Impact of ancestral wheat sodium exclusion genes Nax1 and Nax2 on grain yield of durum wheat on saline soils
Richard A. James A F , Carol Blake A , Alexander B. Zwart B , Ray A. Hare C , Anthony J. Rathjen D and Rana Munns A EA CSIRO Plant Industry, GPO Box 1600, Canberra, ACT 2601, Australia.
B CSIRO Mathematics, Information and Statistics, GPO Box 664, Canberra, ACT 2601, Australia.
C NSW Department of Primary Industries, RMB 944, Tamworth, NSW 2340, Australia.
D University of Adelaide, Waite Campus, Glen Osmond, SA 5064, Australia.
E School of Plant Biology, University of Western Australia, Crawley, WA 6009, Australia.
F Corresponding author. Email: richard.james@csiro.au
Functional Plant Biology 39(7) 609-618 https://doi.org/10.1071/FP12121
Submitted: 18 April 2012 Accepted: 18 May 2012 Published: 4 July 2012
Journal Compilation © CSIRO Publishing 2012 Open Access CC BY-NC-ND
Abstract
Nax1 and Nax2 are two genetic loci that control the removal of Na+ from the xylem and thereby help to exclude Na+ from leaves of plants in saline soil. They originate in the wheat ancestral relative Triticum monococcum L. and are not present in modern durum or bread wheat. The Nax1 and Nax2 loci carry TmHKT1;4-A2 and TmHKT1;5-A, respectively, which are the candidate genes for these functions. This paper describes the development of near-isogenic breeding lines suitable for assessing the impact of the Nax loci and their performance in controlled environment and fields of varying salinity. In young plants grown in 150 mM NaCl, Nax1 reduced the leaf Na+ concentration by 3-fold, Nax2 by 2-fold and both Nax1 and Nax2 together by 4-fold. In 250 mM NaCl, Nax1 promoted leaf longevity and greater photosynthesis and stomatal conductance. In the uppermost leaf, the Na+-excluding effect of the Nax loci was much stronger. In the field, Na+ in the flag leaf was reduced 100-fold by Nax1 and 4-fold by Nax2; however, Nax1 lines yielded 5–10% less than recurrent parent (cv. Tamaroi) in saline soil. In contrast, Nax2 lines had no yield penalty and at high salinity they yielded close to 25% more than Tamaroi, indicating this material is suitable for breeding commercial durum wheat with improved yield on saline soils.
Additional keywords: HKT, osmotic stress, salinity, salt tolerance, turgor.
Introduction
Soil salinity is a major issue in the more arid regions of the world, where soil and environmental factors contribute to the accumulation of salts to a level that adversely affects crop production. Salts can arise from weathering of rocks and from the ocean via wind or rain. Application of poor quality irrigation water and capillary rise of shallow saline groundwater can all contribute to the salinisation of the upper soil layers. Seawater intrusion onto land, due to global warming causing rising sea levels and more violent storms, can deposit a large amount of salts in soils of coastal lands. These particular processes contributing to salinisation, combined with the influence of other climatic and landscape features and the effects of human activities, determine where the salt accumulates in the landscape (Rengasamy 2010). The type of vegetation also determines how far below the soil surface salts accumulate (Rengasamy 2002).
Durum (tetraploid) wheat (Triticum turgidum L. ssp. durum (Desf.)) can attract a significantly higher price on the international market than hexaploid (bread) wheat (Triticum aestivum L.); however, it is poorly adapted to salt-affected soils (Zubaidi et al. 1999a). Its yield is more affected by salinity than bread wheat (Francois et al. 1986; Maas and Grieve 1990). Durum wheat is less able to exclude Na+ (Zubaidi et al. 1999b; Munns and James 2003), a trait associated with salt sensitivity in the Triticeae (Gorham et al. 1990; Dvořák et al. 1994). Durum wheat lacks the Na+-excluding locus Kna1 which enables hexaploid wheat to maintain lower leaf Na+ and a greater K+ to Na+ ratio than durum wheat (Dvořák et al. 1994; Dubcovsky et al. 1996).
A search of an international collection of durum-related genotypes revealed the durum derivative ‘Line 149’ (AUS17045), which was found to have two genetic loci named Nax1 and Nax2 (Munns et al. 2003). Both loci had originated in the diploid wheat Triticum monococcum L. and had been serendipitously crossed into ‘Line 149’ by a breeder crossing genes for rust resistance in T. monococcum into durum wheat (The 1973; James et al. 2006a). As the durum wheat cultivar used for this cross (cv. Marrocos) was not adapted to Australian field conditions, further crossing was made into an Australian durum cultivar, Tamaroi. Nax1 was located on chromosome 2A (Lindsay et al. 2004). Nax2 was located on chromosome 5A (Byrt et al. 2007). Nax1 removes Na+ from the xylem in roots and the lower parts of leaves, the leaf sheaths, whereas Nax2 removes Na+ from the xylem, only in the roots (James et al. 2006a). This is a novel source of Na+ exclusion, not present in durum or hexaploid wheat (Huang et al. 2008). Both loci confer a reduced rate of Na+ transport from roots to shoots by retrieving Na+ from root xylem (Davenport et al. 2005; James et al. 2006a). Fine mapping of the Nax1 locus indicated that the candidate gene was TmHKT1;4-A2 (Huang et al. 2006), which likely encodes for a Na+-specific transporter located on the plasma membrane of cells surrounding xylem vessels (Munns et al. 2012). An HKT1;5-like gene was proposed as the candidate for both Nax2 and Kna1 (Byrt et al. 2007).
This paper describes the pre-breeding work that led to the development of near-isogenic lines with and without Nax1 and Nax2 and the results with Nax1 in comparison to Nax2 on the performance of durum wheat when grown in a farmer’s fields of varying salinity. Recently we showed that the presence of Nax2 could increase the grain yield of wheat on salt-affected farmers’ fields by up to 25% (Munns et al. 2012).
There has been little evaluation of salt tolerance traits on performance in the field, especially for wheat, but also for most other species; yet, scaling up from the level of the gene to yield of a crop is essential to test the validity of a trait for useful purposes (Passioura 2010). Recombinant durum lines with and without the Kna1 locus for K+/Na+ discrimination introgressed from hexaploid wheat were evaluated in saline and non-saline fields by Dvořák et al. (1994). The Kna1 lines had a similar biomass and yield to the kna1 lines on fields of low salinity and a 12–17% increase in biomass on saline fields. Grain yields on saline fields were increased by 9–15% by Kna1, but this was not statistically significant. However, the differences in Na+ exclusion controlled by the Kna1 locus in this genetic background were small. The durum lines containing Kna1 had a K+ : Na+ ratio only twice that of the kna1 lines, with Na+ and K+ concentrations being not significantly different. It is likely that larger differences in exclusion would give larger differences in yield on saline soil.
Several key factors differ between controlled environments and the field. Differences include light quality and quantity, unlimited rooting volume and large diurnal changes in temperature that cannot be supplied by a controlled environment facility. The other is that soil is a very different ionic medium from solution culture. A clay soil can influence Na+ and Cl– availability and can result in different growth responses (Tavakkoli et al. 2010). There is, therefore, the need to confirm the usefulness of results found in controlled environments in the typical soils and ambient conditions that occur in grain production fields. To do this, the genes or trait of interest must be crossed into adapted germplasm, that is, into a cultivar or advanced breeding lines of commercial breeders.
The many steps required in the process of trait breeding have been summarised by Richards et al. (2010). This starts with a genotype chosen because it holds a desirable trait and finishes with a commercial product. The trait is crossed into a current cultivar or advanced breeder’s line that is adapted to the target environment with the appropriate phenology, disease resistance and grain quality. A number (3–6) of backcrosses need to be made, with the trait selected in each generation. Out of this several possible lines are bulked up for testing. These lines undergo the following analysis:
-
evaluation of the trait under controlled environment conditions, or selection based on DNA markers (if reliable markers have already been established for the trait);
-
evaluation of breeding lines in non-saline field conditions; and
-
measurement of yield on well characterised saline field sites.
The present work presents results of breeding lines containing Nax loci in the commercial cultivar Tamaroi. First, we evaluated the lines in a controlled environment by measuring the effect on Na+ exclusion and on rates of photosynthesis. Second, we evaluated the yield in the field in non-saline soil, with unexpected results. Third, we measured yield in a saline field, chosen in the middle of the durum growing area of northern New South Wales, Australia. Key field results with Nax2 have recently been published (Munns et al. 2012). Here we compare the field performance of both Nax1 and Nax2 and describe the breeding program that developed the advanced breeding lines and also circumvented the yield penalty found with earlier material containing Nax1.
Materials and methods
Germplasm development
Backcrossed lines homozygous for Nax1 and Nax2 were developed from a cross between durum wheat (Triticum turgidum L. ssp. durum) Line 149 (Australian Winter Cereals Collection catalogue number AUS17045) and the Australian durum cultivar Tamaroi, with Tamaroi the recurrent parent for backcrosses (James et al. 2006a). Early stages of the breeding process were completed using phenotypic selection of F2 populations, where F2 plants with a leaf Na+ concentration as low as Line 149 were selected for subsequent backcrossing to Tamaroi. A population of 100 BC4F2 seedlings were initially screened for leaf Na+ concentration and also genotyped for the presence of Nax1 (TmHKT1;4-A2), using a co-dominant marker gwm312 developed by Lindsay et al. (2004). Following further selfing and subsequent seed multiplication, BC4F4 families were also assayed for the presence of Nax2 (TmHKT1;5-A), initially using flanking dominant molecular markers gwm410, gwm291 as described in Byrt et al. (2007). Later, a linked co-dominant marker, cslinkNax2, was used for validation of BC4F4 families homozygous for Nax2. Selected lines for evaluation were given the annotation Tamaroi[+]Nax2. Primer sequences and PCR protocols are described by James et al. (2011).
Initial field trials of a selected set of 60 BC4F4 families indicated that most lines containing Nax1 carried a yield penalty of 10 – 15% on non-saline soils (data not shown). One BC4 line (5020), which was homozygous for Nax1 and heterogeneous for Nax2, was found to consistently yield similarly to recurrent parent Tamaroi, indicating that it did not carry the yield penalty typically associated with Nax1. Consequently, a new set of BC4F5 sibling lines were developed from Line 5020 that were either homozygous for Nax1 or homozygous for both Nax1 and Nax2. These lines were given the annotations Tamaroi[+]Nax1 or Tamaroi[+]Nax1,2 respectively.
Germplasm used for field trials at Yanco, Yuluma and Moree
A total of 15 Tamaroi-derived BC4 Nax breeding lines were evaluated for yield potential under non-saline field conditions at Yanco in 2007. This set included six BC4 Nax1 lines (5020–7, 5020–8, 5020–11, 5020–27, 5020–29, 5020–43), four BC4 Nax2 lines (5004, 5042, 6096, 6139) and five BC4 lines fixed for both Nax1 and Nax2 (5020–3, 5020–18, 5020–20, 5020–26, 5020–30). For field trials on saline soils at Yuluma (2009) and Moree (2008, 2009) a subset from the BC4 Nax lines listed above were used for intensive evaluation and comparison to recurrent parent Tamaroi. This subset consisted of two BC4 Nax1 lines (5020–7, 5020–27), two BC4 Nax2 lines (5004, 5042) and two BC4 lines fixed for both Nax1 and Nax2 (5020–20, 5020–26).
Measurement of Na+ uptake into durum wheat leaves in 150 mM NaCl
The effect of Nax1 and Nax2 separately and the potential additive effect of Nax1 and Nax2 together on Na+ accumulation in durum wheat leaves was evaluated on Tamaroi-derived BC4 lines containing Nax1 (4 lines), Nax2 (4 lines) and Nax1 plus Nax2 (4 lines) and compared with Tamaroi. Plants were grown in supported hydroponics in two 40-L trays as described previously (James et al. 2008). At ~8 days after emergence and just before the appearance of leaf 3, 25 mM NaCl was added twice daily to a final concentration of 150 mM. Supplemental Ca2+ was also added as CaCl2 to give a final Na+ : Ca2+ of 15 : 1. Plants were grown in a controlled environment chamber with a 12 h photoperiod and a PPFD of 800 μmol m–2 s–1 at 24°C during the day and 18°C during the night. After 10 days in 150 mM NaCl, the blade of leaf 3 was harvested and dried at 70°C for 2 days, weighed, extracted in 500 mM HNO3 at 80°C for 1.5 h and analysed for Na+ and K+ by an inductively coupled plasma – atomic emission spectrometer (Vista Pro, Varian, Melbourne, Vic.).
Impact of Nax1 on CO2 assimilation rate of salt-stressed durum wheat in 250 mM NaCl
To assess the effect of Nax1 on photosynthesis of salt-stressed durum wheat, Tamaroi and a Tamaroi-derived BC4 Nax1 line (Line 5020–11) was grown in 250 mM NaCl for 30 days. Plants were grown hydroponically using an automatic subirrigation system as described above. Glasshouse air temperatures were maintained at 25°C during the day and 15°C during the night and daily PAR averaged 21.7 mol m–2 day–1. The experiment was set up as a randomised block design, with six replications per genotype per harvest. Main stem leaf 3 emerged at the commencement of the salt treatment, when the seedling was 8 days old (8 days after emergence, DAE). After 20 days in the salt treatment, gas-exchange measurements, chlorophyll content and Na+ concentration were determined on leaf 3. Gas-exchange measurements were taken from the mid portion of leaf 3 using a LI-6400 portable gas exchange system (Li-Cor, Lincoln, NE, USA). All measurements were taken between 1100 and 1500 hours on sunny well lit days. LI-6400 settings were chosen to approximate glasshouse conditions; leaf temperature was maintained at 25°C, light intensity was set at 1000 μmol m–2 s–1 with a red/blue light source, CO2 was maintained at 400 μmol mol–1 and the leaf to air vapour pressure deficit (VPD) maintained between 1.2 and 1.4 KPa. Following each gas-exchange measurement the leaf was harvested and leaf chlorophyll content was estimated using a SPAD 502 m (Minolta, Osaka, Japan) and analysed for Na+ and K+ as described above. Additionally, main stem leaves 1–4 were analysed for Na+ and K+ from a remaining set of plants that were grown in 250 mM for a further 10 days (30 days in salt treatment).
Field trial sites, design and measurements
Yanco 2007
A field trial was conducted at the Yanco Research Station, Yanco, NSW, Australia (34.62°S, 146.43°E). The trial was sown on 10 June 2007 and harvested 11 December 2007. Seed was sown to give a sowing rate of 150 plants m−2 in plots that were ~10 m2. Rainfall of 197 mm was supplemented by one irrigation event in August equivalent to ~40 mm rainfall. The trial was set out in a latinised 16-row × 6-column design. The latinisation of the design ensured an even distribution of the heavily replicated Tamaroi parent line across the trial and avoided replicating each remaining line in the same row or column.
Yuluma 2009
A field trial was conducted on a grower’s (John Stevenson) property ‘Airlie’ near Yuluma, NSW, Australia (35.217°S, 146.495°E). The trial was sown on May 7, 2009 and harvested 24 November 2009. Seed was sown to give a sowing rate of 150 plants m−2 in plots that were ~10 m2. Soil type was a grey vertosol and previously the paddock had been sown to barley (2007) followed by a long fallow (2008). The rainfall was 190 mm. The Yuluma 2009 trial was an 8 × 8 latin-square design. Recurrent parent Tamaroi was randomised to two of the latin square treatments and remaining Nax lines were randomised to one of the latin-square treatments each.
Moree 2008, 2009
Field trials were conducted on a grower’s (Andrew and Jodie Crowe) property ‘Sunbury’ north of Moree, NSW, Australia (29.05°S, 149.78°E). The 2008 field trial was sown on a low salinity part of a large paddock on 23 June and harvested 1 December. The 2009 field trial was located in a more saline part of the same large paddock, ~2 km north of the 2008 trial. This trial was sown on 10 June and harvested on 5 November. The soil type for both trials was a black vertosol (pH 6.5–7.0) and the previous crop in each instance was barley followed by a summer fallow. Seed was sown to give a sowing rate of 100 plants m−2 in plots that were ~10 m2. The Ashley 2008 trial comprised three rows of 50 plots each. Rows were treated as ‘blocks’ with lines randomised to each row, in such a way as to ensure minimum acceptable separations between the replicates of a line. This ensured a good spatial distribution of each line across the trial. The 2009 field trial was designed in three continuous blocks across an area where salinity ranged from low/moderate (Block 1) to high (Block 3). Each block contained 64 individual plots in an 8 × 8 latin-square design, with lines randomised separately to each block. Within each block, Tamaroi was randomised to two of the latin-square treatments so that Tamaroi was doubly replicated compared with the other lines in the trial.
Na+ and K+ analysis of flag leaves from field trials
Flag leaves (3–5) from each field plot at all saline field trials were removed from individual mature wheat plants at mid grain fill stage. Fresh weights were measured on a subset of leaves for each genotype in order to calculate ion concentrations on a tissue water basis. Leaves were dried at 70°C for 3 days and combined samples each containing three leaves were analysed for Na+ and K+ as described above.
Mapping salinity and soil chemical analysis
Salinity levels at all saline field trial sites were characterised through mapping apparent electrical conductivity (ECa) using an EM38 electromagnetic induction meter (Geonics, Mississauga, Canada). ECa values covering the ECa range of the field site, were validated with chemical analysis (pH, soil moisture, EC1 : 5 and Cl– concentration) of soil cores down to 0.8 m. Chloride was measured on a filtered sub-sample of a 1 : 5 soil : water extract using a QC8500 automated ion analyser (Lachat, Loveland, CO, USA) and converted to mM in the soil solution using the moisture content measured at the time of sampling.
Statistical analyses
Grain yield for each trial (and the separate blocks in the Ashley 2009 trial) was analysed independently using spatial mixed model methods in Genstat (VSN International, Hemel Hempstead, UK). ‘Block’, ‘row’ and ‘column’ design elements were included as random effects according to the design characteristics of the relevant trial and where found to be statistically significant. First order autocorrelated error structures were also included where found significant, as were large scale linear trends across row or columns. ECa (0–1.50 m depth) was measured for every field plot and subsequently included as a covariate in the Yuluma and Ashley 2009 analyses, with establishment counts included similarly in the Yanco 2007 analysis.
Results
Performance of selected breeding lines in a controlled environment
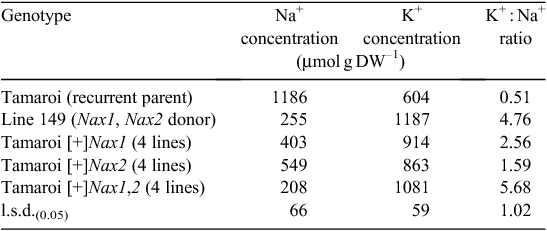
The presence of either Nax1 or Nax2 in the BC4F4 breeding lines greatly reduced the Na+ concentration in the blade of leaf 3 after 10 days in 150 mM NaCl (Table 1). Nax1 was more effective than Nax2 at reducing net Na+ uptake and accumulation in the leaf compared with recurrent parent Tamaroi. Leaf Na+ concentration was reduced on average by 66% by Nax1 compared with 54% by Nax2. The K+ concentration was greatly increased by the presence of either Nax1 or Nax2 and the K+ : Na+ ratio of all Nax lines was much higher than Tamaroi (Table 1). Lines containing both Nax1 and Nax2 had the same Na+, K+ and K+/Na+ as Line 149 (Table 1).
|
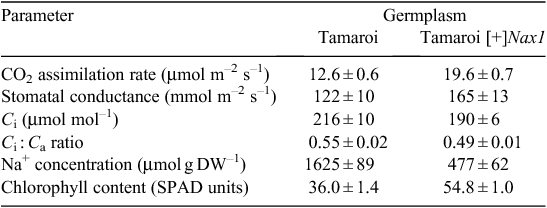
To examine the impact of Na+ exclusion on leaf function under high salinity, the cv. Tamaroi and a Tamaroi-derived BC4 line with Nax1 (Tamaroi [+]Nax1) were grown in the very high salinity (for durum wheat) of 250 mM for 30 days. After 20 days in the salt treatment, CO2 assimilation rates and chlorophyll contents were ~50% higher and leaf Na+ concentration was 3.5-fold lower in the Tamaroi [+]Nax1 line compared with Tamaroi (Table 2). Assuming previously measured FW : DW ratio for leaf 3 of 5.0, the Na+ concentration in leaf 3 on a tissue water basis of Tamaroi [+]Nax1 would be in the order of 100 mM, compared with 400 mM in Tamaroi. Leaf Na+ concentrations above 250 mM are considered to be toxic to photochemistry (James et al. 2002, 2006b). The higher intercellular CO2 concentration (Ci) and resulting Ci : Ca ratio (ratio of intercellular CO2 concentration to ambient CO2 concentration) in Tamaroi compared with the Tamaroi [+]Nax1 line, notwithstanding the lower stomatal conductance (Table 2), indicates that the lower rates of CO2 assimilation in Tamaroi were largely a result of impairment to the photosynthetic machinery, but possibly due to diffusional limitations as well.
|
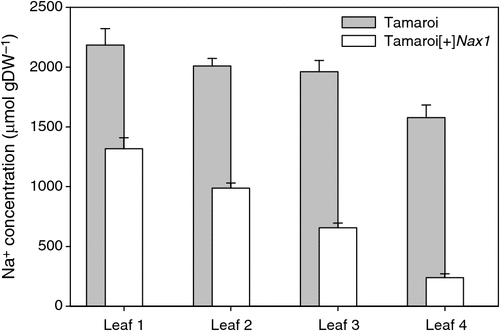
After 30 days in 250 mM NaCl, Na+ concentrations were measured in main stem leaves 1–4, with leaf 4 being the most recent fully expanded leaf (Fig. 1). Leaf Na+ concentration was greater in the older leaves, probably reflecting the greater duration of exposure to salinity. Na+ concentration in the leaves of Tamaroi was always higher than in the Nax1 line. The differences between the genotypes became more profound with each new leaf appearing. A similar trend was also found when these same genotypes were grown in 150 mM NaCl for 35 days (see Fig. S1, available as Supplementary Material to this paper).
|
Evaluation of Nax lines on a non-saline field trial site
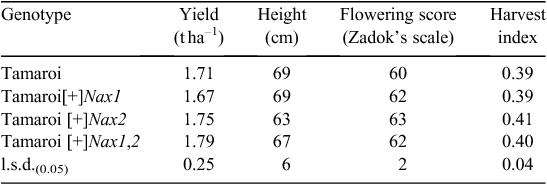
Advanced backcrossed (BC4) Nax breeding lines were evaluated for grain yield and other agronomic features in a non-saline field site in 2007 (Table 3). All Nax lines yielded similarly to recurrent parent Tamaroi at ~1.7 t ha–1 in a season where low water availability late in the season substantially limited the grain yield. Final plant heights were comparable, except for Nax2 lines, which were ~10% shorter than both Tamaroi and other BC4 lines containing Nax1. This is likely to have impacted on a marginally higher harvest index (grain mass as a proportion of total shoot mass) of 0.41 for the Nax2 lines compared with Tamaroi at 0.39. There was only a small variation in flowering time recorded for all lines evaluated. On average Nax lines were slightly more advanced than Tamaroi, the flowering time being 1–2 days earlier than Tamaroi (Table 3).
|
Evaluation of selected Nax lines on saline soil on a commercial farm
Site characterisation for saline field trial sites
The pattern of soil salinity at all saline field sites was undertaken by mapping the apparent electrical conductivity (ECa). At Moree, to identify a location with potentially high salinity for a salt tolerance field trial in 2009, a preliminary ECa survey of a large area (~16 km2) on a 100 m grid was utilised and published by Munns et al. (2012) as fig. 3a. An area was initially identified with ECa ranging up to 500 mS m–1 and further characterisation of this area, undertaken by mapping ECa on a 5 m grid, verified a site where ECa varied between 280 and 500 mS m–1 (Fig. 2). This site (25 m × 280 m) was subsequently separated into three continuous blocks for the field trial and four soil cores from each block were extracted to a depth of 0.8 m and analysed for chloride. There was a strong positive relationship between ECa measured to depths of 0.75 and 1.50 m, respectively, with mean chloride concentration being indicative of NaCl salinity (Fig. 3). Analysis of soil cores into 0.2 m segments showed that chloride concentrations increased substantially with soil depth in all three blocks (Fig. 4). Chloride measured in a 1 : 5 soil water extract in the top soil (0–0.2 m) was low, ranging between 10 and 50 mM on a soil water basis. At depths of ~0.5 m, chloride concentrations increased substantially to high levels, particularly in Blocks 2 and 3. Chloride concentrations in the soil solution at a depth of 0.8 m in Block 3 were calculated to have been 250 mM, which is equivalent to about half sea water concentration. Over the course of the growing season in non-saline soils, roots would typically be expected to be found at or below a depth of 1 m.
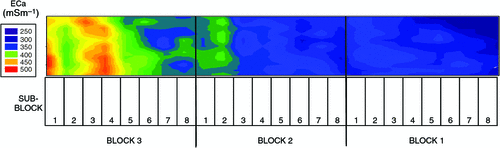
|
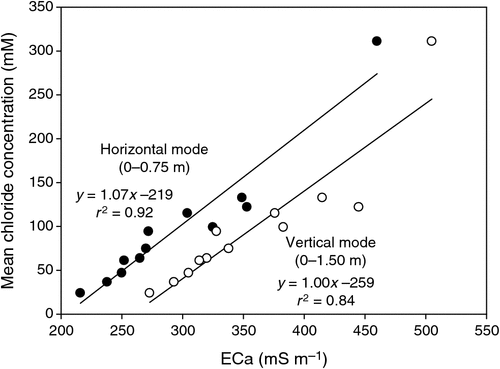
|
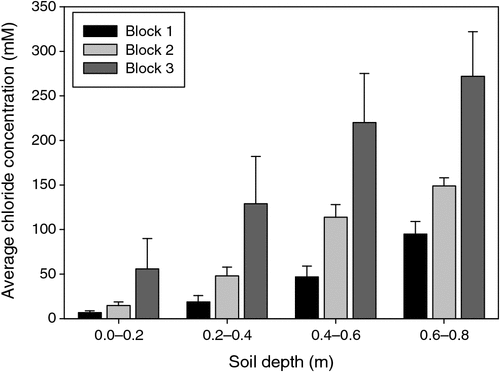
|
Na+ concentration and K+ : Na+ ratio in flag leaves
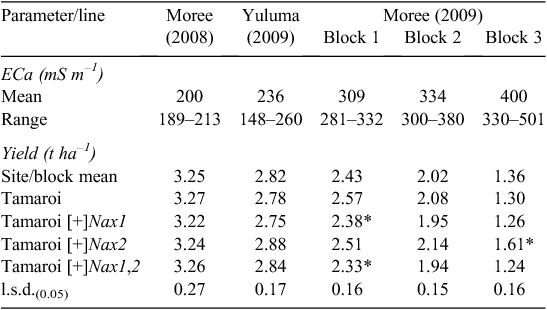
The impact of the Nax loci on Na+ concentration in flag leaves was measured at the mid-grain fill growth stage, a growth stage when a potential restraint on carbon production in the flag leaf could be an important determinant of grain yield. Increasing leaf Na+ concentrations in Tamaroi from Moree 2008 through to Moree 2009 reflected increasing ECa (Table 4) and associated salt concentrations in the soil (Fig. 3).
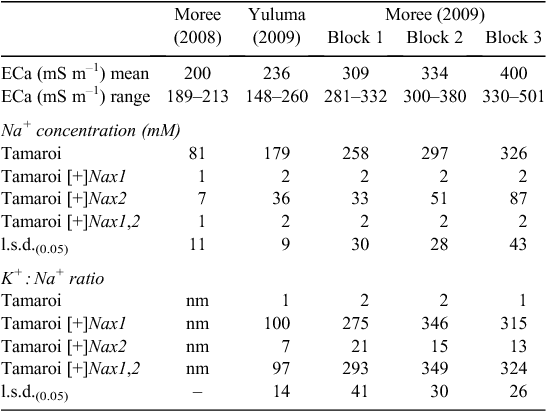
|
Both Nax loci had a profound impact on Na+ accumulation in the flag leaf (Table 4). Nax1 reduced Na+ concentration in the flag leaf by ~100 fold compared with Tamaroi across all sites and ECa range. Na+ concentration in Nax1 lines and also in lines containing both Nax1 and Nax2 was virtually unchanged at very low concentrations of between 1 and 2 mM across sites and all levels of salinity. Nax2 also significantly reduced Na+ uptake into the flag leaf, but not to the same extent as lines containing Nax1 (Table 4). Whereas Na+ concentrations in Nax2 lines increased with salinity, Na+ concentrations were maintained well below 100 mM, even at the highest salinity levels. This is in contrast to recurrent parent Tamaroi, where leaf Na+ concentrations were at very high and potentially toxic concentrations at or above 300 mM in blocks 2 and 3 at Moree in 2009. Flag leaf K+ : Na+ ratios in Tamaroi ranged between 1 – 2, which was ~100 times lower than in lines containing Nax1 and 7 – 10 times lower than in lines containing only Nax2 (Table 4). This large genotypic variation in flag leaf K+ : Na+ ratio was mostly a result of variation in Na+ concentration and to a lesser extent, variation in K+ concentration. For example, average flag leaf K+ concentration across trial blocks at Moree (2009) for Tamaroi was 480 μmol g DW–1, which, although significant (P < 0.05), were only marginally lower than 550 μmol g DW–1 in Nax2 lines and 600 μmol g DW–1 in lines containing Nax1 (data not shown).
Grain yield
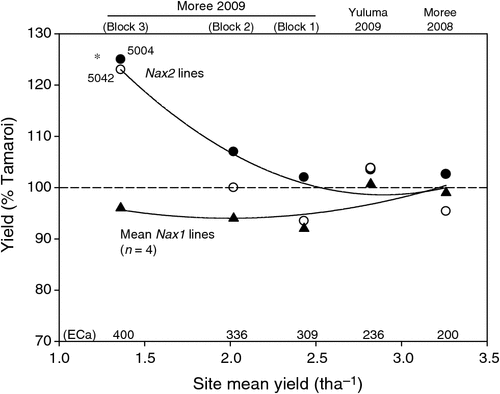
Grain yield of all lines declined substantially with increasing ECa and salinity (Table 5). At low salinity levels such as those found in Moree 2008, average grain yields of all lines evaluated was 3.25 t ha–1. At the highest salinity levels such as those present in Block 3 (Moree 2009), grain yields had decreased substantially to between 1.2 and 1.6 t ha–1. It was also under these high salinity levels that the yields of the Nax2 lines were found to be significantly less reduced than Tamaroi. Under these conditions, Nax2 lines yielded 0.3 t ha–1 or 24% higher than recurrent parent Tamaroi. All four BC4 lines containing Nax1 were between 5 and 10% lower than Tamaroi in all three trial blocks at Moree 2009, which ranged from moderate to high salinity levels (Table 5; Fig. 5). At field sites with low salinity (Yuluma 2009, Moree 2008) or no salinity (Yanco 2007, Table 3) these lines were all yield-neutral compared with recurrent parent Tamaroi.
|
|
Discussion
Relationship between Na+ exclusion and grain yield in saline fields
Salt tolerance in cereals depends upon mechanisms for maintaining photosynthetically-active leaf area, both the total green leaf area and a high rate of photosynthesis per unit leaf area. Both are important for the maintenance of an adequate supply of carbon to growing leaves and roots and reproductive structures. For crop plants such as wheat, this should result first, in the maintenance of the initiation and subsequent growth of tillers, second in the establishment of fertile florets and third, in the subsequent supply of assimilate to the developing grain. The toxic effect of high accumulations of Na+ in leaves inhibits photosynthesis (Table 2) and causes premature senescence and leaf death.
Although several studies in crop species have shown that with greater Na+ exclusion or enhanced K+/Na+ discrimination comes improved salt tolerance, these are based on early stage biomass in controlled environment studies and the relationship between grain yield and Na+ exclusion capacity was rarely measured. Moderate correlations between grain yield and Na+ exclusion from leaves have been shown in bread wheat in pot studies (Chhipa and Lal 1995; Ashraf and O’Leary 1996; Cuin et al. 2009), but other studies have shown no correlation, e.g. Genc et al. (2007). However, in that latter study the lack of correlation between yield and Na+ exclusion was most likely due to the low stress applied (100 mM NaCl) and low concentrations of Na+ in leaves and the relatively low genetic variation for Na+ exclusion in the germplasm that was evaluated. Here we present data for the relationship between Na+ exclusion in the leaves and grain yield in the field using near-isogenic lines differing significantly in leaf Na+ concentrations.
Field performance of Nax1 lines
The yield penalty recorded for the initial set of BC4 Nax1 lines under non-saline field conditions was most likely due to ‘linkage drag’, that is the carrying of linked genes that reduce the yield potential. The introgression of the Nax1 locus from T. monococcum is possibly large, carrying a large part of chromosome 2A from T. monococcum that does not readily recombine with the homologous chromosome in durum wheat (S. Huang, unpubl. data). A yield penalty is relatively common when wheat relatives or progenitors are used as a source of genetic variation in wheat breeding (Colmer et al. 2006) and particularly when used as a source of novel genes for disease resistance (The et al. 1988; Brown 2002).
We overcame this linkage drag issue by identifying what is likely to be a rare recombinant BC4 line (5020) that appeared to show no yield penalty under non-saline conditions. From Line 5020 we developed a series of fixed sibling lines containing Nax1, with and without Nax2 and subsequent field evaluation of these lines established their respective grain yield performances that appeared to validate their lack of yield penalty.
The poor yield performance of the 5020 lines containing Nax1 under high salinity conditions in the field was therefore perplexing. Nax1 lines that yielded identically to Tamaroi under low (Table 5) or non-saline field conditions (Table 3), yielded between 5 and 10% lower than Tamaroi in the moderate to high salinity blocks of the Moree 2009 trial (Fig. 5). This result was all the more surprising given the greater efficiency of Nax1 at excluding Na+ from the leaves compared with Nax2, which would lead to the expectation of a greater yield benefit compared with Nax2. The concentration of Na+ in the flag leaves of Nax1 lines grown in high salinities was kept astonishingly low at 2 mM, which was 25–40 times lower than Nax2 lines and 100–150 times lower than Tamaroi (Table 4). Previously we had found that Nax1 was responsible for the removal of Na+ from the xylem, not only in the roots, but uniquely in sheath tissue as well (James et al. 2006a). Therefore as the wheat plant increases in size and tillers elongate, the size of the sheath also increases, thus effectively increasing the potential of Nax1 to ‘filter’ Na+ from the xylem stream. Evidence for this is seen in the present work, in the large disparity in Na+ concentrations between seedling stage leaves in a controlled environment compared with the flag leaf in the field, and also in glasshouse experiments by Husain et al. (2003). It is possible that the stem, which starts to elongate only while the later leaves are developing, also retains Na+ by a similar mechanism as the sheath, but this idea has not been tested.
One possible explanation for this surprising yield result is that the removal of too much Na+ from the xylem stream may have effectively deprived the upper leaves of a cheap source of osmoticum necessary for osmotic adjustment and turgor maintenance required to cope with severe osmotic stress. Boyer et al. (2008) found that an increase in total inorganic osmotica in durum wheat leaves was largely responsible for the increase in leaf osmotic potential and therefore osmotic adjustment, with increasing salinity levels. In that study, Na+ accounted for ~30% of the increase in leaf osmotic potential and similar values have been reported previously (James et al. 2002; Rivelli et al. 2002; Cuin et al. 2009). The sum of K+ + Na+ was 20–25% lower in Nax1 lines (625–631 mM) than Tamaroi (790–825) in the high salinity blocks, suggestive that the increase in K+ may not have been sufficient to make up for the dramatic decline in the contribution of Na+ to osmotic adjustment in Nax1 lines.
Our conclusion from the present work is that Nax1 may have been too effective at reducing Na+ uptake into the upper leaves of wheat grown in high salinities, not improving yield, but instead causing a small reduction in relative yield. However, for wheat grown in waterlogged saline areas, the greater benefit of Nax1 derived from aboveground Na+ exclusion might outweigh any of the deleterious factors mentioned above (James et al. 2011). Oxygen depletion due to waterlogging would impair ion transport processes in the roots, resulting in high Na+ accumulation in leaves. This process would be ameliorated to a degree by Nax1, through retrieval and storage of Na+ in basal shoot structures, which would remain unaffected by waterlogging.
Field performance of Nax2 lines
The effect of Nax2 on Na+ exclusion and grain yield was evaluated by comparing Tamaroi [+]Nax2 lines with parent cv. Tamaroi across three field sites over 2 years. The data for Nax2 lines at Moree have recently been published (Munns et al. 2012). Here we present the additional data at the Yuluma site in NSW in a contrasting environment to confirm the universality of the results. At the low to moderate salinity levels such as those at the Moree 2008, Yuluma 2009 and Block 1 (Moree 2009) field sites, grain yields of both Tamaroi and Tamaroi [+]Nax2 were virtually identical. This provides further evidence, consistent with earlier trials in SA and NSW in 2004 and 2005 that there was no yield penalty associated with the presence of Nax2 (Munns et al. 2012). This is very important, as farmers will adopt a new wheat variety only if the yield is the same or better than existing varieties where salinity is not limiting biomass or grain yield.
It was only at the highest salinity levels such as those present in Block 3 (Moree 2009) that lines containing Nax2 yielded significantly more than Tamaroi in high salinity, namely 0.3 t ha−1 more or 24% more. This corresponded to very high (toxic) Na+ concentrations in the flag leaves of Tamaroi (>300 mM), whereas flag leaf Na+ concentrations in Nax2 lines were maintained at low (non-toxic) levels (<100 mM). These results show that the Na+ exclusion provided by Nax2 increased grain yields of wheat grown in high salinity levels in the field.
Candidate genes
The most likely candidate gene for Nax2 is TmHKT1;5-A which encodes a Na+-specific transporter located on the plasma membrane of root cells surrounding xylem vessels (Munns et al. 2012). HKT (high-affinity potassium transporter) genes are known to be important in the regulation of Na+ and K+ transport in wheat and other species (Schachtman and Schroeder 1994; Munns and Tester 2008; Horie et al. 2009). The group 1 HKT transporters of Arabidopsis (AtHKT1;1) and rice (OsHKT1;5) reduce transport of Na+ to shoots by unloading Na+ from xylem vessels to xylem parenchyma cells in roots (Mäser et al. 2002; Ren et al. 2005; Sunarpi et al. 2005; Davenport et al. 2007) and increase plant salinity tolerance as determined by the effect of cell-specific expression on shoot growth of Arabidopsis (Moller et al. 2009; Plett et al. 2010). The likely candidate gene for Nax1 is TmHKT1;4-A2 (Huang et al. 2006) although as yet we do not have proof of its function in higher plants as a Na+-specific transporter.
Impact of the osmotic stress on grain yield in a saline field
It is widely appreciated that growth of salt-stressed plants is limited mostly by the osmotic effects of salinity. The osmotic potential of the salt solution in soil, like soil water deficit due to drought, causes a decrease in soil water potential, which induces a reduction in leaf expansion and stomatal conductance and thereby photosynthesis (Munns 2002). Additional growth limitations caused by the accumulation of excessive salt concentrations in mature leaves occurs at a later stage of growth.
Field experiments conducted at close locations with soils of different salinity allow us to estimate the osmotic effect of the salt solution – when soil volume and light are not limiting – as typical of controlled environments and when climatic conditions are identical. The experiment described here at Moree in 2009 with three field blocks of different salinity adjacent to each other and so having identical ambient conditions but with varying soil salinity, allow us to quantify osmotic versus salt-specific effects on yield. Grain yield of all genotypes declined significantly with increasing salinity (Table 5) and we propose that this decline in site mean yield from 2.43 to 1.36 t ha–1 is largely due to the osmotic effect of salts in soil solution. The flag leaf Na+ concentration of Nax2 lines (Table 4) was well below that which would be considered critical, which is greater than 250 mM (James et al. 2006b). In the high salinity block yield of Tamaroi decreased by 50% whereas Nax2 lines decreased by only 36%. The decline in yield of Nax2 lines was entirely due to osmotic stress as Na+ concentrations in leaves were kept at low non-toxic levels, so the additional 14% decline in Tamaroi was due to Na+ toxicity.
A similar conclusion can be drawn for durum wheat lines containing the Kna1 locus for K+/Na+ discrimination introgressed from hexaploid wheat (Dvořák et al. 1994). In moderate salinities in the field yield in Kna1 lines decreased by 30%, most likely due to the osmotic effect of soil solution, as Na+ concentration in leaves was low, ~50 mM (calculated from Dvořák et al. 1994). Lines lacking Kna1 had an additional 10% reduction in both biomass and yield.
The dominance of soil moisture or interaction of water stress and salt stress is important in the field and the relative impact of osmotic stress vs. salt-specific stress on yield deserves more attention. Schubert et al. (2009) grew maize in very large pots through to grain maturity and found that increases in yield were associated only with a combination of Na+ exclusion and osmotic stress tolerance whereas hybrids with just Na+ exclusion were insufficient.
Conclusion
The Na+ exclusion phenotype of Nax1 lines looked the most promising at early seedling stage in controlled environment conditions, however, the more moderate Na+ exclusion capacity of Nax2 proved to be more successful in the field. This study illustrates the effectiveness of the field environment for revealing difference in plant growth and grain yield that cannot be seen in controlled environments. The study also showed the importance of well characterised field sites to account for natural variation in salinity. Salinity varied even across small areas, at a scale of metres and the impact of this on grain yield could be accounted for only by measuring soil salinity under each plot and using this data as a covariate in statistical analysis of the trial.
Both Nax1 and Nax2 have been crossed into hexaploid wheat, as described by James et al. (2011) and field trials in several commercial bread wheat cultivars revealed some promising Nax2 lines, as well as Nax1 lines without a yield penalty, thus indicating the potential for additional genetic gains for improving salt tolerance in bread wheat.
The Nax2 lines produced in this study are in the background of Tamaroi, a cultivar that has been recently superceded by new varieties with improved quality attributes. The lines together with molecular markers are available for breeders to cross into their advanced breeding lines adapted to specific regions, to ensure that the 25% yield advantage on saline soils can be achieved in other salt-affected regions in the world.
Acknowledgements
The authors thank John Stevenson for allowing us to utilise his property ‘Airlee’ (Yuluma) for a field trial in 2009 and also NSW grain growers Andrew and Jodie Crowe for giving us access to ECa mapping data and for hosting our field trials on their property ‘Sunbury’ north of Moree in 2008–09. We acknowledge the assistance of Lorraine Mason and Richard Phillips for ion analysis and thank Caitlin Byrt and Shaobai Huang for genotyping the Nax1 and Nax2 BC4 lines. Evans Lagudah and Wolfgang Spielmeyer provided essential guidance in molecular genetics from the start of this project. We acknowledge the staff of the CSIRO Ginninderra Experimental Station for assistance in sowing and harvesting field trials in Yanco and Yuluma and from the NSW Department of Primary Industries research station at Tamworth, we thank David Gulliford and durum breeder Bert Collard for sowing and harvesting the trials at Moree. Matthew Gilliham, Stuart Roy and John Passioura gave valuable comments on this manuscript. We also acknowledge the support of the Australian Grain Research and Development Corporation (GRDC) for this research.
References
Ashraf M, O’Leary JW (1996) Responses of some newly developed salt-tolerant genotypes of spring wheat to salt stress. 1. Yield components and ion distribution. Journal Agronomy & Crop Science 176, 91–101.| Responses of some newly developed salt-tolerant genotypes of spring wheat to salt stress. 1. Yield components and ion distribution.Crossref | GoogleScholarGoogle Scholar |
Boyer JS, James RA, Munns R, Condon AG, Passioura JB (2008) Osmotic adjustment may lead to anomalously low estimates of relative water content in wheat and barley. Functional Plant Biology 35, 1172–1182.
| Osmotic adjustment may lead to anomalously low estimates of relative water content in wheat and barley.Crossref | GoogleScholarGoogle Scholar |
Brown JKM (2002) Yield penalties of disease resistance in crops. Current Opinion in Plant Biology 5, 339–344.
| Yield penalties of disease resistance in crops.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xls1ejtLk%3D&md5=a54e6b94ae22755eb77ca1af266b8c07CAS |
Byrt C, Platten JD, Spielmeyer W, James RA, Lagudah ES, Dennis ES, Tester M, Munns R (2007) Transporter (HKT1;5) genes linked to Na+ exclusion loci in wheat, Nax2 and Kna1. Plant Physiology 143, 1918–1928.
| Transporter (HKT1;5) genes linked to Na+ exclusion loci in wheat, Nax2 and Kna1.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXksFWjurs%3D&md5=f0f7213246aef9707915ba9c62365e47CAS |
Chhipa BR, Lal P (1995) Na/K ratios as the basis of salt tolerance in wheat. Australian Journal of Agricultural Research 46, 533–539.
| Na/K ratios as the basis of salt tolerance in wheat.Crossref | GoogleScholarGoogle Scholar |
Colmer TD, Flowers TJ, Munns R (2006) Use of wild relatives to improve salt tolerance in wheat. Journal of Experimental Botany 57, 1059–1078.
| Use of wild relatives to improve salt tolerance in wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xis1Gls7s%3D&md5=d75408773f3e0f7a9eb98951ee8fbe2bCAS |
Cuin TA, Tian Y, Betts SA, Chalmandrier R, Shabala S (2009) Ionic relations and osmotic adjustment in durum and bread wheat under saline conditions. Functional Plant Biology 36, 1110–1119.
| Ionic relations and osmotic adjustment in durum and bread wheat under saline conditions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsFSgsb3J&md5=84a5bc1a58911957ff259625b95cac19CAS |
Davenport RJ, James RA, Zakrisson-Plogander A, Tester M, Munns R (2005) Control of sodium transport in durum wheat. Plant Physiology 137, 807–818.
| Control of sodium transport in durum wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXislOruro%3D&md5=d5483a4d15aceec3f23d8768ae2a480aCAS |
Davenport RJ, Munoz-Mayor A, Deepa J, Essah PA, Rus A, Tester M (2007) The Na+ transporter AtHKT1;1 controls retrieval of Na+ from the xylem in Arabidopsis. Plant, Cell & Environment 30, 497–507.
| The Na+ transporter AtHKT1;1 controls retrieval of Na+ from the xylem in Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXksVemu7Y%3D&md5=cb67de2879a6427621e160d9a659a3afCAS |
Dubcovsky J, Santa María G, Epstein E, Luo MC, Dvořák J (1996) Mapping of the K+/Na+ discrimination locus Kna1 in wheat. Theoretical and Applied Genetics 92, 448–454.
| Mapping of the K+/Na+ discrimination locus Kna1 in wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XltFOjtLg%3D&md5=df79f15d11f09fbac3d1eb1be650a5fdCAS |
Dvořák J, Noaman MM, Goyal S, Gorham J (1994) Enhancement of the salt tolerance of Triticum turgidum L. by the Kna1 locus transferred from the Triticum aestivum L. chromosome 4D by homoeologous recombination. Theoretical and Applied Genetics 87, 872–877.
| Enhancement of the salt tolerance of Triticum turgidum L. by the Kna1 locus transferred from the Triticum aestivum L. chromosome 4D by homoeologous recombination.Crossref | GoogleScholarGoogle Scholar |
Francois LE, Maas EV, Donovan TJ, Youngs VL (1986) Effects of salinity on grain yield and quality, vegetative growth, and germination of semi-dwarf and durum wheat. Agronomy Journal 78, 1053–1058.
| Effects of salinity on grain yield and quality, vegetative growth, and germination of semi-dwarf and durum wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2sXjtVWiuw%3D%3D&md5=e0c02eaf0c486ac4005ddc4c24bcc63fCAS |
Genc Y, McDonald GK, Tester M (2007) Re-assessment of tissue Na+ concentration as a criterion for salinity tolerance in bread wheat. Plant, Cell & Environment 30, 1486–1498.
| Re-assessment of tissue Na+ concentration as a criterion for salinity tolerance in bread wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXht1yhsrbE&md5=216386d29b95b68b38c4fb5a519b1366CAS |
Gorham J, Wyn Jones RG, Bristol A (1990) Partial characterisation of the trait for enhanced K+-Na+ discrimination in the D genome of wheat. Planta 180, 590–597.
| Partial characterisation of the trait for enhanced K+-Na+ discrimination in the D genome of wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXitFequr4%3D&md5=ea6f573e9c70cda26036c87f811143b5CAS |
Horie T, Hauser F, Schroeder JI (2009) HKT transporter-mediated salinity resistance mechanisms in Arabidopsis and monocot crop plants. Trends in Plant Science 14, 660–668.
| HKT transporter-mediated salinity resistance mechanisms in Arabidopsis and monocot crop plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsV2mtr7J&md5=8b5093747e84e25562335820492a11a6CAS |
Huang S, Spielmeyer W, Lagudah ES, James RA, Platten JD, Dennis ES, Munns R (2006) A sodium transporter (HKT7) is a candidate for Nax1, a gene for salt tolerance in durum wheat. Plant Physiology 142, 1718–1727.
| A sodium transporter (HKT7) is a candidate for Nax1, a gene for salt tolerance in durum wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtlCns7jE&md5=2e02eb2e3af1edcf847cc9200df0dc01CAS |
Huang S, Spielmeyer W, Lagudah ES, Munns R (2008) Comparative mapping of HKT genes in wheat, barley and rice, key determinants of Na+ transport and salt tolerance. Journal of Experimental Botany 59, 927–937.
| Comparative mapping of HKT genes in wheat, barley and rice, key determinants of Na+ transport and salt tolerance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXjs1KhtLk%3D&md5=b412d6391d0d2ddb2afb3af50af62c6eCAS |
Husain S, Munns R, Condon AG (2003) Effect of sodium exclusion trait on chlorophyll retention and growth of durum wheat in saline soil. Australian Journal of Agricultural Research 54, 589–597.
| Effect of sodium exclusion trait on chlorophyll retention and growth of durum wheat in saline soil.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXmsFGntr0%3D&md5=c71fc906658ce3d5727b7aab27267d97CAS |
James RA, Rivelli AR, Munns R, von Caemmerer S (2002) Factors affecting CO2 assimilation, leaf injury and growth in salt-stressed durum wheat. Functional Plant Biology 29, 1393–1403.
| Factors affecting CO2 assimilation, leaf injury and growth in salt-stressed durum wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXovFWhsA%3D%3D&md5=143979047ddf103eb0c3a164530c254bCAS |
James RA, Davenport RJ, Munns R (2006a) Physiological characterisation of two genes for Na+ exclusion in durum wheat: Nax1 and Nax2. Plant Physiology 142, 1537–1547.
| Physiological characterisation of two genes for Na+ exclusion in durum wheat: Nax1 and Nax2.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtlCns7vI&md5=ba476f11a8fc889d1910d2e8904b6474CAS |
James RA, Munns R, Von Caemmerer S, Trejo C, Miller C, Condon AG (2006b) Photosynthetic capacity is related to the cellular and subcellular partitioning of Na+, K+ and Cl– salt-affected barley and durum wheat. Plant, Cell & Environment 29, 2185–2197.
| Photosynthetic capacity is related to the cellular and subcellular partitioning of Na+, K+ and Cl– salt-affected barley and durum wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtVaisA%3D%3D&md5=bbb943ae2094f2a757fbd3a8eba1afacCAS |
James RA, von Caemmerer S, Condon AG, Zwart AB, Munns R (2008) Genetic variation in tolerance to the osmotic stress component of salinity stress in durum wheat. Functional Plant Biology 35, 111–123.
James RA, Blake C, Byrt CS, Munns R (2011) Major genes for Na+ exclusion Nax1 and Nax2 (wheat HKT1;4 and HKT1;5) decrease Na+ accumulation in bread wheat under saline and waterlogged conditions. Journal of Experimental Botany 62, 2939–2947.
| Major genes for Na+ exclusion Nax1 and Nax2 (wheat HKT1;4 and HKT1;5) decrease Na+ accumulation in bread wheat under saline and waterlogged conditions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXmsVyks7w%3D&md5=50d6aeaf522ca67f0cc3a21eecbec87dCAS |
Lindsay MP, Lagudah ES, Hare RA, Munns R (2004) A locus for sodium exclusion (Nax1), a trait for salt tolerance, mapped in durum wheat. Functional Plant Biology 31, 1105–1114.
| A locus for sodium exclusion (Nax1), a trait for salt tolerance, mapped in durum wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhtVWjt7fO&md5=b92befdf24de1330b0c591bd88e2e0dfCAS |
Maas EV, Grieve CM (1990) Spike and leaf development in salt stressed wheat. Crop Science 30, 1309–1313.
| Spike and leaf development in salt stressed wheat.Crossref | GoogleScholarGoogle Scholar |
Mäser P, Eckelman B, Vaidyanathan R, Horie T, Fairbairn DJ, Kubo M, Yamagami M, Yamaguchi K, Nishimura M, Uozumi N, Robertson W, Sussman MR, Schroeder JI (2002) Altered shoot/root Na+ distribution and bifurcating salt sensitivity in Arabidopsis by genetic disruption of the Na+ transporter AtHKT1. FEBS Letters 531, 157–161.
| Altered shoot/root Na+ distribution and bifurcating salt sensitivity in Arabidopsis by genetic disruption of the Na+ transporter AtHKT1.Crossref | GoogleScholarGoogle Scholar |
Moller IS, Gilliham M, Jha D, Mayo GM, Roy SJ, Coates JC, Haseloff J, Tester M (2009) Shoot Na+ exclusion and increased salinity tolerance engineered by cell type-specific alteration of Na+ transport in Arabidopsis. The Plant Cell 21, 2163–2178.
| Shoot Na+ exclusion and increased salinity tolerance engineered by cell type-specific alteration of Na+ transport in Arabidopsis.Crossref | GoogleScholarGoogle Scholar |
Munns R (2002) Comparative physiology of salt and water stress. Plant, Cell & Environment 25, 239–250.
| Comparative physiology of salt and water stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xhslakurw%3D&md5=8558cb25c538a179db778e53069617f0CAS |
Munns R, James RA (2003) Screening methods for salinity tolerance: a case study with tetraploid wheat. Plant and Soil 253, 201–218.
| Screening methods for salinity tolerance: a case study with tetraploid wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXltVemsbc%3D&md5=70bdd8fd1e2a3bae5d69458170c6bba7CAS |
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annual Review of Plant Biology 59, 651–681.
| Mechanisms of salinity tolerance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXntFaqtrw%3D&md5=74a9a391a4c4a1f572e0302bd42130f5CAS |
Munns R, Rebetzke GJ, Husain S, James RA, Hare RA (2003) Genetic control of sodium exclusion in durum wheat. Australian Journal of Agricultural Research 54, 627–635.
| Genetic control of sodium exclusion in durum wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXmsVKmtbg%3D&md5=c65509e33352e05e2963eeab189dec1fCAS |
Munns R, James RA, Xu B, Athman A, Conn SJ, Jordans C, Byrt CS, Hare RA, Tyerman SD, Tester M, Plett D, Gilliham M (2012) Wheat grain yield on saline soils is improved by an ancestral transporter gene. Nature Biotechnology 30, 360–364.
| Wheat grain yield on saline soils is improved by an ancestral transporter gene.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XjtlOgu7w%3D&md5=e37d5f7828ffe9af1d3f0cedff78aba6CAS |
Passioura JB (2010) Scaling up: the essence of effective agricultural research. Functional Plant Biology 37, 585–591.
| Scaling up: the essence of effective agricultural research.Crossref | GoogleScholarGoogle Scholar |
Plett D, Safwat G, Gilliham M, Moller IS, Roy S, Shirley N, Jacobs A, Johnson A, Tester M (2010) Improved salinity tolerance of rice through cell type-specific expression of AtHKT1;1. PLoS ONE 5, e12571
| Improved salinity tolerance of rice through cell type-specific expression of AtHKT1;1.Crossref | GoogleScholarGoogle Scholar |
Ren ZH, Gao JP, Li LG, Cai XL, Huang W, Chao DY, Zhu MZ, Wang ZY, Luan S, Lin HX (2005) A rice quantitative trait locus for salt tolerance encodes a sodium transporter. Nature Genetics 37, 1141–1146.
| A rice quantitative trait locus for salt tolerance encodes a sodium transporter.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtVCntL%2FJ&md5=8d06ab35399af7295afd1fdee45197a4CAS |
Rengasamy P (2002) Transient salinity and subsoil constraints to dryland farming in Australian sodic soils: an overview. Australian Journal of Experimental Agriculture 42, 351–361.
| Transient salinity and subsoil constraints to dryland farming in Australian sodic soils: an overview.Crossref | GoogleScholarGoogle Scholar |
Rengasamy P (2010) Soil processes affecting crop production in salt-affected soils. Functional Plant Biology 37, 613–620.
| Soil processes affecting crop production in salt-affected soils.Crossref | GoogleScholarGoogle Scholar |
Richards RA, Rebetzke GJ, Watt M, Condon AG, Spielmeyer W, Dolferus R (2010) Breeding for improved water productivity in temperate cereals: phenotyping, quantitative trait loci, markers and the selection environment. Functional Plant Biology 37, 85–97.
| Breeding for improved water productivity in temperate cereals: phenotyping, quantitative trait loci, markers and the selection environment.Crossref | GoogleScholarGoogle Scholar |
Rivelli AR, James RA, Munns R, Condon AG (2002) Effect of salinity on water relations and growth of wheat genotypes with contrasting sodium uptake. Functional Plant Biology 29, 1065–1074.
| Effect of salinity on water relations and growth of wheat genotypes with contrasting sodium uptake.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xntlejt7s%3D&md5=45aaff114ec8052afdc5cb40d874ce63CAS |
Schachtman DP, Schroeder JI (1994) Structure and transport mechanism of a high-affinity potassium uptake transporter from higher plants. Nature 370, 655–658.
| Structure and transport mechanism of a high-affinity potassium uptake transporter from higher plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXmt1Cguro%3D&md5=a3a414db39ce9ce5149edd2906158853CAS |
Schubert S, Neubert A, Schierholt A, Sümer A, Zörb C (2009) Development of salt-resistant maize hybrids: the combination of physiological strategies using conventional breeding methods. Plant Science 177, 196–202.
| Development of salt-resistant maize hybrids: the combination of physiological strategies using conventional breeding methods.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXnsFyhtLs%3D&md5=17a7119f446a94288b8927e8a36f2c77CAS |
Sunarpi , Horie T, Motoda J, Kubo M, Yang H, Yoda K, Horie R, Chan W-Y, Leung H-Y, Hattori K, Konomi M, Osumi M, Yamagami M, Schroeder JI, Uozumi N (2005) Enhanced salt tolerance mediated by AtHKT1 transporter-induced Na+ unloading from xylem vessels to xylem parenchyma cells. The Plant Journal 44, 928–938.
| Enhanced salt tolerance mediated by AtHKT1 transporter-induced Na+ unloading from xylem vessels to xylem parenchyma cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XjslGlsw%3D%3D&md5=4544d7cc5a0a2e8ac45aea020ff220e8CAS |
Tavakkoli E, Rengasamy P, McDonald GK (2010) The response of barley to salinity stress differs between hydroponics and soil systems. Functional Plant Biology 37, 621–633.
| The response of barley to salinity stress differs between hydroponics and soil systems.Crossref | GoogleScholarGoogle Scholar |
The TT (1973) Transference of resistance to stem rust from Triticum monococcum L. to hexpaploid wheat. PhD thesis, University of Sydney.
The TT, Latter BDH, McIntosh RA, Ellison FW, Brennan PS, Fisher J, Hollamby GJ, Rathjen AJ, Wilson RE (1988) Grain yields of near isogenic lines with added genes for stem rust resistance. In ‘Proceedings of the 7th International Wheat Genetics Symposium’. (Eds TE Miller, RMD Koebner) pp. 901–906. (IPSR: Cambridge)
Zubaidi A, McDonald GK, Hollamay GJ (1999a) Shoot growth, root growth and grain yield of bread and durum wheat in South Australia. Australian Journal of Experimental Agriculture 39, 709–720.
| Shoot growth, root growth and grain yield of bread and durum wheat in South Australia.Crossref | GoogleScholarGoogle Scholar |
Zubaidi A, McDonald GK, Hollamby GJ (1999b) Nutrient uptake and distribution by bread and durum wheat under drought conditions in South Australia. Australian Journal of Experimental Agriculture 39, 721–732.
| Nutrient uptake and distribution by bread and durum wheat under drought conditions in South Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXhsVOitg%3D%3D&md5=1205d9d24b57873564493a8287865297CAS |


