Carotenoids in nature: insights from plants and beyond
Christopher I. CazzonelliAustralian Research Council Centre of Excellence in Plant Energy Biology, College of Medicine, Biology and Environment, Research School of Biology, The Australian National University, Building 134, Linnaeus Way, Canberra, ACT 0200, Australia. Email: christopher.cazzonelli@anu.edu.au
This review originates from the Peter Goldacre Award 2010 of the Australian Society of Plant Scientists that was received by the author.
Functional Plant Biology 38(11) 833-847 https://doi.org/10.1071/FP11192
Submitted: 19 August 2011 Accepted: 30 August 2011 Published: 18 October 2011
Abstract
Carotenoids are natural isoprenoid pigments that provide leaves, fruits, vegetables and flowers with distinctive yellow, orange and some reddish colours as well as several aromas in plants. Their bright colours serve as attractants for pollination and seed dispersal. Carotenoids comprise a large family of C40 polyenes and are synthesised by all photosynthetic organisms, aphids, some bacteria and fungi alike. In animals carotenoid derivatives promote health, improve sexual behaviour and are essential for reproduction. As such, carotenoids are commercially important in agriculture, food, health and the cosmetic industries. In plants, carotenoids are essential components required for photosynthesis, photoprotection and the production of carotenoid-derived phytohormones, including ABA and strigolactone. The carotenoid biosynthetic pathway has been extensively studied in a range of organisms providing an almost complete pathway for carotenogenesis. A new wave in carotenoid biology has revealed implications for epigenetic and metabolic feedback control of carotenogenesis. Developmental and environmental signals can regulate carotenoid gene expression thereby affecting carotenoid accumulation. This review highlights mechanisms controlling (1) the first committed step in phytoene biosynthesis, (2) flux through the branch to synthesis of α- and β-carotenes and (3) metabolic feedback signalling within and between the carotenoid, MEP and ABA pathways.
Additional keywords: abscisic acid, apocarotenoids, carotenoid, chloroplast, chromatin, epigenetic, hormones, isoprenoid, metabolic feedback, plant, photoisomerisation, regulation, signal molecule, strigolactone.
Functions for carotenoids in nature
Vitamins, antioxidants and spices
Animals are generally unable to synthesise carotenoids and require a dietary intake of plant products to meet daily health demands. There are exceptions such as the synthesis of carotenoids in human protist parasites (e.g. Plasmodium and Toxoplasma) and aphids, which can be explained by the existence of a remnant plastid and lateral gene transfer of carotenoid biosynthesis genes from a fungus, respectively (Tonhosolo et al. 2009; Moran and Jarvik 2010). In most animals, dietary carotenoids are cleaved to provide precursors for vitamin A biosynthesis (of which a deficiency leads to blindness) and are valuable for many physiological functions and thus promote human health (e.g. antioxidant activity, immunostimulants, yolk nourishment to embryos, photoprotection, visual tuning as well as limiting age-related macular degeneration of the eye) (Johnson 2002; Krinsky and Johnson 2005).
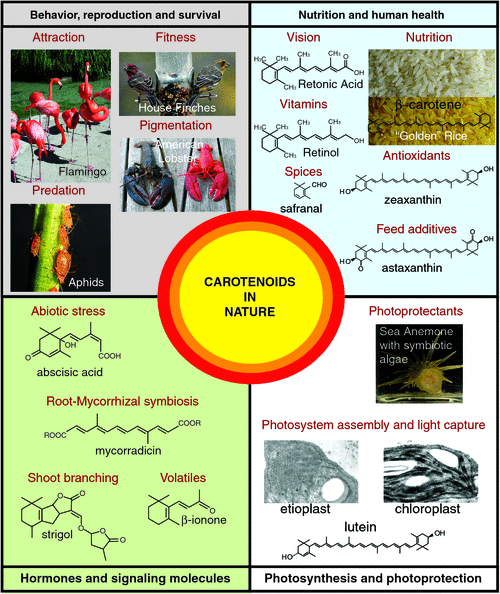
A deficiency in vitamin A is responsible for child (and maternal) mortality and it is estimated that a 23–34% reduction in preschool mortality can be expected from vitamin A program reaching children in undernourished settings (The State of the World’s Children, UNICEF, www.un.org/en/mdg/summit2010, accessed 19 August 2011). The development of high yielding β-carotene crops such as ‘golden rice’ could provide close to the recommended daily allowance of vitamin A for malnourished children and help combat vitamin A deficiency-induced mortality and morbidity (Fig. 1) (www.goldenrice.org, accessed 19 August 2011).
|
The nutraceutical industry synthetically manufactures five major carotenoids on an industrial scale (e.g. lycopene, β-carotene, canthaxanthin, zeaxanthin and astaxanthin) for use in a range of food products and cosmetics, such as vitamin supplements and health products and as feed additives for poultry, livestock, fish and crustaceans (reviewed by Del Campo et al. 2007; Jackson et al. 2008). One of the most commercially valuable pigments, astaxanthin, is primarily synthesised by marine microorganisms, such as the green alga Haematococcus pluvialis and accumulates in fish such as salmon, thus, colouring their flesh red. Astaxanthin has been implicated as a potential therapeutic agent treating cardiovascular disease and prostatic cancer (Fassett and Coombes 2011).
Carotenoids accumulate in light exposed tissues, such as skin and as such have gained increased value in the cosmetic industries as suitable compounds for photoprotection due to their scavenging action on reactive oxygen species (ROS) and anti-inflammatory properties (Stahl and Sies 2007). Photo-oxidative damage affects cellular lipids, proteins and DNA and is involved in the patho-biochemistry of erythema formation, premature aging of the skin, development of photodermatoses and skin cancer. Evidence shows that β-Carotene, lutein and perhaps even lycopene, can prevent UV-induced erythema formation and contribute to life-long protection against exposure to harmful affects of sunlight (Stahl and Sies 2007).
Apocarotenoids are also highly valued as additives in the food industry. Spices such as bixin (annatto), a red-collared, di-carboxylic monomethyl ester apocarotenoid is traditionally derived from the plant Bixa orellana (also known as achiote). Saffron comes from the thread-like reddish coloured female reproductive organs of the Crocus sativa flower (petals are coloured light purple), which is considered one of the worlds most expensive spices and widely used as a natural colourant. The colour is due to the degradation of carotenoids (e.g. zeaxanthin to crocin and crocetin), whereas the flavour arises from the accumulation of carotenoid cleavage oxidation products from zeaxanthin (e.g. mainly safranal and the bitter glucoside, picrocrocin). Safranal (2,6,6-trimethylcyclohexa-1,3-dien-1-carboxaldehyde) is easily synthesised by de-glucosylation of picrocrocin and composes ~70% of total volatiles from crocin flowers (Fig. 1) (Leffingwell 2002; Bouvier et al. 2003a; Schwab et al. 2008). There is a growing interest in the impact of saffron carotenoids on human health due to their high antioxidant capacity (Verma and Bordia 1998). Other carotenoid-derived volatiles, such as α- and β-ionone are predominant norisoprenoid volatiles in the mature stigma tissue that are also important components of aroma and taste produced during flower and fruit development (Goff and Klee 2006).
Animal behaviour, reproduction and survival
Animals accumulate carotenoids where they are critical in determining sexual behaviour, reproduction and avoiding predation as well as parasitism. Fish and birds accumulate dietary carotenoids, which boost their immune system and advertise health, often leading to preferential selection by the sexual partner (McGraw et al. 2006b; Baron et al. 2008). Animals typically place different priorities on fitness-enhancing activities (e.g. gametic investment in females, sexual attraction in males) and carotenoid allocation appears to track such investment patterns in the two sexes (McGraw and Toomey 2010). For example, environmental and physiological factors influence colour expression in house finches (Fig. 1) and the type of dietary carotenoids is one determinant of their ability to become bright red rather than drab yellow (McGraw et al. 2006a). A particular primacy of higher levels of β-cryptoxanthin was assigned to the coloration in the male house finches that become red and more sexually attractive. It is possible that the red house finches adopt selective foraging strategies for the most β-cryptoxanthin-rich foods rather then grains and fruits, which typically contain the more common dietary yellow xanthophylls and carotenes (Fig. 2) (McGraw et al. 2006a). Finally, Hill (2000) demonstrated that birds fed diets rich in lutein and zeaxanthin turn only yellow, but grow red feathers when fed β-cryptoxanthin-rich foods (e.g. tangerine juice). Perhaps, it is the metabolic precursors of β-cryptoxanthin such as 3-hydroxyechinenone, which provide the red carotenoid pigmentation (Hill 2000).
|
Another intriguing example is the flamingo, whose feathers are coloured bright rosy pink (Fig. 1) from the carotenoid pigments (derivatives such as canthaxanthin, main pigment found in the secretions, as well as small quantities of β-cryptoxanthin are likely to contribute to pink colouration) in the algae and various invertebrates (e.g. crayfish) that make up the bulk of a flamingo’s diet. Curiously, the crustaceans accumulate astaxanthin whose spectral properties become modified by the β-barrel protein, crustacyanin, resulting in blue pigmentation that shifts to red upon protein–pigment denaturation during cooking (Fig. 1; American lobster) (Krawczyk and Britton 2001). A dietary source of carotenoids by the flamingo was found in the oils of uropygial secretions, which deposit over the plumage and growing feathers more frequently during periods when in a group, contributing some colour and possibly indicating a cosmetic function for mate choice. The cosmetic nature of accumulating carotenoids by painting canthaxanthin-rich secretions onto their feathers to create a visually more attractive breeding partner appeared to enhance the frequency of their courting behaviour during mating seasons (Amat et al. 2010).
Carotenoids also play important roles in animals to avoid predation and reduce parasitism. For example, aphids (e.g. Acyrthosiphon pisum) are the first known animal to have acquired the carotenoid biosynthetic machinery to produce carotenoids such as torulene and dehydro-γ, ψ-carotene, which provide a reddish colouration distinguished them from their green forms, which accumulate γ-carotene, β-carotene and α-carotene (Moran and Jarvik 2010). The colour polymorphism is maintained by frequency-dependent selection imposed by natural predators that preferentially prey upon the red morphs (Fig. 1) and higher rates of parasitism in the green forms (Losey et al. 1997).
Photosynthesis and photoprotection in plants
Carotenoids are essential for energy capture from the solar emission spectrum. Of the many naturally occurring carotenoids, less than 50 play a light-harvesting role in photosynthetic organisms (e.g. plants; protists such as the human parasites Plasmodium and Toxoplasma as well as the coral endosymbiotic Dinoflagellates; and the Symbiodinium marine algae which forms a relationship with the sea anemone, Aiptasia insignis) (Fig. 1) (Chidambara Murthy et al. 2005; Polívka and Frank 2010). Carotenoid colours range from pale yellow to a reddish brown depending upon the number of conjugated double bonds along the C40 backbone, as well as other cyclic and oxygenic modifications. The distinctive yellow colour of light-harvesting carotenoids become more apparent in leaves during autumn when chlorophyll degrades revealing their strong colours. Carotenoids are required for the correct assembly of photosystems (Pogson et al. 2005; Li et al. 2009b). Here they absorb light across a broader range of the spectral region in which the sun irradiates maximally and transfer the energy to chlorophyll, initiating the photochemical events of photosynthesis (Polívka and Frank 2010). In plants, carotenoids are bound in discrete pigment–protein complexes referred to as the LHCII trimeric complex, which typically benefits from lutein, neoxanthin, as well as violaxanthin to transfer energy to chlorophyll (Pogson et al. 2005; Demmig-Adams and Adams 2006; Dall’Osto et al. 2010). A curious marine dinoflagellate, Amphidinium carterae alternatively evolved a peridinin–chlorophyll protein complex that contains more of the bound carotenoid (peridinin) when compared with chlorophyll (Hofmann et al. 1996; Polívka and Frank 2010) (Fig. 1).
Perhaps the roles that carotenoids play to protect the photosynthetic machinery from excessive light are among key biological functions of carotenoids relevant for life on earth. Plants are able to balance between absorbing sufficient light for photosynthetic processes while avoiding photo oxidative damage to membranes and proteins caused by excessive light. To meet this balance carotenoids (1) quench triplet chlorophyll, (2) scavenge ROS like singlet oxygen which damage membranes and proteins, thereby behaving as antioxidants (along with ascorbate and tocopherols) and (3) dissipate excess energy via xanthophyll-mediated non-photochemical quenching (NPQ) (e.g. xanthophyll carotenoids include zeaxanthin, antheraxanthin and lutein). The physiological relevance of xanthophylls to photosynthesis and plant biology in general is displayed by bleaching, delayed greening, viviparous and semi-lethal phenotypes observed in several carotenoid and NPQ-deficient mutants (Niyogi 1999; Pogson et al. 2005; Bailey and Grossman 2008; Alboresi et al. 2011).
Colour, volatiles, phytohormones and signalling molecules in plants
The bright colours of carotenoids serve as powerful visual attractants for birds and insects, which assist in the dispersal of pollen and seeds aiding plant reproduction. For example, a single locus, YELLOW UPPER5–7 (YUP) controls the presence or absence of yellow carotenoid pigments in the petals of pink-flowered Mimulus lewisii (monkey flower), which is pollinated by bumblebees and its red-flowered sister species M. cardinalis, which is pollinated by hummingbirds (Schemske and Bradshaw 1999). Researchers creating near-isogenic lines (NILs) in which the YUP allele from each species was substituted altering flower colour and revealed an adaptive shift in pollinator preference as a result of a single mutation altering carotenoid composition (Bradshaw and Schemske 2003). The next question from a plant regulatory perspective is what is the underling molecular nature or mutation associated with the YUP allele? The regulation of carotenoid colours in flowers has been reviewed recently (Zhu et al. 2010). In addition to providing colour to flowers and fruits, C40 carotenoid derivatives serve as substrates in the biosynthesis of plant volatile scents and aroma constituents (geranyl acetone and β-ionone) that attract insects and animals for pollination as well as seed dispersal (Simkin et al. 2004; Walter et al. 2010).
In plants, carotenoids serve as substrates for the production of phytohormones such as abscisic acid and strigolactone as well as other signalling molecules (e.g. blumenin and mycorradicin) (Figs 1, 3). ABA is a cleavage product of 9-cis-violaxanthin and/or 9’-cis-neoxanthin by 9-cis-epoxycarotenoid dioxygenase (NCED), which was first identified in the maize viviparous14 (vp14) mutant (Schwartz et al. 1997; Seo and Koshiba 2002; Tan et al. 2003; Chinnusamy et al. 2008). In some situations, carotenoid biosynthetic genes, such as β-carotene hydroxylase from rice, were shown to be rate-limiting for ABA biosynthesis and can alter the plants resistance to drought and oxidative stress by modulating the levels of xanthophylls and abscisic acid synthesis (Du et al. 2010). ABA mediates responses to environmental stresses, notably the control of stomatal aperture and transpiration during drought, also the induction of many stress-related gene products. Finally, ABA promotes developmental processes such as seed maturation and dormancy (Seo and Koshiba 2002; Nambara and Marion-Poll 2005; Chinnusamy et al. 2008).
|
The strigolactone class of carotenoid-derived terpenoids functions to (1) inhibit shoot branching, presumably by inhibiting bud outgrowth (Gomez-Roldan et al. 2008; Umehara et al. 2008), (2) influence mycorrhizal hyphal branching in order to stimulate a symbiotic relationship in the root rhizosphere (Akiyama et al. 2005) and (3) encourage germination of parasitic plant seeds such as striga (Matusova et al. 2005). Indeed, strigolactone application of GR24 can restore wild-type branching phenotype in pea ccd8 mutants and also in the orthologous ccd8 Arabidopsis, in a dose-dependent manner (Gomez-Roldan et al. 2008). Beta-carotene has been proposed as the substrate for strigolactone biosynthesis (Matusova et al. 2005; Rani et al. 2008) and it has yet to be determined if changes in β-carotene levels perturb strigolactone production.
Other carotenoid-derived signalling molecules such as C13 cyclohexenone derivatives (e.g. blumenol) and C14 apocarotenoids (e.g. mycorradicin) play important roles in controlling beneficial arbuscule turnover during arbuscular mycorrhizal (AM) fungi symbiosis as well as triggering hyphal branching in the rhizosphere (Fig. 1) (Giuliano et al. 2003; Akiyama et al. 2005; Walter et al. 2010). Further, evidence exists for the potential for a novel mobile carotenoid-derived signalling metabolite required for normal root and shoot development. The ArabidopsisBYPASS1 (BPS1) gene encodes an unknown protein and roots of bps1 mutants produce a graft transmissible signal arresting shoot development (Van Norman et al. 2004). That fluridone (blocks phytoene production) can partially rescue both leaf and root defects displayed by bps1 and CPTA (a lycopene cyclase inhibitor) treatment indicates that the signal is likely to require the biosynthesis of β-carotene and its derivatives (Fig. 2). The mobile signal is neither ABA- nor strigolactone related and the signal does not require the activity of any single carotenoid cleavage dioxygenase (Van Norman et al. 2004; Van Norman and Sieburth 2007). Unravelling the biosynthesis and regulation of carotenoid-derived signalling molecules will undoubtedly provide new insights into the essential roles that carotenoids play in nature.
Carotenoid biosynthesis and regulation
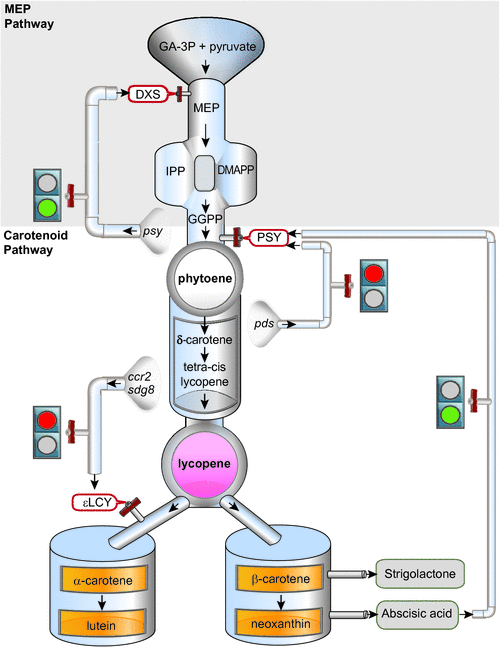
Carotenoid biosynthesis requires an available source of isoprenoid substrates derived from the plastid-localised 2-C-methyl-D-erythritol 4-phosphate (MEP) pathway (Rodriguez-Concepcion 2010). Isoprenoids (or terpenoids) are naturally-occurring organic chemicals that serve as precursors to produce a diverse range of compounds such as tocopherols, chlorophylls, phylloquinone (PhQ), gibberellins, abscisic acid, monoterpenes and plastoquinone (PQ). Glyceraldehyde-3-phosphate and pyruvate (generated from the Calvin cycle or glycolysis) act as initial substrates leading to five-carbon isoprene isomers, isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP), which are then condensed to synthesise geranylgeranyl diphosphate (GGPP) (Fig. 3). The first steps in the MEP pathway are catalysed by 1-deoxyxylulose-5-phosphate synthase (DXS) and 1-deoxy-D-xylulose 5-phosphate reductoisomerase (DXR). 1-Hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate reductase (HDR) then catalyses the production of IPP (isopentenyl diphosphate) and DMAPP (dimethylallyl diphosphate). Abiotic and biotic factors are likely to influence the production of isoprenoid substrates for carotenogenesis. Most importantly, light and circadian oscillations appear to regulate the expression of most MEP and several carotenoid biosynthetic genes. The biosynthesis and regulation of the MEP pathway and isoprenoid substrates required for carotenogenesis has been covered elsewhere (Cordoba et al. 2009).
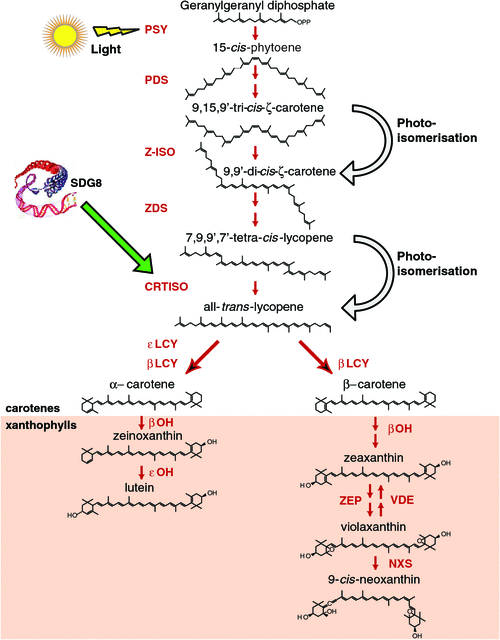
The condensation of two GGPP molecules catalysed by the phytoene synthase (PSY) enzyme produces 15-cis-phytoene, the first colourless carotenoid (Fig. 2). PSY is an important rate-limiting regulatory enzyme in the pathway, forming the first committed step and a bottleneck in carotenogenesis. In addition to high light, PSY can be transcriptionally responsive to ABA, salt, drought, temperature, photoperiod, development cues and post-transcriptional feedback regulation (Cazzonelli and Pogson 2010). Carotenoid pathway flux can be controlled by multiple PSYs, as is the case for tomato, rice and maize, whereas only a single allele exists in Arabidopsis (Welsch et al. 2000, 2008; Giorio et al. 2008; Li et al. 2008a). Multiple homologues of PSY are likely to be somewhat redundant; however, individual PSY alleles are expressed in a tissue-specific manner and only some alleles show a unique response to abiotic stress-induced ABA signalling of PSY gene expression (Li et al. 2008b; Welsch et al. 2008; Qin et al. 2011). Alternative splicing as well as allelic variation are mechanisms by which to titrate PSY enzyme activity and are likely to underlie a major QTL determinant of flour colour in bread and durum wheats (Howitt et al. 2009; Rodríguez-Suárez et al. 2011). The expression of PSY is highly co-regulated with photosynthesis-related genes and isoprenoid biosynthesis pathway genes insuring an optimum photosynthetic metabolism (Meier et al. 2011). Environmental, developmental and metabolic cues are major regulators of PSY gene expression and will be consider later in the review.
The production of all-trans-lycopene from 15-cis-phytoene involves four enzymes, phytoene desaturase (PDS), ζ-carotene isomerase (ζ-ISO), ζ-carotene desaturase (ZDS) and carotenoid isomerase (CRTISO) as well as a light-mediated photoisomerisation (Fig. 2) (Bartley et al. 1999; Park et al. 2002; Isaacson et al. 2004; Breitenbach and Sandmann 2005; Dong et al. 2007; Chen et al. 2010). PDS mRNA transcript levels are slightly upregulated during photomorphogenesis via the phytochrome-mediated pathway, which could imply that PDS plays a rate-limiting role in the generation of 9,15,9′-tri-cis-ζ-carotene (ζ-carotene) (Welsch et al. 2000; Qin et al. 2007). The plastid-targeted alternative oxidase (PTOX) protein was required for phytoene desaturase (PDS) activity and links desaturation to chloroplast electron transport (Yu et al. 2007). Regulatory roles for ZDS and Z-ISO in the catalysis of ζ-carotene to tetra-cis-lycopene (pro-lycopene), the substrate for CRTISO are beginning to emerge. ZISO gene expression appears to be regulated by an extended the dark period and the loss-of-function of ZDS affects carotenoid accumulation as well as plastid-to-nucleus communications (referred to as retrograde signalling; Pogson et al. 2008), most likely due to impaired plastid development (Dong et al. 2007; Chen et al. 2010).
CRTISO catalyses the cis–trans reactions to isomerase the four cis-bonds introduced by the desaturases and emerges as a major regulatory node in the pathway (Cazzonelli et al. 2009c). crtiso Mutants accumulate poly-cis-isomers (e.g. phytoene, phytofluene, ζ-carotene and neurosporene in addition to prolycopene) instead of the typical xanthophyll profile in non-photosynthetic tissues such as flowers (e.g. petals), the inner pericarp of green fruit and etiolated seedlings (Isaacson et al. 2002; Park et al. 2002). The function of these cis-carotenoids remains largely unknown and it is easy to speculate that they could play a role as novel signalling molecules. Most recently, CRTISO was shown to be essential for establishing an equilibrium between cis and trans carotenoid isomers (Yu et al. 2011). Perhaps CRTISO has other functions, such as to control the production of apocarotenoid precursors required for phytohormone biosynthesis (e.g. ABA or strigolactone) or to establish an equilibrium between cis- to trans-isomerisation and, hence, control the accumulation of cis-carotenoids. A recent insight revealed that the OsCRTISO may function to regulate the expression of the PSII core proteins (CP43 and CP47), thereby affecting the redox state of the PQ to stabilise oxygen-evolving extrinsic proteins and activity of photosynthetic oxygen evolution in rice (Wei et al. 2010).
Despite the block from cis- to an all-trans-configuration in crtiso mutants, carotenogenesis can proceed in chloroplasts via photoisomerisation, but there is a delay in the greening of etiolated seedlings and a substantial reduction in lutein in Arabidopsis as well as some degree of chlorosis in tomato and rice (Isaacson et al. 2002; Park et al. 2002; Fang et al. 2008; Wei et al. 2010; Chai et al. 2011). Perhaps, CRTISO plays important roles in non-green tissues (e.g. fruit and root appear to be protected from photoisomerisation) and in germinating seeds as they grow through the soil towards the light (Isaacson et al. 2002; Park et al. 2002). The role of light in substituting for the absence of CRTISO activity in green tissues is under debate and now emerges as an exciting area of intense carotenoid research to unravel the elusive chemical mechanisms and regulatory nature associated with photoisomerisation (Fig. 2) (Cunningham and Schiff 1985; Sandmann 1991; Park et al. 2002; Isaacson et al. 2004; Yu et al. 2011).
Carotenoid biosynthesis bifurcates after lycopene to produce epsilon- and β-carotenoids by enzymatic activity of two lycopene cyclases, ॉLCY and βLCY (Fig. 2). Regulation by ॉLCY is the first committed step coordinating carotenoid flux through the β-ॉ branch and modulates the ratio of the most abundant carotenoid, lutein to the β-carotenoids leading to changes in lutein content (Cuttriss et al. 2007; Harjes et al. 2008; Cazzonelli et al. 2009a; Howitt et al. 2009). A molecular synergism between ॉLCY and βLCY activities is an overall major determinant of flux through the branch leading to production of lutein, β-carotene and other xanthophylls cycle (XC) carotenoids (Fig. 1) (Yu et al. 2008; Bai et al. 2009). Investigations have yielded mutants perturbing lutein biosynthesis including lut1, ॉ-hydroxylase (Tian et al. 2004); lut2, ॉLCY(Cunningham et al. 1996; Pogson et al. 1996); ccr2, CRTISO (Park et al. 2002); and lut5, an additional β-hydroxylase (Kim and DellaPenna 2006) as well as the SDG8 chromatin regulatory mutant, ccr1 (Cazzonelli et al. 2009a). Implications for the epigenetic regulation of carotenoid flux through the β-ॉ branch are discussed below.
The xanthophyll carotenoids are major carotenoid sink metabolites accumulating in plant leaf tissues produced by the oxidative functionalisation of either α-carotene (lutein) or β-carotenes (zeaxanthin, violaxanthin and neoxanthin) (Fig. 2). Modulation of xanthophyll composition can greatly affect photosystem assembly light harvesting, photoprotection and impact a plant’s response to stressful conditions (Pogson et al. 1998; Dall’Osto et al. 2006). Zeaxanthin can be epoxidised to produce violaxanthin by zeaxanthin epoxidase (ZEP) (Marin et al. 1996). Under high-light stress, this reaction is reversed by violaxanthin de-epoxidase (VDE) (Pfundel et al. 1994). Violaxanthin is converted to neoxanthin by neoxanthin synthase (NXS) (Al-Babili et al. 2000). Neoxanthin is the final carotenoid of the β-β branch of the classical biosynthetic pathway (Fig. 2). Many other xanthophyll carotenoids have been identified in some plants tissues and these include the highly valued ketocarotenoids (e.g. astaxanthin, capsanthin, capsorubin and canthaxanthin) and their biosynthesis has been reviewed elsewhere (Giuliano et al. 2008; Jackson et al. 2008).
Turnover and degradation of carotenoids
Carotenoids are relatively stable compounds that accumulate in diverse types of tissues (photosynthetic and non-photosynthetic). Recently, it was demonstrated by 14CO2 uptake experiments that carotenoid turnover appears to be much greater than expected (Beisel et al. 2010). Given the continued synthesis in mature leaves the active degradation of carotenoids by CCD (carotenoid cleavage dioxygenases) and NCED (9-cis-epoxycarotenoid dioxygenase) enzymatic turnover has emerged an exciting area of discovery (Bouvier et al. 2005; Walter et al. 2010; Lewinsohn et al. 2005). Members of these gene families are involved in the biosynthesis of the phytohormone ABA (NCEDs), which controls abiotic stress signalling pathways and strigolactone (CCDs), which controls shoot growth and root-mycorrhizal symbiosis (Figs 1, 3).
The NCED gene family (NCED2, 3, 5, 6, 9) show different substrate specificity as well as tissue distribution and changes in NCED transcript levels regulate carotenoid composition and abundance (Schwartz et al. 1997, 2003; Tan et al. 1997, 2003). Further, NCED3 gene expression appears to be controlled by chromatin modifying protein, ATX1 (homologue of the Drosophila Trithorax, TRX protein also referred to as SDG27), which is implicated in diverse dehydration stress-response mechanisms in Arabidopsis (Ding et al. 2011; Thorstensen et al. 2011). ATX1 was shown to affect the quantity of RNA polymerase II bound to the NCED3 locus and is necessary for the increased levels of NCED3 transcripts and nucleosomal histone-3-lysine-4 trimethylation (H3K4 me3) that occur during dehydration stress. In the atx1 mutant the NCED3 transcript levels decline and this in part reduces ABA production. The loss of function of atx1 mutants shows a reduced germination rate, enlarged stomatal aperture, increased transpiration rates and a lower tolerance to dehydration stress (Ding et al. 2011).
The CCD gene family (CCD1, 4, 7 and 8) play essential roles in synthesis of colour, apocarotenoid flavour, aroma volatiles and phytohormones such as strigolactone. The active degradation of the xanthophylls by CCD activity can reduce lutein content in strawberries as well as cause changes in the pigmentation in chrysanthemums from white to yellow (Ohmiya et al. 2006; García-Limones et al. 2008). In maturing Arabidopsis seeds a loss of function of CCD1 activity leads to higher carotenoid levels and may have a role in synthesis of apocarotenoid flavour and aroma volatiles (Auldridge et al. 2006). Similarly, in tomato (Lycopersicon esculentum) LeCCD1 activity contributes to the formation of the flavour volatiles β-ionone, pseudoionone and geranylacetone (Simkin et al. 2004). The Crocus, zeaxanthin 7,8(7′,8′)-cleavage dioxygenase (CsZCD) and 9,10(9′,10′)-cleavage dioxygenase (CsCCD) initiate the biogenesis of carotenoid derivatives such as crocetin glycosides, picrocrocin and safranal (saffron). Indeed, the expression of CsZCD mRNA was mostly restricted to the style branch tissues and transcript levels were inducible under dehydration stress (Bouvier et al. 2003b). Two of the genes that affect shoot branching encoding CCD7 and CCD8 can sequentially cleave β-carotene to form the C18 compound 13-apo-carotenone (Schwartz et al. 2004). CCD7 appears to be a biosynthetic cross point, controlling both strigolactone and AM-induced C13 cyclohexenone and C14 mycorradicin apocarotenoids (Vogel et al. 2010). Finally, there are examples of cross talk where inhibition of ABA biosynthesis reduces CCD7 and CCD8 transcript abundance as well as strigolactone levels (López-Ráez et al. 2010).
Accumulation, storage and insights from biofortication
The storage of carotenoids requires a lipophilic environment, usually within the membranes (envelope and in some cases the thylakoid membranes) of plastid organelles, which behave as a sink for carotenoid accumulation. The biogenesis of organelles is a major determinant of the storage compartment size of plastids and can affect the accumulation of carotenoids (reviewed by Pogson and Albrecht 2011) The colourless pluripotent progenitor proplastid differentiates into specialised plastids that can store chlorophylls and carotenoids (Kirk and Tiliney-Bassett 1978). Carotenoids are usually synthesised de novo in differentiated plastids of roots, flowers, fruits and seeds, accumulating mostly in chloroplasts (green photosynthetic plastids) and chromoplasts (coloured plastids), but also in amyloplasts (starch-storing plastids), leucoplasts (colourless plastids), etioplasts (dark-grown precursors of the chloroplast) and elaioplasts (lipid-storing plastids) (Cazzonelli and Pogson 2010). There are links between changes in carotenoid composition and plastid biogenesis, morphology and protein translocation, in particular, it is noteworthy that carotenoids (e.g. lutein) are necessary for the differentiation of an etioplast into a chloroplast (Fig. 1) (Park et al. 2002).
The regulation of carotenoid targeting, storage and sequestration within various plastid types is a process by which to modulate a sink for carotenoid accumulation (Lu et al. 2006; Cuttriss et al. 2007; Cazzonelli and Pogson 2010). For example, the high-pigment 1 tomato mutant, hp1, displays an increased pigmentation because of increased chromoplast compartment size (Cookson et al. 2003). A naturally occurring mutation in the Brassica oleracea orange-curd (or) gene changes a normally white cauliflower curd into an orange Or mutant, which accumulates high levels of β-carotene (Lu and Li 2008). The OR gene encodes a DnaJ cysteine-rich domain-containing protein and plays a role in coordinating the differentiation of proplastids in the shoot and inflorescence meristems of the curd into chromoplasts (Li et al. 2001; Lu et al. 2006). Carotenoids accumulate in lipoprotein structures within the chromoplast (Bartley and Scolnik 1995; Vishnevetsky et al. 1999), which might allow for additional carotenoid biosynthesis. Therefore, chromoplasts serve as a metabolic sink to control carotenoid accumulation in plants and reveals the importance of plastid differentiation in controlling carotenoid accumulation in plants (Lu et al. 2006; Li and Van Eck 2007). The Crocus chromoplast localised enzymes, CsZCD and CsCCD control the accumulation of saffron metabolites as well as the differentiation of amyloplasts and chromoplasts (Bouvier et al. 2003b). The oxidative cleavage of zeaxanthin in chromoplasts is intriguingly followed by the sequestration of modified water-soluble derivatives into the central vacuole (Bouvier et al. 2003b).
The introduction of foreign exogenous carotenoid biosynthetic genes into crops is another means by which to modulate the sink for carotenoid storage. Carotenoid accumulation has been achieved in oil seeds of canola (Brassica napus) through the overexpression of PSY, which resulted in 43–50-fold increase in total seed carotenoid content (Shewmaker et al. 1999; Lindgren et al. 2003). ‘Golden rice 2’ is a high β-carotene accumulating line overexpressing PSY from maize as well as bacterial CrtI (from Erwinia uredovora) and accumulates 23-fold more carotenoids (37 µg g–1) than was the case for the initial ‘golden rice’, which overexpressed PSY from daffodil (Beyer et al. 2002; Paine et al. 2005). Underground food crops, such as potato and carrot have also been engineered to have increased carotenoid content, in particular β-carotene and ketocarotenoids (e.g. astaxanthin), which produce a deep yellow (‘golden’) potato tuber phenotype and a distinct pink to deep red colour compared with the typical orange colour in wild-type carrot roots (Taylor and Ramsay 2005; Diretto et al. 2007; Jayaraj et al. 2008). Finally, the overexpression of PSY in Arabidopsis increased carotenoid content by 10- to 100-fold in nonphotosynthetic calli and roots, predominantly β-carotene and its derivatives, which were deposited as crystals in storage plastids (Maass et al. 2009).
Photostimulation of carotenoid gene expression
Light regulates the key rate-limiting step in phytoene biosynthesis. PSY transcript abundance increases during photomorphogenesis via a phytochrome-mediated (red-light) pathway and this response was abolished in the phyA mutant (Welsch et al. 2000, 2008). Phytochrome-interacting factor 1 (PIF1) has been shown to bind to the PSY promoter and represses PSY mRNA expression. The authors show that light triggers the degradation of PIF1 after interacting with photoactivated phytochromes during de-etiolation and that a rapid derepression of PSY gene expression enhances carotenoid production during the optimal transition to photosynthetic metabolism (Toledo-Ortiz et al. 2010). The light-triggered degradation of PIFs causes a rapid accumulation of carotenoids, which is coordinated with chlorophyll biosynthesis to enable the rapid assembly of the photosynthetic machinery (Toledo-Ortiz et al. 2010). Very recently, the DELLA proteins were shown to function in part through the regulation of PIF activity, by a DELLA-PIF complex that coordinates the levels of POR, chlorophyll precursors and carotenoids in cotyledons of dark-grown seedlings (Cheminant et al. 2011). PSY is strongly light-induced in greening seedlings (Welsch et al. 2000) and a transcription factor, RAP2.2, (AP2/EREBP family) which binds to the PSY promoter was able to slightly perturb pigment alterations in Arabidopsis root calli (Welsch et al. 2007).
High light stress also alters the synthesis of β-xanthophylls, such as the synthesis of zeaxanthin from β-carotene (Depka et al. 1998). Plants with reduced zeaxanthin exhibit increased sensitivity to light stress (Havaux and Niyogi 1999). The amount of zeaxanthin can be modulated by regulating the expression of the β-hydroxylase (β-OH) gene, which is photo-inducible and violaxanthin de-epoxidase (VDE), whose transcript levels are slightly downregulated by light (Rossel et al. 2002). In addition, the luminal pH and ascorbate content appear to affect post-translation modulation of VDE activity and is critical for determining the levels of zeaxanthin upon exposure to excessive light (Rockholm and Yamamoto 1996). Further, the βLCY mutant (suppressor of zeaxanthin-less1, szl1) lacks zeaxanthin, yet accumulates higher levels of lutein and α-carotene, which partially restores quenching efficiency, revealing that lutein could substitute for zeaxanthin (Li et al. 2009a). The modulation of zeaxanthin levels by the violaxanthin de-epoxidase (VDE) is thought to be required for the thermal dissipation of excess light energy and the protection of photosynthetic membranes against lipid peroxidation, a process known as the xanthophyll cycle (Havaux and Niyogi 1999).
Epigenetic maintenance of carotenoid composition
Beyond the heavy demands from developmental and environmental cues to coordinate carotenoid gene expression (Cazzonelli and Pogson 2010), there are further implications for the epigenetic control over apocarotenoid formation (described above) and especially carotenoid biosynthesis (Cazzonelli et al. 2009c; Ding et al. 2011). A chromatin modifying protein was found to regulate CRTISO expression and carotenoid flux through the branch to α and β-carotenoids, thereby limiting lutein production. The SET DOMAIN GROUP (SDG) family of histone lysine methyltransferases are chromatin modifying proteins that methylate lysine residues (chromatin marks) on the tails of histone proteins in order to recruit other regulatory factors and make the DNA either more or less accessible to RNA polymerase II transcriptional complex (Cazzonelli et al. 2009b; Thorstensen et al. 2011). The methylation of lysine residues can be maintained through cell division (mitosis) establishing long-term memory required for adaptation and these marks are usually reset during gamete formation (meiosis) (Saze 2008).
SDG8 (homologue of the Drosophila absent, small or homeotic disks 2 often referred to as ASHH2 or in plants as carotenoid and chloroplast regulation, ccr1, or early flowering in short days, efs) maintains a transcriptionally permissive chromatin state surrounding the CRTISO locus and thus is able to regulate carotenoid composition, particularly accumulation of the most abundant carotenoid, lutein (Fig. 2) (Cazzonelli et al. 2009a). In non-photosynthetic tissues, SDG8 is required for CRTISO activity and in the absence of SDG8 activity, etiolated tissues accumulate cis-isomers (Park et al. 2002). SDG8 maintains H3K4 trimethylation of regions surrounding the CRTISO promoter and SDG8 is necessary for regulating the CRTISO promoter in rapidly dividing tissues during epigenetic programming (e.g. mitosis in the shoot apex) and reprogramming (e.g. meiosis in pollen) events (Cazzonelli et al. 2010). The reporter gene expression patterns enabled by SDG8 and CRTISO promoters overlap considerably, with the strongest expression being apparent in many tissues essential for defining plant architecture and development, including germinating seedlings, vasculature, meristems, shoot apices, floral anthers and pollen (Cazzonelli et al. 2010).
The sdg8 mutant shows several pleiotropic phenotypes, such as early flowering, altered shoot and root architecture, altered pathogen resistance, male sterility, abnormal sporophyte and gametophyte development (Kim et al. 2005; Cazzonelli et al. 2009a; Grini et al. 2009; Berr et al. 2010). A few putative target genes responsible for these phenotypes have been described and target gene loci show a lower abundance of H3K4 or H3K36 methylation marks, as well as reduced mRNA levels in the sdg8 mutant. However, only the early flowering and lutein deficient phenotypes have been sufficiently complemented by overexpression of the corresponding target genes, FLOWERING LOCUS C (FLC) and CRTISO, respectively (Kim et al. 2005; Cazzonelli et al. 2009a). The affect of the chromatin marks can spread and appear to only have a slight affect on the relative transcript abundance of genes neighbouring CRTISO and FLC, suggesting that indeed both CRTISO and FLC are likely to be direct targets of SDG8 activity (Kim et al. 2005; Cazzonelli et al. 2009a).
The chromatin regulatory proteins required to recruit and or interact with SDG8 in order to post-translationally modify the CRTISO locus with permissive chromatin marks is yet to be discovered. The epigenetic regulation of FLC by vernalisation has been extensively studied and should pave the way to discover new insights into the epigenetic regulatory mechanisms controlling CRTISO expression and perhaps link carotenoids with the developmental regulation of floral initiation (Dennis and Peacock 2007). It was recently demonstrated that SDG8 interacts with FRIGIDA (FRI), a protein that delays flowering by activating FLC expression and in the absence of a prolonged cold winter period (a process known as vernalisation) FRI promotes a vegetative state of growth (Choi et al. 2011). SDG8 is essential for FRI function and SDG8 was recently demonstrated to have a novel dual substrate specificity for H3K4 and H3K36 specific histone lysine methylation activity (Ko et al. 2010). The affect of FRI on carotenoid accumulation, if any, remains to be determined.
There are bigger questions that remain to be answered. First, does SDG8 also affect H3K36 chromatin marks surrounding the CRTISO locus? Second, what is the molecular nature of the mechanisms used to target SDG8 to key target genes like CRTISO and FLC? Third, is carotenoid composition regulated in response to an epigenetic phenomena? Finally, what novel roles might SDG8 and CRTISO play in fine tuning plant development, architecture and the production of precursors for phytohormone biosynthesis? It is possible to speculate the role, if any, for photoisomerisation in overriding SDG8 regulation in green tissues, however, in non-photosynthetic tissues, perhaps SDG8 and CRTISO serve important functions in coordinating organelle differentiation, cellular specialisation and plastid to nucleus communications during development. The discovery that carotenoid biogenesis and apocarotenoid formation are regulated by chromatin modification opens the door to begin exploring what new roles might carotenoids and apocarotenoids (e.g. ABA) play in memory forming processes.
Carotenoid signals and metabolite feedback control
Several genes encoding enzymes in isoprenoid and carotenoid biosynthesis are the subject of negative and positive feedback regulatory processes and are likely to be mediated by a carotenoid or a molecule derived from a carotenoid (Römer et al. 2000; Beyer et al. 2002; Cuttriss et al. 2007; Qin et al. 2007; Bai et al. 2009). There is strong evidence to show carotenoid metabolite feedback regulation requires post-transcriptional and transcriptional processes to modulate target genes that (1) supply isoprenoid precursors for carotenogenesis, (2) control activity of the rate-limiting phytoene synthase step and (3) control carotenoid flux through the branch to synthesis of α- and β-carotenes. Essentially, positive and negative metabolite feedback mechanisms exist within the carotenoid pathway and among carotenoids, MEP and ABA (Fig. 3).
The overexpression of PSY transcript levels enhanced carotenoid levels in etiolated Arabidopsis seedlings via the concomitant post-transcriptional accumulation of DXS protein, which initiated feedback stimulating the supply of MEP substrates (Fig. 3) (Rodríguez-Villalón et al. 2009). Additional evidence that PSY controls metabolic flux was obtained through the paclobutrazol treatment of Arabidopsis seedlings, which not only inhibits gibberellin synthesis but stimulates de-etiolation despite the absence of light. PSY activity and carotenoid levels were shown to increase in the dark following paclobutrazol treatment and this increase was supported by feedback regulation of DXS and DXR protein abundance (Rodríguez-Villalón et al. 2009). Surprisingly, overexpression of DXS mRNA in dark-grown seedlings does not affect carotenoid accumulation (Rodríguez-Villalón et al. 2009). An increase in DXS protein activity (but not gene expression levels) was also detected in tomato fruit containing increased PSY transcript levels and it was speculated that this could occur through the enhanced accumulation of active DXS enzymes in chromoplasts (Fraser et al. 2007; Rodríguez-Villalón et al. 2009). Finally, the establishment of plant root arbuscular mycorrhizal (AM) fungi in the rhizosphere activates the MEP pathway enhancing transcript levels of the first two enzymes of the MEP pathway (e.g. DXS and DXR), the two desaturases in the carotenoid pathway (e.g. PDS and ZDS) as well as the carotenoid-cleaving dioxygenases in AM roots (Strack and Fester 2006). The molecular nature of the metabolite feedback regulatory mechanisms controlling the accumulation of MEP precursors and DXS at the protein or gene expression levels, remain open for discovery.
PSY regulates the first committed step in carotenogenesis (e.g. phytoene production) and appears to be controlled by source and sink metabolites (Fig. 3). For example, in the pds3 mutant, downstream (e.g. ZDS and βLCY) and upstream (IPI, GGPS and PSY) pathway genes were downregulated, but also those related to the biosynthesis of gibberellins as well as chlorophylls (Qin et al. 2007). The absence of downstream carotenoids in pds3 mutants could provide the signal as evidence from the analysis of a tomato PDS promoter::GUS fusion demonstrated end product regulation in photosynthetic tissues (Corona et al. 1996). OsPSY3 gene expression is upregulated in rice during increased abscisic acid (ABA) formation as well as upon salt treatment and drought, especially in roots indicating that the third PSY allele in rice plays a specialised role in abiotic stress-induced ABA formation (Welsch et al. 2008). A positive feedback regulation mediated by the application of ABA induces OsPSY3 as well as OsNCED gene expression. An ABA-response element as well as a coupling element was identified in the promoter region for OsPSY3, but its function remains to be validated (Welsch et al. 2008). Therefore, the regulation of phytoene synthesis in plants appears to involve both negative and positive feedback regulatory mechanisms.
The control of carotenoid flux through the branch from all-trans-lycopene to synthesis of α- and β-carotenes is also under negative metabolite feedback regulation and mediated through the transcription control of ॉLCY gene expression (Cuttriss et al. 2007). Investigations have indicated that both CRITSO (ccr2) and SDG8 (ccr1) mutants have an inhibitory effect on ॉLCY transcript levels (Fig. 3), revealing that feedback may partially explain the reduced lutein observed in ccr2 and ccr1 mutants (Cuttriss et al. 2007; Cazzonelli et al. 2009a). Further, a reduction in seed ॉLCY mRNA levels show higher total carotenoid content, specifically increased levels of β-carotene, zeaxanthin, violaxanthin and unexpectedly, lutein (Yu et al. 2008). However, another report showed that tuber specific silencing of ॉLCY resulted in an expected increase in β-carotene levels in potato (Solanum tuberosum) (Diretto et al. 2006). In B. napus, total carotenoids increased in the βLCY (lcyB-m2.1) mutant by nearly 200 and 40% in mutant embryo and endosperm tissues of maize respectively (Bai et al. 2009). Lycopene was the primary carotenoid that accumulated in lcyB-m2.1 embryo tissues, together with a small amount of δ-carotene (Bai et al. 2009) and perhaps these molecules are candidates for degradation and subsequent feedback regulation. Together, these lines of evidence support a hypothesis that lutein composition is largely rate-determined by ॉLCY expression and that metabolite feedback might be another key regulator of the branch node in carotenogenesis (Fig. 3).
Future prospects
Carotenoids are everywhere and are essential in nature, clearly serving numerous functions during animal and plant development (Fig. 1). A deeper investigation into the control of carotenoid biosynthesis will undoubtedly provide new regulatory mechanisms by which to facilitate improvements to crop nutrition. Understanding how carotenoids are sequestered within plastid types is an area ripe for discovery and has the potential to further improve metabolic engineering attempts to enhance the composition of essential dietary micronutrients, such as the xanthophylls and carotenes. The discovery of new regulators of carotenoid biosynthesis that modulate the production of strigolactones and ABA will really highlight the important roles that carotenoids play in plants, especially in response to developmental and environmental signals. Two major nodes in the pathway play essential roles in regulating carotenoid composition and flux. One node affects the production of phytoene at the bottleneck in the pathway and the other modulates the accumulation of cis-carotenoids and lutein at the branch to α- and β-carotenoids. The cross-talk between and within the MEP, carotenoid and ABA biosynthetic pathways is an intriguing example of metabolite feedback control. This could involve a signalling protein bound to specific carotenoids or their cis-/trans-isomers and perhaps even soluble or volatile carotenoid cleavage products could mediate signalling between the plastid and nucleus. For now the nature of the different forms of metabolic feedback regulation remain unknown. It will be necessary to establish an evolutionary importance for some of the carotenoid regulatory processes described in this review using non-model plant species. Finally, a deeper understanding of the molecular nature by which metabolite feedback and chromatin regulatory mechanisms control carotenoid abundance and composition is paramount to determine if indeed there is a memory forming process epigenetically regulating carotenogenesis in plants.
Acknowledgements
CC was supported by the Australian Research Council Centre of Excellence in Plant Energy Biology (CE0561495). A special thanks goes to Barry Pogson (Australian National University) in nominating me for the Peter Goldacre Award and for introducing me to the brighter world of carotenoids. Many thanks also to Mark Taylor (The James Hutton Institute) and Li Li (USDA-ARS) for reviewing this article and providing constructive comments. The author would finally like to give a strong appreciation to the following contributors for providing images in Fig. 1: aphids (Nancy Moran, Yale University), etioplast and chloroplast (Reprinted from Trends in Plant Sciences, 15(5), 266–274, 2010, with permission from Elsevier), finches (Kevin McGraw, Arizona State University), golden rice (Courtesy Golden Rice Humanitarian Board: www.goldenrice.org, accessed 29 July 2011), sea anemone (Shunichi Takahashi, Australian National University) and flamingos as well as crayfish (Harry Frank, University of Connecticut).
References
Akiyama K, Matsuzaki K, Hayashi H (2005) Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435, 824–827.| Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXkvVGgsL4%3D&md5=60987d7085f7de2338b693abfc82c76dCAS |
Al-Babili S, Hugueney P, Schledz M, Welsch R, Frohnmeyer H, Laule O, Beyer P (2000) Identification of a novel gene coding for neoxanthin synthase from Solanum tuberosum. FEBS Letters 485, 168–172.
| Identification of a novel gene coding for neoxanthin synthase from Solanum tuberosum.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXotlGjs7o%3D&md5=0c574f364654e67e4671f7f269adf2c3CAS |
Alboresi A, Dall’Osto L, Aprile A, Carillo P, Roncaglia E, Cattivelli L, Bassi R (2011) Reactive oxygen species and transcript analysis upon excess light treatment in wild-type Arabidopsis thaliana vs a photosensitive mutant lacking zeaxanthin and lutein. BMC Plant Biology 11, 62
| Reactive oxygen species and transcript analysis upon excess light treatment in wild-type Arabidopsis thaliana vs a photosensitive mutant lacking zeaxanthin and lutein.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXltV2msrs%3D&md5=956d5625ee8e5c622d9ae5dc9a024e65CAS |
Amat J, Rendón M, Garrido-Fernández J, Garrido A, Rendón-Martos M, Pérez-Gálvez A (2010) Greater flamingos Phoenicopterus roseus use uropygial secretions as makeup. Behavioural Ecology and Sociobiology 65, 665–673.
| Greater flamingos Phoenicopterus roseus use uropygial secretions as makeup.Crossref | GoogleScholarGoogle Scholar |
Auldridge ME, Block A, Vogel JT, Dabney-Smith C, Mila I, Bouzayen M, Magallanes-Lundback M, DellaPenna D, McCarty DR, Klee HJ (2006) Characterisation of three members of the Arabidopsis carotenoid cleavage dioxygenase family demonstrates the divergent roles of this multifunctional enzyme family. The Plant Journal 45, 982–993.
| Characterisation of three members of the Arabidopsis carotenoid cleavage dioxygenase family demonstrates the divergent roles of this multifunctional enzyme family.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xjs1SmsLg%3D&md5=a9d4a734018eba85ba4b3bcc2e549e16CAS |
Bai L, Kim EH, DellaPenna D, Brutnell TP (2009) Novel lycopene epsilon cyclase activities in maize revealed through perturbation of carotenoid biosynthesis. The Plant Journal 59, 588–599.
| Novel lycopene epsilon cyclase activities in maize revealed through perturbation of carotenoid biosynthesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtFChtrrO&md5=6adfb118880b1a88d2a7195809eeaeebCAS |
Bailey S, Grossman A (2008) Photoprotection in cyanobacteria: regulation of light harvesting. Photochemistry and Photobiology 84, 1410–1420.
| Photoprotection in cyanobacteria: regulation of light harvesting.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsVWkt73K&md5=73dd9fe4fb5061c2912b8647b41dba9aCAS |
Baron M, Davies S, Alexander L, Snellgrove D, Sloman KA (2008) The effect of dietary pigments on the coloration and behaviour of flame-red dwarf gourami, Colisa lalia. Animal Behaviour 75, 1041–1051.
| The effect of dietary pigments on the coloration and behaviour of flame-red dwarf gourami, Colisa lalia.Crossref | GoogleScholarGoogle Scholar |
Bartley GE, Scolnik PA (1995) Plant carotenoids: pigments for photoprotection, visual attraction and human health. The Plant Cell 7, 1027–1038.
Bartley GE, Scolnik PA, Beyer P (1999) Two Arabidopsis thaliana carotene desaturases, phytoene desaturase and zeta-carotene desaturase, expressed in Escherichia coli, catalyse a poly-cis pathway to yield pro-lycopene. European Journal of Biochemistry 259, 396–403.
| Two Arabidopsis thaliana carotene desaturases, phytoene desaturase and zeta-carotene desaturase, expressed in Escherichia coli, catalyse a poly-cis pathway to yield pro-lycopene.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXlt1Kksw%3D%3D&md5=826f59e833cc6b97161fd8989eb2b99bCAS |
Beisel KG, Jahnke S, Hofmann D, Koppchen S, Schurr U, Matsubara S (2010) Continuous turnover of carotenes and chlorophyll a in mature leaves of Arabidopsis revealed by 14CO2 pulse-chase labelling. Plant Physiology 152, 2188–2199.
| Continuous turnover of carotenes and chlorophyll a in mature leaves of Arabidopsis revealed by 14CO2 pulse-chase labelling.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXmsVantrs%3D&md5=d9f194a6025274e5975a4077c4430e9fCAS |
Berr A, McCallum EJ, Alioua A, Heintz D, Heitz T, Shen WH (2010) Arabidopsis histone methyltransferase SET DOMAIN GROUP8 mediates induction of the jasmonate/ethylene pathway genes in plant defense response to necrotrophic fungi. Plant Physiology 154, 1403–1414.
| Arabidopsis histone methyltransferase SET DOMAIN GROUP8 mediates induction of the jasmonate/ethylene pathway genes in plant defense response to necrotrophic fungi.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsV2ntrzP&md5=cb5db47e1059cf4e17f2b1d6379718e4CAS |
Beyer P, Al-Babili S, Ye X, Lucca P, Schaub P, Welsch R, Potrykus I (2002) Golden rice: introducing the beta-carotene biosynthesis pathway into rice endosperm by genetic engineering to defeat vitamin A deficiency. Journal of Nutrition 132, 506S–510S.
Bouvier F, Dogbo O, Camara B (2003a) Biosynthesis of the food and cosmetic plant pigment bixin (annatto). Science 300, 2089–2091.
| Biosynthesis of the food and cosmetic plant pigment bixin (annatto).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXkvVOrsbo%3D&md5=b1f64f983e7ba0cfa01650a49cb18d9dCAS |
Bouvier F, Suire C, Mutterer J, Camara B (2003b) Oxidative remodelling of chromoplast carotenoids: identification of the carotenoid dioxygenase CsCCD and CsZCD genes involved in Crocus secondary metabolite biogenesis. The Plant Cell 15, 47–62.
| Oxidative remodelling of chromoplast carotenoids: identification of the carotenoid dioxygenase CsCCD and CsZCD genes involved in Crocus secondary metabolite biogenesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXmtFensQ%3D%3D&md5=6f4367e8ffdd5c743d0dcc8682ebd7edCAS |
Bouvier F, Isner JC, Dogbo O, Camara B (2005) Oxidative tailoring of carotenoids: a prospect towards novel functions in plants. Trends in Plant Science 10, 187–194.
| Oxidative tailoring of carotenoids: a prospect towards novel functions in plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXjtVyru7w%3D&md5=109343e78a50c56f72286036edcb315dCAS |
Bradshaw HD, Schemske DW (2003) Allele substitution at a flower colour locus produces a pollinator shift in monkeyflowers. Nature 426, 176–178.
| Allele substitution at a flower colour locus produces a pollinator shift in monkeyflowers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXovVKmtbs%3D&md5=b33338acc6f9907cd91b6e72498f06cdCAS |
Breitenbach J, Sandmann G (2005) Zeta-carotene cis-isomers as products and substrates in the plant poly-cis carotenoid biosynthetic pathway to lycopene. Planta 220, 785–793.
| Zeta-carotene cis-isomers as products and substrates in the plant poly-cis carotenoid biosynthetic pathway to lycopene.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhvVSqs70%3D&md5=f4f98af39eea13ba7f2ab38a747f1851CAS |
Cazzonelli CI, Pogson BJ (2010) Source to sink: regulation of carotenoid biosynthesis in plants. Trends in Plant Science 15, 266–274.
| Source to sink: regulation of carotenoid biosynthesis in plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXlvVaqsbc%3D&md5=b097fba5ec3b848bce3f48a7652a46afCAS |
Cazzonelli CI, Cuttriss AJ, Cossetto SB, Pye W, Crisp P, Whelan J, Finnegan EJ, Turnbull C, Pogson BJ (2009a) Regulation of carotenoid composition and shoot branching in Arabidopsis by a chromatin modifying histone methyltransferase, SDG8. The Plant Cell 21, 39–53.
| Regulation of carotenoid composition and shoot branching in Arabidopsis by a chromatin modifying histone methyltransferase, SDG8.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXlsFCitrw%3D&md5=fbc041d94b2a8bb53d75f1e19a97834fCAS |
Cazzonelli CI, Millar T, Finnegan EJ, Pogson BJ (2009b) Promoting gene expression in plants by permissive histone lysine methylation. Plant Signalling & Behaviour 4, 484–488.
| Promoting gene expression in plants by permissive histone lysine methylation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXpvFelu7o%3D&md5=28957c5fa07b0f803ad15f7a69b030ceCAS |
Cazzonelli CI, Yin K, Pogson BJ (2009c) Potential implications for epigenetic regulation of carotenoid biosynthesis during root and shoot development. Plant Signalling & Behaviour 4, 339–341.
| Potential implications for epigenetic regulation of carotenoid biosynthesis during root and shoot development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXpsV2jsr0%3D&md5=801773800c34fe4a97f3e5f77afebc86CAS |
Cazzonelli CI, Roberts AC, Carmody ME, Pogson BJ (2010) Transcriptional control of SET DOMAIN GROUP8 and CAROTENOID ISOMERASE during Arabidopsis development. Molecular Plant 3, 174–191.
| Transcriptional control of SET DOMAIN GROUP8 and CAROTENOID ISOMERASE during Arabidopsis development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtVSqu78%3D&md5=012d174fe1c100801ea29da28aef043aCAS |
Chai C, Fang J, Liu Y, Tong H, Gong Y, Wang Y, Liu M, Wang Y, Qian Q, Cheng Z, Chu C (2011) ZEBRA2, encoding a carotenoid isomerase, is involved in photoprotection in rice. Plant Molecular Biology 75, 211–221.
| ZEBRA2, encoding a carotenoid isomerase, is involved in photoprotection in rice.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtFSjsb8%3D&md5=4c5c4877ef7459b35383801bb76cc721CAS |
Cheminant S, Wild M, Bouvier F, Pelletier S, Renou JP, Erhardt M, Hayes S, Terry MJ, Genschik P, Achard P (2011) DELLAs regulate chlorophyll and carotenoid biosynthesis to prevent photo-oxidative damage during seedling de-etiolation in Arabidopsis. The Plant Cell 23, 1849–1860.
| DELLAs regulate chlorophyll and carotenoid biosynthesis to prevent photo-oxidative damage during seedling de-etiolation in Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXptFWqsLo%3D&md5=2c7a037069a04471cfd15ff2676f6547CAS |
Chen Y, Li F, Wurtzel ET (2010) Isolation and characterisation of the Z-ISO gene encoding a missing component of carotenoid biosynthesis in plants. Plant Physiology 153, 66–79.
| Isolation and characterisation of the Z-ISO gene encoding a missing component of carotenoid biosynthesis in plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXnt1Ghu7c%3D&md5=29603c28aa51b79209e17115a06b66f2CAS |
Chidambara Murthy KN, Vanitha A, Rajesha J, Mahadeva Swamy M, Sowmya PR, Ravishankar GA (2005) In vivo antioxidant activity of carotenoids from Dunaliella salina – a green microalga. Life Sciences 76, 1381–1390.
| In vivo antioxidant activity of carotenoids from Dunaliella salina – a green microalga.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2M%2FksVWjtA%3D%3D&md5=54dda1c52b1dc147c383b867ef35fa2fCAS |
Chinnusamy V, Gong Z, Zhu JK (2008) Abscisic acid-mediated epigenetic processes in plant development and stress responses. Journal of Integrative Plant Biology 50, 1187–1195.
| Abscisic acid-mediated epigenetic processes in plant development and stress responses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtlaitbrI&md5=e9cdfea28d14f51f9041dcd85ffb6fe7CAS |
Choi K, Kim J, Hwang HJ, Kim S, Park C, Kim SY, Lee I (2011) The FRIGIDA complex activates transcription of FLC, a strong flowering repressor in Arabidopsis, by recruiting chromatin modification factors. The Plant Cell 23, 289–303.
| The FRIGIDA complex activates transcription of FLC, a strong flowering repressor in Arabidopsis, by recruiting chromatin modification factors.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXpsFCksro%3D&md5=0b3f83631505eab5258b32b27abe08edCAS |
Cookson PJ, Kiano JW, Shipton CA, Fraser PD, Romer S, Schuch W, Bramley PM, Pyke KA (2003) Increases in cell elongation, plastid compartment size and phytoene synthase activity underlie the phenotype of the high pigment-1 mutant of tomato. Planta 217, 896–903.
| Increases in cell elongation, plastid compartment size and phytoene synthase activity underlie the phenotype of the high pigment-1 mutant of tomato.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXns1Gqur8%3D&md5=f9b419f180929798be2b3bfe7bbbbe82CAS |
Cordoba E, Salmi M, Leon P (2009) Unravelling the regulatory mechanisms that modulate the MEP pathway in higher plants. Journal of Experimental Botany 60, 2933–2943.
| Unravelling the regulatory mechanisms that modulate the MEP pathway in higher plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXosFWjtb0%3D&md5=a074e1cc18367f6cf49214007616c5c2CAS |
Corona V, Aracri B, Kosturkova G, Bartley GE, Pitto L, Giorgetti L, Scolnik PA, Giuliano G (1996) Regulation of a carotenoid biosynthesis gene promoter during plant development. The Plant Journal 9, 505–512.
| Regulation of a carotenoid biosynthesis gene promoter during plant development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XivFWntLo%3D&md5=575dfbdc902fb73914ad5f57c77126d0CAS |
Cunningham FX Cunningham FX (1985) Photoisomerisation of ζ−carotene stereoisomers in cells of euglena gracilis mutant W3BUL and in solution. Photochemistry and Photobiology 42, 295–307.
| Photoisomerisation of ζ−carotene stereoisomers in cells of euglena gracilis mutant W3BUL and in solution.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL28Xjt1Wq&md5=58cbb68a2fb070997cf0dd236a63f784CAS |
Cunningham FX, Pogson B, Sun ZR, McDonald KA, DellaPenna D, Gantt E (1996) Functional analysis of the beta and epsilon lycopene cyclase enzymes of Arabidopsis reveals a mechanism for control of cyclic carotenoid formation. The Plant Cell 8, 1613–1626.
| Functional analysis of the beta and epsilon lycopene cyclase enzymes of Arabidopsis reveals a mechanism for control of cyclic carotenoid formation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XlvFOks7k%3D&md5=349ffd8e265fb53585a042d08f196fceCAS |
Cuttriss AJ, Chubb A, Alawady A, Grimm B, Pogson B (2007) Regulation of lutein biosynthesis and prolamellar body formation in Arabidopsis. Functional Plant Biology 34, 663–672.
| Regulation of lutein biosynthesis and prolamellar body formation in Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXos1KntL8%3D&md5=c58bac911750b7edfc82f77740f429a3CAS |
Dall’Osto L, Lico C, Alric J, Giuliano G, Havaux M, Bassi R (2006) Lutein is needed for efficient chlorophyll triplet quenching in the major LHCII antenna complex of higher plants and effective photoprotection in vivo under strong light. BMC Plant Biology 6, 32
| Lutein is needed for efficient chlorophyll triplet quenching in the major LHCII antenna complex of higher plants and effective photoprotection in vivo under strong light.Crossref | GoogleScholarGoogle Scholar |
Dall’Osto L, Cazzaniga S, Havaux M, Bassi R (2010) Enhanced photoprotection by protein-bound vs free xanthophyll pools: a comparative analysis of chlorophyll b and xanthophyll biosynthesis mutants. Molecular Plant 3, 576–593.
| Enhanced photoprotection by protein-bound vs free xanthophyll pools: a comparative analysis of chlorophyll b and xanthophyll biosynthesis mutants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXmslWit70%3D&md5=43c766633b9610d57b2c1c385cce85efCAS |
Del Campo JA, García-González M, Guerrero MG (2007) Outdoor cultivation of microalgae for carotenoid production: current state and perspectives. Applied Microbiology and Biotechnology 74, 1163–1174.
| Outdoor cultivation of microalgae for carotenoid production: current state and perspectives.Crossref | GoogleScholarGoogle Scholar |
Demmig-Adams B, Adams WW (2006) Photoprotection in an ecological context: the remarkable complexity of thermal energy dissipation. New Phytologist 172, 11–21.
| Photoprotection in an ecological context: the remarkable complexity of thermal energy dissipation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtVyisrnE&md5=b5053ae7026361074997e57fb71457edCAS |
Dennis ES, Peacock WJ (2007) Epigenetic regulation of flowering. Current Opinion in Plant Biology 10, 520–527.
| Epigenetic regulation of flowering.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtFekurjE&md5=a32b468540bd780bd866f1f9c16f2f78CAS |
Depka B, Jahns P, Trebst A (1998) Beta-carotene to zeaxanthin conversion in the rapid turnover of the D1 protein of PSII. FEBS Letters 424, 267–270.
| Beta-carotene to zeaxanthin conversion in the rapid turnover of the D1 protein of PSII.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXhs1Gjur4%3D&md5=bbfe6e4630a253834b40915e6f41fcc6CAS |
Ding Y, Avramova Z, Fromm M (2011) The Arabidopsis trithorax-like factor ATX1 functions in dehydration stress responses via ABA-dependent and ABA-independent pathways. The Plant Journal 66, 735–744.
| The Arabidopsis trithorax-like factor ATX1 functions in dehydration stress responses via ABA-dependent and ABA-independent pathways.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXnslahtL8%3D&md5=9702703370af711345b076eb83e9e50dCAS |
Diretto G, Tavazza R, Welsch R, Pizzichini D, Mourgues F, Papacchioli V, Beyer P, Giuliano G (2006) Metabolic engineering of potato tuber carotenoids through tuber-specific silencing of lycopene epsilon cyclase. BMC Plant Biology 6, 13
| Metabolic engineering of potato tuber carotenoids through tuber-specific silencing of lycopene epsilon cyclase.Crossref | GoogleScholarGoogle Scholar |
Diretto G, Al-Babili S, Tavazza R, Papacchioli V, Beyer P, Giuliano G (2007) Metabolic engineering of potato carotenoid content through tuber-specific overexpression of a bacterial mini-pathway. PLoS ONE 2, e350
| Metabolic engineering of potato carotenoid content through tuber-specific overexpression of a bacterial mini-pathway.Crossref | GoogleScholarGoogle Scholar |
Dong H, Deng Y, Mu J, Lu Q, Wang Y, Xu Y, Chu C, Chong K, Lu C, Zuo J (2007) The Arabidopsis Spontaneous Cell Death1 gene, encoding a ζ-carotene desaturase essential for carotenoid biosynthesis, is involved in chloroplast development, photoprotection and retrograde signalling. Cell Research 17, 458–470.
| The Arabidopsis Spontaneous Cell Death1 gene, encoding a ζ-carotene desaturase essential for carotenoid biosynthesis, is involved in chloroplast development, photoprotection and retrograde signalling.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXlt1Wktbs%3D&md5=1ec1b12c39ea116f9a948bf1f5562733CAS |
Du H, Wang N, Cui F, Li X, Xiao J, Xiong L (2010) Characterisation of the beta-carotene hydroxylase gene DSM2 conferring drought and oxidative stress resistance by increasing xanthophylls and abscisic acid synthesis in rice. Plant Physiology 154, 1304–1318.
| Characterisation of the beta-carotene hydroxylase gene DSM2 conferring drought and oxidative stress resistance by increasing xanthophylls and abscisic acid synthesis in rice.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsV2ntr%2FJ&md5=4dc68d57a307906f51bc75cc0727b19eCAS |
Fang J, Chai C, Qian Q, Li C, Tang J, Sun L, Huang Z, Guo X, Sun C, Liu M, Zhang Y, Lu Q, Wang Y, Lu C, Han B, Chen F, Cheng Z, Chu C (2008) Mutations of genes in synthesis of the carotenoid precursors of ABA lead to pre-harvest sprouting and photo-oxidation in rice. The Plant Journal 54, 177–189.
| Mutations of genes in synthesis of the carotenoid precursors of ABA lead to pre-harvest sprouting and photo-oxidation in rice.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXlsVWhsr4%3D&md5=c9be4b9c8949973275c184dee9755365CAS |
Fassett RG, Coombes JS (2011) Astaxanthin: a potential therapeutic agent in cardiovascular disease. Marine Drugs 9, 447–465.
| Astaxanthin: a potential therapeutic agent in cardiovascular disease.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXktl2rtb4%3D&md5=febd7aec3c677ae200425e70d78161caCAS |
Fraser PD, Enfissi EM, Halket JM, Truesdale MR, Yu D, Gerrish C, Bramley PM (2007) Manipulation of phytoene levels in tomato fruit: effects on isoprenoids, plastids and intermediary metabolism. The Plant Cell 19, 3194–3211.
| Manipulation of phytoene levels in tomato fruit: effects on isoprenoids, plastids and intermediary metabolism.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhsVersLbO&md5=666996d836bbfd6893b20d2608620941CAS |
García-Limones C, Schnäbele K, Blanco-Portales R, Luz Bellido M, Caballero JL, Schwab W, Muñoz-Blanco J (2008) Functional characterisation of FaCCD1: a carotenoid cleavage dioxygenase from strawberry involved in lutein degradation during fruit ripening. Journal of Agricultural and Food Chemistry 56, 9277–9285.
| Functional characterisation of FaCCD1: a carotenoid cleavage dioxygenase from strawberry involved in lutein degradation during fruit ripening.Crossref | GoogleScholarGoogle Scholar |
Giorio G, Stigliani AL, D’Ambrosio C (2008) Phytoene synthase genes in tomato (Solanum lycopersicum L.) – new data on the structures, the deduced amino acid sequences and the expression patterns. FEBS Journal 275, 527–535.
| Phytoene synthase genes in tomato (Solanum lycopersicum L.) – new data on the structures, the deduced amino acid sequences and the expression patterns.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhvVOksbw%3D&md5=bd324cae140df61eef213e0f5077fd6dCAS |
Giuliano G, Al-Babili S, von Lintig J (2003) Carotenoid oxygenases: cleave it or leave it. Trends in Plant Science 8, 145–149.
| Carotenoid oxygenases: cleave it or leave it.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXjtFeqs78%3D&md5=0b6e4ee0af4cee7886f133440dfcee6dCAS |
Giuliano G, Tavazza R, Diretto G, Beyer P, Taylor MA (2008) Metabolic engineering of carotenoid biosynthesis in plants. Trends in Biotechnology 26, 139–145.
| Metabolic engineering of carotenoid biosynthesis in plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXitVOktLw%3D&md5=93fa3023c876f9815b97ea8eeb797dd0CAS |
Goff SA, Klee HJ (2006) Plant volatile compounds: sensory cues for health and nutritional value? Science 311, 815–819.
| Plant volatile compounds: sensory cues for health and nutritional value?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtFylt7s%3D&md5=4af9bf3b0bf4078afde4f2ff65c7f55bCAS |
Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pages V, Dun EA, Pillot JP, Letisse F, Matusova R, Danoun S, Portais JC, Bouwmeester H, Becard G, Beveridge CA, Rameau C, Rochange SF (2008) Strigolactone inhibition of shoot branching. Nature 455, 189–194.
| Strigolactone inhibition of shoot branching.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtV2qtLzK&md5=a691bf6a8414df67fe4f64a5f68c81b1CAS |
Grini PE, Thorstensen T, Alm V, Vizcay-Barrena G, Windju SS, Jorstad TS, Wilson ZA, Aalen RB (2009) The ASH1 HOMOLOG 2 (ASHH2) histone H3 methyltransferase is required for ovule and anther development in Arabidopsis. PLoS ONE 4, e7817
| The ASH1 HOMOLOG 2 (ASHH2) histone H3 methyltransferase is required for ovule and anther development in Arabidopsis.Crossref | GoogleScholarGoogle Scholar |
Harjes CE, Rocheford TR, Bai L, Brutnell TP, Kandianis CB, Sowinski SG, Stapleton AE, Vallabhaneni R, Williams M, Wurtzel ET, Yan J, Buckler ES (2008) Natural genetic variation in lycopene epsilon cyclase tapped for maize biofortification. Science 319, 330–333.
| Natural genetic variation in lycopene epsilon cyclase tapped for maize biofortification.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXmt1yjsQ%3D%3D&md5=e95714ef2401650ceaaf0c827da3a719CAS |
Havaux M, Niyogi KK (1999) The violaxanthin cycle protects plants from photo-oxidative damage by more than one mechanism. Proceedings of the National Academy of Sciences of the United States of America 96, 8762–8767.
| The violaxanthin cycle protects plants from photo-oxidative damage by more than one mechanism.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXkslOkur4%3D&md5=db26e5c288f477f9929bc150ec31694eCAS |
Hill GE (2000) Energetic constraints on expression of carotenoid-based plumage coloration. Journal of Avian Biology 31, 559–566.
| Energetic constraints on expression of carotenoid-based plumage coloration.Crossref | GoogleScholarGoogle Scholar |
Hofmann E, Wrench PM, Sharples FP, Hiller RG, Welte W, Diederichs K (1996) Structural basis of light harvesting by carotenoids: peridinin-chlorophyll-protein from Amphidinium carterae. Science 272, 1788–1791.
| Structural basis of light harvesting by carotenoids: peridinin-chlorophyll-protein from Amphidinium carterae.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XjslWnu78%3D&md5=bd2fa8d3a366a9b7e88852a36f0545c5CAS |
Howitt CA, Cavanagh CR, Bowerman AF, Cazzonelli C, Rampling L, Mimica JL, Pogson BJ (2009) Alternative splicing, activation of cryptic exons and amino acid substitutions in carotenoid biosynthetic genes are associated with lutein accumulation in wheat endosperm. Functional & Integrative Genomics 9, 363–376.
| Alternative splicing, activation of cryptic exons and amino acid substitutions in carotenoid biosynthetic genes are associated with lutein accumulation in wheat endosperm.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXns1Chur4%3D&md5=5f24a8bc4c8f38762c0fb2d27f96d291CAS |
Isaacson T, Ronen G, Zamir D, Hirschberg J (2002) Cloning of tangerine from tomato reveals a carotenoid isomerase essential for the production of beta-carotene and xanthophylls in plants. The Plant Cell 14, 333–342.
| Cloning of tangerine from tomato reveals a carotenoid isomerase essential for the production of beta-carotene and xanthophylls in plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XisVKgtbo%3D&md5=535dc78ede64ac5e3b257a2d587316a6CAS |
Isaacson T, Ohad I, Beyer P, Hirschberg J (2004) Analysis in vitro of the enzyme CRTISO establishes a poly-cis-carotenoid biosynthesis pathway in plants. Plant Physiology 136, 4246–4255.
| Analysis in vitro of the enzyme CRTISO establishes a poly-cis-carotenoid biosynthesis pathway in plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXjtVartA%3D%3D&md5=bdc80bdb3ecde32a6932b8952a5e3a83CAS |
Jackson H, Braun CL, Ernst H (2008) The chemistry of novel xanthophyll carotenoids. American Journal of Cardiology 101, S50–S57.
| The chemistry of novel xanthophyll carotenoids.Crossref | GoogleScholarGoogle Scholar |
Jayaraj J, Devlin R, Punja Z (2008) Metabolic engineering of novel ketocarotenoid production in carrot plants. Transgenic Research 17, 489–501.
| Metabolic engineering of novel ketocarotenoid production in carrot plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXnvFyit7s%3D&md5=06e7e53702c22254d5f05c98196d3561CAS |
Johnson EJ (2002) The role of carotenoids in human health. Nutrition in Clinical Care 5, 56–65.
| The role of carotenoids in human health.Crossref | GoogleScholarGoogle Scholar |
Kim J, DellaPenna D (2006) Defining the primary route for lutein synthesis in plants: the role of Arabidopsis carotenoid beta-ring hydroxylase CYP97A3. Proceedings of the National Academy of Sciences of the United States of America 103, 3474–3479.
| Defining the primary route for lutein synthesis in plants: the role of Arabidopsis carotenoid beta-ring hydroxylase CYP97A3.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XksF2ku7Y%3D&md5=73b61b60ec6c59324660ba4188b297a0CAS |
Kim SY, He Y, Jacob Y, Noh YS, Michaels S, Amasino R (2005) Establishment of the vernalisation-responsive, winter-annual habit in Arabidopsis requires a putative histone H3 methyl transferase. The Plant Cell 17, 3301–3310.
| Establishment of the vernalisation-responsive, winter-annual habit in Arabidopsis requires a putative histone H3 methyl transferase.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtlersLzM&md5=0f76142375e4f634772d1433fef2bc56CAS |
Kirk J, Tiliney-Bassett R (1978) Proplastids, etioplasts, amyloplasts, chromoplasts and other plastids. In ‘The plastids: their chemistry, structure, growth and inheritance’. (Eds ST Kirck, RA Tiliney-Bassett) pp. 217–239. (Elsevier: Amsterdam)
Ko JH, Mitina I, Tamada Y, Hyun Y, Choi Y, Amasino RM, Noh B, Noh YS (2010) Growth habit determination by the balance of histone methylation activities in Arabidopsis. EMBO Journal 29, 3208–3215.
| Growth habit determination by the balance of histone methylation activities in Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtVais7bJ&md5=fb722a07b4f187ebe8548506e7f1803aCAS |
Krawczyk S, Britton G (2001) A study of protein-carotenoid interactions in the astaxanthin-protein crustacyanin by absorption and Stark spectroscopy; evidence for the presence of three spectrally distinct species. Biochimica et Biophysica Acta 1544, 301–310.
| A study of protein-carotenoid interactions in the astaxanthin-protein crustacyanin by absorption and Stark spectroscopy; evidence for the presence of three spectrally distinct species.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXksValug%3D%3D&md5=8f65e0b819cca7481c0e26963706da90CAS |
Krinsky NI, Johnson EJ (2005) Carotenoid actions and their relation to health and disease. Molecular Aspects of Medicine 26, 459–516.
| Carotenoid actions and their relation to health and disease.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXht1OnurfL&md5=5aaa9e81a54684d3074348d08fd62396CAS |
Leffingwell JC (2002) Saffron. Leffingwell Reports 2, 1–6.
Lewinsohn E, Sitrit Y, Bar E, Azulay Y, Ibdah M, Meir A, Yosef E, Zamir D, Tadmor Y (2005) Not just colours – carotenoid degradation as a link between pigmentation and aroma in tomato and watermelon fruit. Trends in Food Science & Technology 16, 407–415.
| Not just colours – carotenoid degradation as a link between pigmentation and aroma in tomato and watermelon fruit.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXpslyhtr8%3D&md5=24d3c3b961fda580ada1b8a8d4b8acf9CAS |
Li L, Van Eck J (2007) Metabolic engineering of carotenoid accumulation by creating a metabolic sink. Transgenic Research 16, 581–585.
| Metabolic engineering of carotenoid accumulation by creating a metabolic sink.Crossref | GoogleScholarGoogle Scholar |
Li L, Paolillo DJ, Parthasarathy MV, Dimuzio EM, Garvin DF (2001) A novel gene mutation that confers abnormal patterns of beta-carotene accumulation in cauliflower (Brassica oleracea var. botrytis). The Plant Journal 26, 59–67.
| A novel gene mutation that confers abnormal patterns of beta-carotene accumulation in cauliflower (Brassica oleracea var. botrytis).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXks1Kls7c%3D&md5=0eb612cf076204ede4678c0ee9a9706aCAS |
Li F, Vallabhaneni R, Wurtzel ET (2008a) PSY3, a new member of the phytoene synthase gene family conserved in the Poaceae and regulator of abiotic stress-induced root carotenogenesis. Plant Physiology 146, 1333–1345.
| PSY3, a new member of the phytoene synthase gene family conserved in the Poaceae and regulator of abiotic stress-induced root carotenogenesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXjsVKgtb0%3D&md5=090daa1d2b5a575ec0b1f7a051a315c5CAS |
Li F, Vallabhaneni R, Yu J, Rocheford T, Wurtzel ET (2008b) The maize phytoene synthase gene family: overlapping roles for carotenogenesis in endosperm, photomorphogenesis and thermal stress tolerance. Plant Physiology 147, 1334–1346.
| The maize phytoene synthase gene family: overlapping roles for carotenogenesis in endosperm, photomorphogenesis and thermal stress tolerance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXoslyisb4%3D&md5=7c34b8b217438bcbbe3d9a4d5326206fCAS |
Li Z, Ahn TK, Avenson TJ, Ballottari M, Cruz JA, Kramer DM, Bassi R, Fleming GR, Keasling JD, Niyogi KK (2009a) Lutein accumulation in the absence of zeaxanthin restores non-photochemical quenching in the Arabidopsis thaliana npq1 mutant. The Plant Cell 21, 1798–1812.
| Lutein accumulation in the absence of zeaxanthin restores non-photochemical quenching in the Arabidopsis thaliana npq1 mutant.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXpvFOks7g%3D&md5=5f7ba5b064720ebe5cf5f7f464d266d4CAS |
Li Z, Wakao S, Fischer BB, Niyogi KK (2009b) Sensing and responding to excess light. Annual Review of Plant Biology 60, 239–260.
| Sensing and responding to excess light.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXntFGls7k%3D&md5=ed61fd64814be47cfd282a954a0fc2d5CAS |
Lindgren LO, Stalberg KG, Hoglund A-S (2003) Seed-specific overexpression of an endogenous Arabidopsis phytoene synthase gene results in delayed germination and increased levels of carotenoids, chlorophyll and abscisic acid. Plant Physiology 132, 779–785.
| Seed-specific overexpression of an endogenous Arabidopsis phytoene synthase gene results in delayed germination and increased levels of carotenoids, chlorophyll and abscisic acid.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXkslert70%3D&md5=0ac273c27146d68cba050836db63864bCAS |
López-Ráez JA, Kohlen W, Charnikhova T, Mulder P, Undas AK, Sergeant MJ, Verstappen F, Bugg TD, Thompson AJ, Ruyter-Spira C, Bouwmeester H (2010) Does abscisic acid affect strigolactone biosynthesis? New Phytologist 187, 343–354.
| Does abscisic acid affect strigolactone biosynthesis?Crossref | GoogleScholarGoogle Scholar |
Losey J, Ives A, Harmon J, Ballantyne F, Brown C (1997) A polymorphism maintained by opposite patterns of parasitism and predation. Nature 388, 269–272.
| A polymorphism maintained by opposite patterns of parasitism and predation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXkslShu7w%3D&md5=f7208a83b41b621e08e0e3d2973d38d8CAS |
Lu S, Li L (2008) Carotenoid metabolism: biosynthesis, regulation and beyond. Journal of Integrative Plant Biology 50, 778–785.
| Carotenoid metabolism: biosynthesis, regulation and beyond.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXpt1ajurc%3D&md5=2998152f29e0de306d6fadb816be830dCAS |
Lu S, Van Eck J, Zhou X, Lopez AB, O’Halloran DM, Cosman KM, Conlin BJ, Paolillo DJ, Garvin DF, Vrebalov J, Kochian LV, Kupper H, Earle ED, Cao J, Li L (2006) The cauliflower Or gene encodes a DnaJ cysteine-rich domain-containing protein that mediates high levels of beta-carotene accumulation. The Plant Cell 18, 3594–3605.
| The cauliflower Or gene encodes a DnaJ cysteine-rich domain-containing protein that mediates high levels of beta-carotene accumulation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhvVKru7o%3D&md5=4eec98659677e9aa2be6cecb440d16d4CAS |
Maass D, Arango J, Wust F, Beyer P, Welsch R (2009) Carotenoid crystal formation in Arabidopsis and carrot roots caused by increased phytoene synthase protein levels. PLoS ONE 4, e6373
| Carotenoid crystal formation in Arabidopsis and carrot roots caused by increased phytoene synthase protein levels.Crossref | GoogleScholarGoogle Scholar |
Marin E, Nussaume L, Quesada A, Gonneau M, Sotta B, Hugueney P, Frey A, Marion-Poll A (1996) Molecular identification of zeaxanthin epoxidase of Nicotiana plumbaginifolia, a gene involved in abscisic acid biosynthesis and corresponding to the ABA locus of Arabidopsis thaliana. EMBO Journal 15, 2331–2342.
Matusova R, Rani K, Verstappen FW, Franssen MC, Beale MH, Bouwmeester HJ (2005) The strigolactone germination stimulants of the plant-parasitic Striga and Orobanche spp. are derived from the carotenoid pathway. Plant Physiology 139, 920–934.
| The strigolactone germination stimulants of the plant-parasitic Striga and Orobanche spp. are derived from the carotenoid pathway.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtFCgsb%2FI&md5=872784839572252fad9359d67786f9b4CAS |
McGraw KJ, Toomey MB (2010) Carotenoid accumulation in the tissues of zebra finches: predictors of integumentary pigmentation and implications for carotenoid allocation strategies. Physiological and Biochemical Zoology 83, 97–109.
| Carotenoid accumulation in the tissues of zebra finches: predictors of integumentary pigmentation and implications for carotenoid allocation strategies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsVyqtbw%3D&md5=ff5096e03fad4153384587f62b50801fCAS |
McGraw K, Nolan P, Crino O (2006a) Carotenoid accumulation strategies for becoming a colourful house finch: analyses of plasma and liver pigments in wild moulting birds. Functional Ecology 20, 678–688.
| Carotenoid accumulation strategies for becoming a colourful house finch: analyses of plasma and liver pigments in wild moulting birds.Crossref | GoogleScholarGoogle Scholar |
McGraw KJ, Crino OL, Medina-Jerez W, Nolan PM (2006b) Effect of dietary carotenoid supplementation on food intake and immune function in a songbird with no carotenoid coloration. Ethology 112, 1209–1216.
| Effect of dietary carotenoid supplementation on food intake and immune function in a songbird with no carotenoid coloration.Crossref | GoogleScholarGoogle Scholar |
Meier S, Tzfadia O, Vallabhaneni R, Gehring C, Wurtzel ET (2011) A transcriptional analysis of carotenoid, chlorophyll and plastidial isoprenoid biosynthesis genes during development and osmotic stress responses in Arabidopsis thaliana. BMC Systems Biology 5, 77
| A transcriptional analysis of carotenoid, chlorophyll and plastidial isoprenoid biosynthesis genes during development and osmotic stress responses in Arabidopsis thaliana.Crossref | GoogleScholarGoogle Scholar |
Moran NA, Jarvik T (2010) Lateral transfer of genes from fungi underlies carotenoid production in aphids. Science 328, 624–627.
| Lateral transfer of genes from fungi underlies carotenoid production in aphids.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXlt1Crt74%3D&md5=4fc470bc504e48f1cc5cd884ae778d46CAS |
Nambara E, Marion-Poll A (2005) Abscisic acid biosynthesis and catabolism. Annual Review of Plant Biology 56, 165–185.
| Abscisic acid biosynthesis and catabolism.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXmtVaru7o%3D&md5=f7daff4290bbf99e16c035b8378baff5CAS |
Niyogi KK (1999) Photoprotection revisited: genetic and molecular approaches. Annual Review of Plant Physiology and Plant Molecular Biology 50, 333–359.
| Photoprotection revisited: genetic and molecular approaches.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXkt1yksbo%3D&md5=c7899f1e30d07f91ed3a1921b371d24aCAS |
Ohmiya A, Kishimoto S, Aida R, Yoshioka S, Sumitomo K (2006) Carotenoid cleavage dioxygenase (CmCCD4a) contributes to white colour formation in chrysanthemum petals. Plant Physiology 142, 1193–1201.
| Carotenoid cleavage dioxygenase (CmCCD4a) contributes to white colour formation in chrysanthemum petals.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht1eju77J&md5=0bfd3a563a3ae5da9115b25bc2833013CAS |
Paine JA, Shipton CA, Chaggar S, Howells RM, Kennedy MJ, Vernon G, Wright SY, Hinchliffe E, Adams JL, Silverstone AL, Drake R (2005) Improving the nutritional value of golden rice through increased pro-vitamin A content. Nature Biotechnology 23, 482–487.
| Improving the nutritional value of golden rice through increased pro-vitamin A content.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXivFOhtLY%3D&md5=b3477706278a921956fb6cd2dafa57ebCAS |
Park H, Kreunen SS, Cuttriss AJ, DellaPenna D, Pogson BJ (2002) Identification of the carotenoid isomerase provides insight into carotenoid biosynthesis, prolamellar body formation and photomorphogenesis. The Plant Cell 14, 321–332.
| Identification of the carotenoid isomerase provides insight into carotenoid biosynthesis, prolamellar body formation and photomorphogenesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XisVKgtb0%3D&md5=79f538888d558e7d9fe219a0bb212334CAS |
Pfundel EE, Renganathan M, Gilmore AM, Yamamoto HY, Dilley RA (1994) Intrathylakoid pH in isolated pea chloroplasts as probed by violaxanthin de-epoxidation. Plant Physiology 106, 1647–1658.
Pogson BJ, Albrecht V (2011) Genetic dissection of chloroplast biogenesis and development: an overview. Plant Physiology 155, 1545–1551.
| Genetic dissection of chloroplast biogenesis and development: an overview.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXkvVOrsLk%3D&md5=612aee60c6960a3b45fff78c424c9537CAS |
Pogson B, McDonald KA, Truong M, Britton G, DellaPenna D (1996) Arabidopsis carotenoid mutants demonstrate that lutein is not essential for photosynthesis in higher plants. The Plant Cell 8, 1627–1639.
Pogson BJ, Niyogi KK, Bjorkman O, DellaPenna D (1998) Altered xanthophyll compositions adversely affect chlorophyll accumulation and non-photochemical quenching in Arabidopsis mutants. Proceedings of the National Academy of Sciences of the United States of America 95, 13324–13329.
| Altered xanthophyll compositions adversely affect chlorophyll accumulation and non-photochemical quenching in Arabidopsis mutants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXntFWrsb4%3D&md5=99a63514c299ac5052acef650a168cf1CAS |
Pogson BJ, Rissler HM, Frank HA (2005) The roles of carotenoids in photosystem II of higher plants. In ‘Photosystem II: the light-driven water: plastoquinone oxidoreductase’. (Eds T Wydrzynski, K Satoh) pp. 515–537. (Springer-Verlag: Dordrecht, The Netherlands)
Pogson BJ, Woo NS, Forster B, Small ID (2008) Plastid signalling to the nucleus and beyond. Trends in Plant Science 13, 602–609.
| Plastid signalling to the nucleus and beyond.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtlartL7I&md5=4e61866e85a53e9cfbaf5448a3ee2f0eCAS |
Polívka T, Frank HA (2010) Molecular factors controlling photosynthetic light harvesting by carotenoids. Accounts of Chemical Research 43, 1125–1134.
| Molecular factors controlling photosynthetic light harvesting by carotenoids.Crossref | GoogleScholarGoogle Scholar |
Qin G, Gu H, Ma L, Peng Y, Deng XW, Chen Z, Qu LJ (2007) Disruption of phytoene desaturase gene results in albino and dwarf phenotypes in Arabidopsis by impairing chlorophyll, carotenoid and gibberellin biosynthesis. Cell Research 17, 471–482.
| Disruption of phytoene desaturase gene results in albino and dwarf phenotypes in Arabidopsis by impairing chlorophyll, carotenoid and gibberellin biosynthesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXlt1WktbY%3D&md5=0be46ac22cbdafc9e7a07bb6015196c6CAS |
Qin X, Coku A, Inoue K, Tian L (2011) Expression, subcellular localisation and cis-regulatory structure of duplicated phytoene synthase genes in melon (Cucumis melo L.). Planta 234, 737–748.
| Expression, subcellular localisation and cis-regulatory structure of duplicated phytoene synthase genes in melon (Cucumis melo L.).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXht1ansrvN&md5=0bafe0a355991725ab9cc59e02084f1bCAS |
Rani K, Zwanenburg B, Sugimoto Y, Yoneyama K, Bouwmeester HJ (2008) Biosynthetic considerations could assist the structure elucidation of host plant produced rhizosphere signalling compounds (strigolactones) for arbuscular mycorrhizal fungi and parasitic plants. Plant Physiology and Biochemistry 46, 617–626.
| Biosynthetic considerations could assist the structure elucidation of host plant produced rhizosphere signalling compounds (strigolactones) for arbuscular mycorrhizal fungi and parasitic plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXosVygurw%3D&md5=27e93744188b53622babd919c98e88f4CAS |
Rockholm DC, Yamamoto HY (1996) Violaxanthin de-epoxidase. Plant Physiology 110, 697–703.
| Violaxanthin de-epoxidase.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XhtF2msLw%3D&md5=77e2a7725fdea153d0161ce0347688e1CAS |
Rodriguez-Concepcion M (2010) Supply of precursors for carotenoid biosynthesis in plants. Archives of Biochemistry and Biophysics 504, 118–122.
Rodríguez-Suárez C, Atienza SG, Pistón F (2011) Allelic variation, alternative splicing and expression analysis of Psy1 gene in Hordeum chilense Roem. et Schult. PLoS ONE 6, e19885
| Allelic variation, alternative splicing and expression analysis of Psy1 gene in Hordeum chilense Roem. et Schult.Crossref | GoogleScholarGoogle Scholar |
Rodríguez-Villalón A, Gas E, Rodríguez-Concepción M (2009) Phytoene synthase activity controls the biosynthesis of carotenoids and the supply of their metabolic precursors in dark-grown Arabidopsis seedlings. The Plant Journal 60, 424–435.
| Phytoene synthase activity controls the biosynthesis of carotenoids and the supply of their metabolic precursors in dark-grown Arabidopsis seedlings.Crossref | GoogleScholarGoogle Scholar |
Römer S, Fraser PD, Kiano JW, Shipton CA, Misawa N, Schuch W, Bramley PM (2000) Elevation of the pro-vitamin A content of transgenic tomato plants. Nature Biotechnology 18, 666–669.
| Elevation of the pro-vitamin A content of transgenic tomato plants.Crossref | GoogleScholarGoogle Scholar |
Rossel JB, Wilson IW, Pogson BJ (2002) Global changes in gene expression in response to high light in Arabidopsis. Plant Physiology 130, 1109–1120.
| Global changes in gene expression in response to high light in Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XovVOnu7g%3D&md5=726f33728aa32a6f4d43290b5a476bc1CAS |
Sandmann G (1991) Light-dependent switch from formation of poly-cis carotenes to all-trans carotenoids in the Scenedesmus mutant C-6D. Archives of Microbiology 155, 229–233.
| Light-dependent switch from formation of poly-cis carotenes to all-trans carotenoids in the Scenedesmus mutant C-6D.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXhvVWru7c%3D&md5=adb67a965035d60af706d0c2b8134a6eCAS |
Saze H (2008) Epigenetic memory transmission through mitosis and meiosis in plants. Seminars in Cell & Developmental Biology 19, 527–536.
| Epigenetic memory transmission through mitosis and meiosis in plants.Crossref | GoogleScholarGoogle Scholar |
Schemske DW, Bradshaw HD (1999) Pollinator preference and the evolution of floral traits in monkeyflowers (Mimulus). Proceedings of the National Academy of Sciences of the United States of America 96, 11910–11915.
| Pollinator preference and the evolution of floral traits in monkeyflowers (Mimulus).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXmvVGjs70%3D&md5=457a338fbf6fdcfbdac90fe989e369d5CAS |
Schwab W, Davidovich-Rikanati R, Lewinsohn E (2008) Biosynthesis of plant-derived flavor compounds. The Plant Journal 54, 712–732.
| Biosynthesis of plant-derived flavor compounds.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXmvFKgs7w%3D&md5=e9639b691510874897bb5fe82c542278CAS |
Schwartz SH, Tan BC, Gage DA, Zeevaart JA, McCarty DR (1997) Specific oxidative cleavage of carotenoids by VP14 of maize. Science 276, 1872–1874.
| Specific oxidative cleavage of carotenoids by VP14 of maize.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXksVCmsrg%3D&md5=8855cc1aa5efca8bb131660b64f389dcCAS |
Schwartz SH, Tan BC, McCarty DR, Welch W, Zeevaart JAD (2003) Substrate specificity and kinetics for VP14, a carotenoid cleavage dioxygenase in the ABA biosynthetic pathway. Biochimica et Biophysica Acta 1619, 9–14.
Schwartz SH, Qin X, Loewen MC (2004) The biochemical characterisation of two carotenoid cleavage enzymes from Arabidopsis indicates that a carotenoid-derived compound inhibits lateral branching. Journal of Biological Chemistry 279, 46940–46945.
| The biochemical characterisation of two carotenoid cleavage enzymes from Arabidopsis indicates that a carotenoid-derived compound inhibits lateral branching.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXptFejs7o%3D&md5=cbe5723795be88e40afc30b83cd88bf3CAS |
Seo M, Koshiba T (2002) Complex regulation of ABA biosynthesis in plants. Trends in Plant Science 7, 41–48.
| Complex regulation of ABA biosynthesis in plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xntl2nsg%3D%3D&md5=78febde60fbf9ba44d9cf6066cc1cf71CAS |
Shewmaker CK, Sheehy JA, Daley M, Colburn S, Ke DY (1999) Seed-specific overexpression of phytoene synthase: increase in carotenoids and other metabolic effects. The Plant Journal 20, 401–412.
| Seed-specific overexpression of phytoene synthase: increase in carotenoids and other metabolic effects.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXksVOhsw%3D%3D&md5=28554e2d0f702fe8a43b1a4f292c39bbCAS |
Simkin AJ, Schwartz SH, Auldridge M, Taylor MG, Klee HJ (2004) The tomato carotenoid cleavage dioxygenase 1 genes contribute to the formation of the flavor volatiles beta-ionone, pseudoionone and geranylacetone. The Plant Journal 40, 882–892.
| The tomato carotenoid cleavage dioxygenase 1 genes contribute to the formation of the flavor volatiles beta-ionone, pseudoionone and geranylacetone.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXpslGhsw%3D%3D&md5=d9a03eafbd2080a552a4c5f773ee1c4cCAS |
Stahl W, Sies H (2007) Carotenoids and flavonoids contribute to nutritional protection against skin damage from sunlight. Molecular Biotechnology 37, 26–30.
| Carotenoids and flavonoids contribute to nutritional protection against skin damage from sunlight.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtVais7%2FI&md5=74bcd3952ad44f225f5a46e17bd812f1CAS |
Strack D, Fester T (2006) Isoprenoid metabolism and plastid reorganisation in arbuscular mycorrhizal roots. New Phytologist 172, 22–34.
| Isoprenoid metabolism and plastid reorganisation in arbuscular mycorrhizal roots.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtVyisrnF&md5=5f8242389aba060f35655d3a2e8af983CAS |
Tan BC, Schwartz SH, Zeevaart JA, McCarty DR (1997) Genetic control of abscisic acid biosynthesis in maize. Proceedings of the National Academy of Sciences of the United States of America 94, 12235–12240.
| Genetic control of abscisic acid biosynthesis in maize.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXntFSmtb4%3D&md5=9b1de882969a05c6743e85ffa0113411CAS |
Tan BC, Joseph LM, Deng WT, Liu L, Li QB, Cline K, McCarty DR (2003) Molecular characterisation of the Arabidopsis 9-cis epoxycarotenoid dioxygenase gene family. The Plant Journal 35, 44–56.
| Molecular characterisation of the Arabidopsis 9-cis epoxycarotenoid dioxygenase gene family.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXmt12hu7Y%3D&md5=2625c17dbddb5f2d3c97c9616517e73bCAS |
Taylor M, Ramsay G (2005) Carotenoid biosynthesis in plant storage organs: recent advances and prospects for improving plant food quality. Physiologia Plantarum 124, 143–151.
| Carotenoid biosynthesis in plant storage organs: recent advances and prospects for improving plant food quality.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXlt1ynsbk%3D&md5=1f090eaa71855e2c5213c8a98bbca6fcCAS |
Thorstensen T, Grini PE, Aalen RB (2011) SET domain proteins in plant development. Biochimica et Biophysica Acta 1809, 407–420.
Tian L, Musetti V, Kim J, Magallanes-Lundback M, DellaPenna D (2004) The Arabidopsis LUT1 locus encodes a member of the cytochrome P450 family that is required for carotenoid epsilon-ring hydroxylation activity. Proceedings of the National Academy of Sciences of the United States of America 101, 402–407.
| The Arabidopsis LUT1 locus encodes a member of the cytochrome P450 family that is required for carotenoid epsilon-ring hydroxylation activity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXjvFaktg%3D%3D&md5=7dd074487dc06044fbf17af98010465dCAS |
Toledo-Ortiz G, Huq E, Rodriguez-Concepcion M (2010) Direct regulation of phytoene synthase gene expression and carotenoid biosynthesis by phytochrome-interacting factors. Proceedings of the National Academy of Sciences of the United States of America 107, 11626–11631.
| Direct regulation of phytoene synthase gene expression and carotenoid biosynthesis by phytochrome-interacting factors.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXot1eksbY%3D&md5=d1cf0727bc476fc3a60196a304fc7285CAS |
Tonhosolo R, D’Alexandri FL, de Rosso VV, Gazarini ML, Matsumura MY, Peres VJ, Merino EF, Carlton JM, Wunderlich G, Mercadante AZ, Kimura EA, Katzin AM (2009) Carotenoid biosynthesis in intraerythrocytic stages of Plasmodium falciparum. The Journal of Biological Chemistry 284, 9974–9985.
| Carotenoid biosynthesis in intraerythrocytic stages of Plasmodium falciparum.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXjvFKrsb4%3D&md5=184c8c22b3bfcd4ef51a6f441020ebc6CAS |
Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, Takeda-Kamiya N, Magome H, Kamiya Y, Shirasu K, Yoneyama K, Kyozuka J, Yamaguchi S (2008) Inhibition of shoot branching by new terpenoid plant hormones. Nature 455, 195–200.
| Inhibition of shoot branching by new terpenoid plant hormones.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtV2qtLnE&md5=40c7e7a8e1e7be1ceca0c701e801a631CAS |
Van Norman JM, Sieburth LE (2007) Dissecting the biosynthetic pathway for the bypass1 root-derived signal. The Plant Journal 49, 619–628.
| Dissecting the biosynthetic pathway for the bypass1 root-derived signal.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXjtlGmsbk%3D&md5=94d7a27d9ca24db02fdd59f14c9660a3CAS |
Van Norman JM, Frederick RL, Sieburth LE (2004) BYPASS1 negatively regulates a root-derived signal that controls plant architecture. Current Biology 14, 1739–1746.
| BYPASS1 negatively regulates a root-derived signal that controls plant architecture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXotFCkur0%3D&md5=d4744537c5e50a85e4bcf884985f7849CAS |
Verma SK, Bordia A (1998) Antioxidant property of saffron in man. Indian Journal of Medical Sciences 52, 205–207.
Vishnevetsky M, Ovadis M, Vainstein A (1999) Carotenoid sequestration in plants: the role of carotenoid-associated proteins. Trends in Plant Science 4, 232–235.
| Carotenoid sequestration in plants: the role of carotenoid-associated proteins.Crossref | GoogleScholarGoogle Scholar |
Vogel JT, Walter MH, Giavalisco P, Lytovchenko A, Kohlen W, Charnikhova T, Simkin AJ, Goulet C, Strack D, Bouwmeester HJ, Fernie AR, Klee HJ (2010) SlCCD7 controls strigolactone biosynthesis, shoot branching and mycorrhiza-induced apocarotenoid formation in tomato. The Plant Journal 61, 300–311.
| SlCCD7 controls strigolactone biosynthesis, shoot branching and mycorrhiza-induced apocarotenoid formation in tomato.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsFarsbc%3D&md5=e07ce64e462a912edeac962647ffe142CAS |
Walter MH, Floss DS, Strack D (2010) Apocarotenoids: hormones, mycorrhizal metabolites and aroma volatiles. Planta 232, 1–17.
| Apocarotenoids: hormones, mycorrhizal metabolites and aroma volatiles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXmtFKltb8%3D&md5=e39a0733b096c0ce973efdd7d64c461cCAS |
Wei J, Xu M, Zhang D, Mi H (2010) The role of carotenoid isomerase in maintenance of photosynthetic oxygen evolution in rice plant. Acta Biochimica et Biophysica Sinica 42, 457–463.
| The role of carotenoid isomerase in maintenance of photosynthetic oxygen evolution in rice plant.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXotlCjur0%3D&md5=4cb3b49ab044ccdd345ec61548df6d43CAS |
Welsch R, Beyer P, Hugueney P, Kleinig H, von Lintig J (2000) Regulation and activation of phytoene synthase, a key enzyme in carotenoid biosynthesis, during photomorphogenesis. Planta 211, 846–854.
| Regulation and activation of phytoene synthase, a key enzyme in carotenoid biosynthesis, during photomorphogenesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXotVKnt7o%3D&md5=e9d242df2c667e3cac335259987f0851CAS |
Welsch R, Maass D, Voegel T, Dellapenna D, Beyer P (2007) Transcription factor RAP2.2 and its interacting partner SINAT2: stable elements in the carotenogenesis of Arabidopsis leaves. Plant Physiology 145, 1073–1085.
| Transcription factor RAP2.2 and its interacting partner SINAT2: stable elements in the carotenogenesis of Arabidopsis leaves.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtlemsrfL&md5=f42387099475acb859f389d44c1dfe94CAS |
Welsch R, Wust F, Bar C, Al-Babili S, Beyer P (2008) A third phytoene synthase is devoted to abiotic stress-induced abscisic acid formation in rice and defines functional diversification of phytoene synthase genes. Plant Physiology 147, 367–380.
| A third phytoene synthase is devoted to abiotic stress-induced abscisic acid formation in rice and defines functional diversification of phytoene synthase genes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXms1eksbY%3D&md5=7cfa3f013690269689235bd5cd872f24CAS |
Yu F, Fu A, Aluru M, Park S, Xu Y, Liu H, Liu X, Foudree A, Nambogga M, Rodermel S (2007) Variegation mutants and mechanisms of chloroplast biogenesis. Plant, Cell & Environment 30, 350–365.
| Variegation mutants and mechanisms of chloroplast biogenesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXjtlemu7k%3D&md5=369a6a0c76bd3b7e8b3e570fbf403b85CAS |
Yu B, Lydiate DJ, Young LW, Schafer UA, Hannoufa A (2008) Enhancing the carotenoid content of Brassica napus seeds by downregulating lycopene epsilon cyclase. Transgenic Research 17, 573–585.
| Enhancing the carotenoid content of Brassica napus seeds by downregulating lycopene epsilon cyclase.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXnvFyiur0%3D&md5=9651bc63c36d7f9b244948e3fa37ea58CAS |
Yu Q, Ghisla S, Hirschberg J, Mann V, Beyer P (2011) Plant carotene cis-trans isomerase CRTISO: a new member of the FAD(RED)-dependent flavoproteins catalysing non-redox reactions. Journal of Biological Chemistry 286, 8666–8676.
| Plant carotene cis-trans isomerase CRTISO: a new member of the FAD(RED)-dependent flavoproteins catalysing non-redox reactions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXislyksrw%3D&md5=69c552b7f00e0956295269cebb43a697CAS |
Zhu C, Bai C, Sanahuja G, Yuan D, Farre G, Naqvi S, Shi L, Capell T, Christou P (2010) The regulation of carotenoid pigmentation in flowers. Archives of Biochemistry and Biophysics 504, 132–141.


