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 12077151PubMed |
Xu Y, Liu F, Han G, Cheng B (2018) Genome-wide identification and comparative analysis of phosphate starvation-responsive transcription factors in maize and three other gramineous plants. Plant Cell Reports 37, 711–726.
| Genome-wide identification and comparative analysis of phosphate starvation-responsive transcription factors in maize and three other gramineous plants.Crossref | GoogleScholarGoogle Scholar | 29396709PubMed |
Yu B, Xu C, Benning C (2002) Arabidopsis disrupted in SQD2 encoding sulfolipid synthase is impaired in phosphate-limited growth. Proceedings of the National Academy of Sciences of the United States of America 99, 5732–5737.
| Arabidopsis disrupted in SQD2 encoding sulfolipid synthase is impaired in phosphate-limited growth.Crossref | GoogleScholarGoogle Scholar | 11960029PubMed |
Zhan X, Shen Q, Wang X, Hong Y (2017) The sulfoquinovosyltransferase-like enzyme SQD2.2 is involved in flavonoid glycosylation, regulating sugar metabolism and seed setting in rice. Scientific Reports 7, 4685
| The sulfoquinovosyltransferase-like enzyme SQD2.2 is involved in flavonoid glycosylation, regulating sugar metabolism and seed setting in rice.Crossref | GoogleScholarGoogle Scholar | 28680100PubMed |
* These authors contributed equally to this work.