The evolution and functional significance of leaf shape in the angiosperms
Adrienne B. Nicotra A H , Andrea Leigh B , C. Kevin Boyce C , Cynthia S. Jones D , Karl J. Niklas E , Dana L. Royer F and Hirokazu Tsukaya GA Research School of Biology, The Australian National University, Canberra, ACT 0200, Australia.
B School of the Environment, University of Technology, Sydney, PO Box 123, Broadway, NSW 2007, Australia.
C Department of the Geophysical Sciences, 5734 S. Ellis Avenue, Chicago, IL 60637, USA.
D Department of Ecology and Evolutionary Biology, University of Connecticut, 75 N. Eagleville Road, Unit-3043, Storrs, CT 06269, USA.
E Department of Plant Biology, Cornell University, 412 Mann Library Building, Cornell University, Ithaca, NY 14853, USA.
F Department of Earth and Environmental Sciences, Wesleyan University, 265 Church Street, Middletown, CT 06459, USA.
G Graduate School of Science, University of Tokyo, Science Build #2, 7-3-1 Hongo, Tokyo 113-0033, Japan.
H Corresponding author. Email: adrienne.nicotra@anu.edu.au
This paper is part of an ongoing series: ‘The Evolution of Plant Functions’.
Functional Plant Biology 38(7) 535-552 https://doi.org/10.1071/FP11057
Submitted: 28 February 2011 Accepted: 30 May 2011 Published: 12 July 2011
Abstract
Angiosperm leaves manifest a remarkable diversity of shapes that range from developmental sequences within a shoot and within crown response to microenvironment to variation among species within and between communities and among orders or families. It is generally assumed that because photosynthetic leaves are critical to plant growth and survival, variation in their shape reflects natural selection operating on function. Several non-mutually exclusive theories have been proposed to explain leaf shape diversity. These include: thermoregulation of leaves especially in arid and hot environments, hydraulic constraints, patterns of leaf expansion in deciduous species, biomechanical constraints, adaptations to avoid herbivory, adaptations to optimise light interception and even that leaf shape variation is a response to selection on flower form. However, the relative importance, or likelihood, of each of these factors is unclear. Here we review the evolutionary context of leaf shape diversification, discuss the proximal mechanisms that generate the diversity in extant systems, and consider the evidence for each the above hypotheses in the context of the functional significance of leaf shape. The synthesis of these broad ranging areas helps to identify points of conceptual convergence for ongoing discussion and integrated directions for future research.
Additional keywords: compound leaf, leaf dissection, leaf margin, leaf size, leaves, lobbing.
Introduction
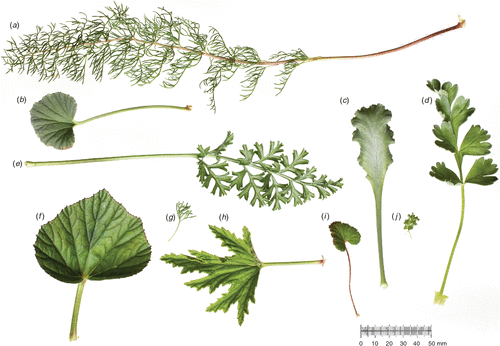
A casual look at any local flora reveals great diversity in angiosperm leaf shape. ‘Shape’ describes a dimensionless descriptor, for example, length/width, which quantifies form in terms of the natural dimensions or size of any object. The functional significance of shape variation among leaves has been the subject of debate for many years, and there are a range of different approaches to describing leaf shape (e.g. Nicotra 2010). The diversity of shape suggests that there is no one ecological strategy that is dependent exclusively on leaf shape. Even within a single genus, leaf shape variation can be tremendous (Fig. 1). Here we provide an overview of the evolutionary history of leaf shape and then examine genetic, developmental, physiological and ecological determinants of leaf shape to better understand the evolutionary drivers of diversification in leaf shape and the functional significance therein.
|
Research over the past decade has produced sound ecological explanations for the significance of some key non-shape leaf traits. The leaf economic spectrum describes a continuum ranging from leaves with low to high mass per unit area (LMA). Leaves with high LMA represent a high investment in structure and are typically long-lived with lower photosynthetic rates (Wright et al. 2004). Leaf size varies in a similar way: small leaves are associated with harsh conditions such as cold (Gates 1980), hot (Smith and Nobel 1977), dry (Thoday 1931; Raunkiaer 1934; Gates et al. 1968; Parkhurst and Loucks 1972; Specht and Specht 1999; Fonseca et al. 2000; McDonald et al. 2003), high light (Smith and Nobel 1977; Bragg and Westoby 2002), exposed (Ackerly et al. 2002), nutrient poor (Beadle 1966; Cunningham et al. 1999; Fonseca et al. 2000) and saline environments (Ball et al. 1988), or many of these factors in combination (e.g. McDonald et al. 2003). Leaf shape, in contrast, has been shown to vary less predictably across environments or biomes than size or LMA (McDonald et al. 2003). Thus, the functional significance of leaf shape and the evolutionary drivers of its diversity remain the subject of discussion.
The theories about leaf shape are many, and not mutually exclusive: thermoregulation of leaves especially in arid and hot environments, hydraulic constraints, patterns of leaf expansion in deciduous species, mechanical constraints, adaptations to avoid herbivory, adaptations to optimise light interception and, given that leaves are hypothesised to be developmental homologues of floral organs, and it has even been suggested that leaf shape reflects the effects of selection on flower form. Finally, there is the chance that leaf shape variation has little functional or adaptive significance and instead reflects random variation within the context of phylogenetic history. However, given the importance of the leaf we believe the latter option rather unlikely. To distinguish among the former, we here synthesise the varied fields involved so that we may assess the relative importance of each and identify the ecological situations in which one or the other of these mechanisms is likely to be significant.
In this review we first examine leaf shape from an evolutionary perspective and in the process demonstrate that shape is highly labile and widely explored in evolutionary history. We bring the focus onto the angiosperms in particular, and then consider the proximate determinants of angiosperm leaf shape. The current understanding of the genetic signals and developmental processes underlying shape differences are then reviewed to explore the genetic controls and constraints on leaf shape evolution. The hypotheses about the function of leaf shape are next reviewed with an evolutionary and genetic perspective in mind. We highlight those functional issues that we see as particularly relevant to leaf shape evolution, and then turn to three examples of leaf shape variation: across communities, within lineages and within individuals to explore how an evolutionary perspective alters understanding of the relationship between leaf form and function. We conclude with suggestions on how this perspective can be used to direct future research on the function and evolution of leaf shape.
A brief history of the angiosperm leaf and shape diversity therein
To understand the evolution of angiosperm leaf shape, some perspective on the evolution of the leaf itself is needed, as leaves of many shapes have evolved numerous times independently in evolutionary history. For the purpose of this review of angiosperm leaf shape we define a leaf as a vascular asymmetric appendicular structure initiated at the shoot apical meristem. This definition excludes the functionally analogous structures of mosses and leafy liverworts. Morphologically simple leaves, microphylls, arose in the lycopsids. Larger leaves with more complex shapes and venation networks, called megaphylls, arose in the seed plants, in the extinct progymnosperm and sphenophyll lineages, and in one or more lineages of ‘ferns’ (Galtier 1981; Kenrick and Crane 1997; Niklas 1997; Boyce and Knoll 2002; Tomescu 2009; Boyce 2010). Thus, the seed plants represent just one of several independent evolutionary origins of large leaves with diverse shapes among the vascular plants.
Despite the many independent origins of leaves, several traits now associated with leaves can be considered homologous across all vascular plants through a shared monoplyletic origin from a leafless common ancestor. First, all vascular plant leaves are distinct from the leaf-like organs of bryophytes that rely on external surfaces for photosynthetic gas exchange (as would also be true of the external appendages independently derived in many algal lineages; Niklas 2000). Vascular plant leaves use internal airspaces regulated by stomata for gas exchange (Raven 1996; Boyce 2008a). Second, evidence from both living plants and the venation patterns of fossil leaves indicates that the ancestral form of tissue production in the growing leaves of all vascular plants was limited to discrete marginal zones of growth (Pray 1960; Zurakowski and Gifford 1988; Boyce and Knoll 2002; Boyce 2007). Other aspects of leaf organography, such as the evolution of differentiated abaxial/adaxial domains (see section on ‘Proximal mechanisms underlying leaf shape diversity’ below) and determinate leaf growth appear to arise independently in seed plants and other lineages, however, several of these lineages have co-opted the same underlying genetic pathways to regulate these developmental processes (see section on ‘Proximal mechanisms underlying leaf shape diversity’, Bharathan et al. 2002; Harrison et al. 2005; Sanders et al. 2007; Tomescu 2009; Boyce 2010).
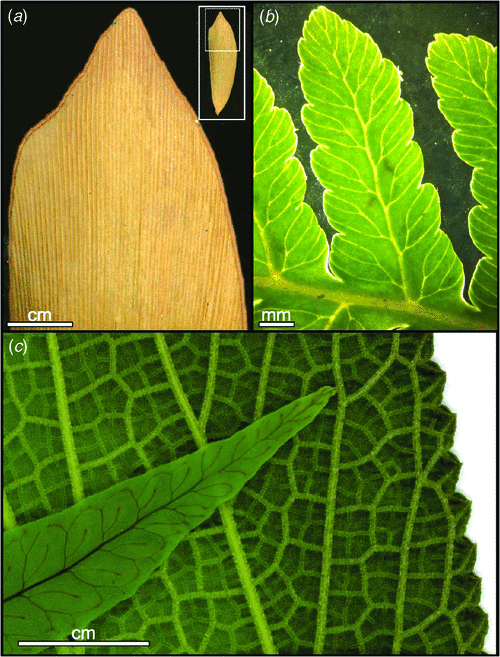
Within this broader evolutionary context, angiosperm leaves represent a range of particularly aberrant variants of vascular plant leaves. Some of the distinctive characteristics of angiosperm leaves have arisen independently in other lineages, whereas other traits are unique to the flowering plants. Angiosperms have evolved leaf growth that occurs diffusely throughout the leaf without being limited to the margin (Pray 1955; Poethig and Sussex 1985a, 1985b), thus, enabling the enormous diversity and complexity of angiosperm leaf venation patterns and shapes. The phylogenetic distribution of complex venation patterns suggest that the evolutionary departures from marginal growth observed in the angiosperms have also arisen repeatedly in two or three other seed plant lineages and 10 or more groups of ferns (Boyce 2005). In these, likewise, diffuse growth and complex venation patterns can be seen to occur with diverse leaf shapes. Despite these similarities to the other seed plants, angiosperm leaf evolution remains distinctive in that it ran counter to the general temporal trend seen in the vasculature of other seed plants. The vascular network of other seed plant lineages evolved towards simplification. After initially exhibiting the complete range of morphologies consistent with marginal growth, including many involving two vein orders with a midvein and open or reticulate networks of secondary veins (morphologies now common only among the ferns), the leaf architecture in the other major vascular plant lineages became progressively limited to a narrow subset of forms. In cycads, conifers, Ginkgo and several other extinct seed plant lineages a single order of veins lead straight, parallel courses to a distal margin. Angiosperms, however, exhibit many hierarchical orders of reticulate, internally directed veins (Boyce 2005). Further, angiosperm leaves are unique in their possession of extraordinarily high vein densities (Fig. 2): whereas all other plants, living or extinct, average ~2 mm of vein length per square mm of leaf surface, angiosperms average between 8 and 10 mm mm–2 and can range to above 20 mm mm–2 (Boyce 2008a, 2008b, 2009; Brodribb and Feild 2010; Brodribb et al. 2010).
|
Together these changes that accompanied angiosperm evolution represent a substantial revision of the functional possibilities of a leaf. First, the release of venation patterns from strictly marginal growth allows the production of novel leaf shapes and sizes that would not otherwise be possible. Second, differences in angiosperm venation allowed a complete revolution in how water is distributed within a leaf (Boyce 2008a). In any leaf, the tissue furthest from the leaf base has access only to that water which has not been lost to transpiration in more proximal tissues (Zwieniecki et al. 2004a, 2006). Reticulate venation and the minute size of the final order of veins in flowering plants leads to equitable distribution of water between successive vein orders (Zwieniecki et al. 2002). Finally, the high vein densities of angiosperms shorten the path length along xylem and mesophyll, thus, enabling assimilation and transpiration capacities higher than in any other group of plants (Bond 1989; Sack and Frole 2006; Brodribb et al. 2007; Boyce et al. 2009). The uniquely high transpiration capacities of angiosperms may also have influenced the evolution of leaf shape by relaxing some of the thermal constraints on leaf size and shape (see ‘Temperature and water’ section).
It remains controversial when, and in which lineages, these characteristics of angiosperm leaves arose. The distinctiveness of angiosperms leaves has led to polarised interpretations of their ancestry. Some researcher propose that similarities in leaf venation tie the early angiosperms to particular fossil seed plants (e.g. Melville 1969), but the group of plants with the most similar venation are the clearly unrelated dipterid ferns. The various seed plant lineages most often favoured as angiosperm relatives based upon reproductive characteristics (Doyle and Hickey 1976; Hilton and Bateman 2006; Frohlich and Chase 2007) tend to have very dissimilar leaves. Others suggest that angiosperm leaves are so distinct as to require a complete reinvention of a leaf after passing through a leafless intermediate, such as one that was aquatic- or desert-adapted (Doyle and Hickey 1976). But the appearance of angiosperm-like leaf traits in a variety of fern lineages suggest that invoking a leafless intermediate is unnecessary. Although the history of early angiosperm leaf evolution is still debated, we are certain that there have been numerous independent origins of diverse leaf shapes and that leaf shape diversification is associated with both high vein densities and reticulate venation patterns.
Proximal mechanisms underlying leaf shape diversity
What are the genetic mechanisms underlying this leaf shape diversification and how similar are they given the many independent origins of diverse leaf shapes? What we know of the processes of leaf development (and leaf shape determination) is largely a product of studies on model species systems. In particular, Arabidopsis thaliana L. (Heynh) (hereafter referred to as Arabidopsis) has been widely used for the identification of genes determining leaf form. In the following sections we examine the development of the angiosperm leaf: specifically, the establishment of dorsiventral domains and flattening; expansion of the lamina, and formation of leaf margin; and discuss how these factors interact to determine leaf shape.
Establishment of dorsiventral domains and lamina flattening
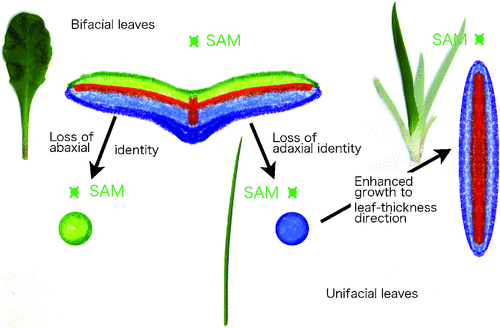
Since leaf primordia arise as protrusions from shoot apical meristems (SAM), suppression of the regular SAM developmental program is the first step required for normal development of a leaf primordium. Recently, Sarojam et al. (2010) found that genes in the YABBY family suppress SAM identity and promote lamina growth in Arabidopsis. In most bifacial leaves, such as leaves of Arabidopsis and snapdragon (Antirrhinum majus L.), the radial primordium differentiates into abaxial (lower) and adaxial (upper) surfaces to establish dorsiventrality (Waites and Hudson 1995). Our current understanding is that the molecular genetic regulation of identification of the abaxial and adaxial domains depends on actions of both small RNAs and direct/indirect targets such as class III Zip genes, KANADI genes and AUXIN RESPONSE FACTORS3 (ARF3)/ETT in Arabidopsis (Husbands et al. 2009; Floyd and Bowman 2010; Kidner 2010). Without establishment of the adaxial and abaxial domains, it is believed that leaves cannot expand in any angiosperm species; rather they become stalk like, since the so-called plate meristem is never activated to expand the laminas (Fig. 3).
|
The monocot clade is typified by many unifacial leaves that have only abaxial identity in the lamina, as seen in leek (Allium ampeloprasum var. porrum L.) and Iris spp. Leek leaves are cylindrical, since they lack adaxial identity in their leaf blade. Iris, in contrast, has flat leaves that expand in a distinct, perpendicular direction relative to that of normal bifacial leaves. Likewise, the rush Juncus prismatocarpus R.Br. has unifacial flat leaves; the primordium of the leaf blade grows into a flat lamina as a result of extensive thickening growth. This developmental pathway is dependent on the gene, DROOPING LEAF (DL, Yamaguchi et al. 2010; Fig. 3), that is also a member of the YABBY gene family, but with a monocot-specific function. As seen in this case, diversified morphology in a particular taxon sometimes depends on some taxon-specific molecular mechanisms that are modifications of gene function from ancestral lineages.
Mechanisms for expansion of lamina
Once dorsiventral identity is generated the leaf lamina can then diversify in morphology to form simple or compound leaves with entire or serrated margins, differing in thickness, length and width, and in orientation (Tsukaya 2006). The leaf length : width ratio is regulated by polar-dependent cell expansion and cell proliferation/distribution. Several key genes for its regulation have been identified from Arabidopsis: ANGUSTIFOLIA (AN) and ROTUNDIFOLIA3 (ROT3) regulate the shape of cells whereas ANGUSTIFOLIA3 (AN3) and ROTUNDIFOLIA4 (ROT4) affect the number of cells in the lamina (Tsukaya 2006). Loss-of-function mutations of AN and AN3 result in narrower leaves, whereas loss-of-function of ROT3 or overexpression of ROT4 make leaves shorter. In addition, LONGIFOLIA1 (LON1) and LON2 regulate the length of the leaf lamina in Arabidopsis (Lee et al. 2006). A vast array of genes is known to influence leaf area (Horiguchi et al. 2006). For example, heteroblastic change in leaf area is governed by the miR156-SQUAMOSA PROMOTER BINDING PROTEIN-LIKE (SPL)-miR172 pathway via regulation of cell size and cell numbers in Arabidopsis (Usami et al. 2009). Alterations of activities of any of these factors could contribute to natural variation in leaf length : width ratio and area. None of these genetic variations result in loss of reproductive structures, suggesting that these genes could be under direct environmentally mediated selection. As yet, we do not know whether the above patterns hold in other non-model species.
Leaves also vary in the extent to which the lamina is flat or curved. CINCINATA (CIN) of snapdragon was once thought to function to keep the leaf flat by orchestrating cell proliferation along the medio-lateral axis of the leaf primordia (Nath et al. 2003). More recently, however, analyses of the TCP genes, which are homologues of CIN in Arabidopsis, indicate that the major role of the TCP is in promotion of maturation in laminar tissues, not in regulation of cell proliferation (Efroni et al. 2008; Sarojam et al. 2010). The role of CIN in snapdragon may also be the same as that of TCPs in Arabidopsis. The other candidate genetic mechanism for control of leaf curvature is an auxin-related pathway. Several hyponastic leaf mutants have been isolated from Arabidopsis and many of the responsible genes are related to auxin signalling (Perez-Perez et al. 2002, 2009). Since auxin signalling is also very important for venation patterning, species-specific modification of auxin signalling in leaf primordia may have caused diversification of both leaf curvature and vasculature patterning.
Genetic regulation for patterning of leaf margin: serration and leaflet formation
Variation in leaf shape, as distinct from length and area or curvature, arises from variation in leaf margin and lobe formation. Kawamura et al. (2010) showed that spatial patterning of serrations, or leaf teeth (see ‘Leaf shape variation within lineages’ section), on the leaf margin depends on formation of auxin maxima on the leaf margin in Arabidopsis. Location of these maxima is governed by polar auxin transport and the stabilisation of auxin maxima is maintained by prolonged expression of CUP-SHAPED COTYLEDON2 (CUC2) around it (Kawamura et al. 2010). The mechanism of rhythmic pattern formation of the auxin maxima/minima on the leaf margin is thought to involve self-organising pattern formation mechanisms by polar auxin transport, similar to that involved in pattern formation of phyllotaxy (de Reuille et al. 2006; Jonsson et al. 2006). Either the loss of distinct auxin maxima or an instability of the auxin maxima will result in an entire leaf margin (Kawamura et al. 2010). Changes in functions of these genes, however, severely influence many biological processes including floral organ development as seen in the pin-formed 1 mutant (Okada et al. 1991). Thus, variation among species in pattern of margin serration might accompany variation in floral organs, or more likely, reflect differential regulation for example by downstream genetic pathways.
Combined control of auxin-maxima formation by PIN and CUC genes, as described for serration above, is also known to have an important role in leaflet patterning in bittercress (Cardamine flexuosa With.), an Arabidopsis relative with compound leaves (Barkoulas et al. 2008). Again this control varies among species: in tomato (Solanum lycopersicum L.), loss of CUC3 function does not convert compound leaves into simple leaves (Blein et al. 2008). Thus, it is clear that the independent evolution of compound leaves from simple leaves is likely driven by species-specific mechanisms (Efroni et al. 2010), though these may reflect modifications of similar pathways.
Recent studies have identified several important key genes for regulation of compound versus simple leaf forms. For example, absence or prolonged expression of class I Knotted1-like homeobox (KNOX) genes in leaf primordia is important to compound leaf formation in a wide range of angiosperms and gymnosperms (Bharathan et al. 2002). In some legume species, however, the LEAFY (LFY)/UNIFOLIATA (UNI) gene is the key for compound leaf formation, instead of the class I KNOX genes (Hofer et al. 1997). Changes in these genes could be linked to the evolution of compound leaves, although loss-of-function of LFY/UNI also causes infertility in Arabidopsis and, thus, it is unlikely that selection directly on these particular genes could result in the evolution of variation in leaf shape. Diversified spatial patterning of petiole/petiolule and lamina/leaflet in compound leaves have also been suggested to be linked to the distribution pattern of AS1-ROUGH SHEATH2-PHANTASTICA (ARP) expression domains (Kim et al. 2003). However, based on the fact that the ARP gene has only a limited role in compound leaf formation in some species, it is unclear whether ARP will turn out to be major factor in others (Efroni et al. 2010).
As described above, the independent evolutionary origins of several angiosperm leaf features should caution against the assumption that a single ancestral genetic system underlies leaf development. The developmental genetic approaches outlined have revealed several key mechanisms for leaf shape control, but have also demonstrated variation in the function of these genes among lineages. In a few studies researchers have gone beyond one or two species studies, and have looked for deeper evolutionary links (Illing et al. 2009). Studies of the role of the class 1 KNOX genes have considered not just angiosperms but other lineages. Among simple-leafed dicots and monocots (e.g. Arabidopsis and Zea), KNOX genes are strongly expressed during the indeterminate growth of the apical meristem, but are rapidly downregulated in the cells flanking the apical dome in regions defined by leaf primordia. Although some arginine-rich protein (ARP) genes repress the expression of KNOX transcription factors during the development of both micro- and megaphyll primordia (Harrison et al. 2005), the role of homeodomain-leucine zipper (HD-ZIP) III genes, which are involved in the specification of foliar adaxial/abaxial fate, differs in the development of these two leaf types (Floyd and Bowman 2006). HD-ZIP III genes have been identified in the moss Physcomitrella (Sakakibara et al. 2001) and in the fern Ceratopteris (Aso et al. 1999); they are also expressed during normal vascular tissue development in Arabidopsis (Zhong and Ye 1999), suggesting that they are very ancient but also multifunctional.
Accumulating knowledge on molecular genetic mechanisms of leaf morphogenesis is leading to an increasingly evo-devo approach to studies of leaf diversity (Tsukaya 2010); this progress is aided by establishing new model species (e.g. Cardamine hirsuta and the rush; Canales et al. 2010; Yamaguchi and Tsukaya 2010). Over the next decade we anticipate that genomic approaches on multiple species analysed in phylogenetic frameworks (e.g. Illing et al. 2009) will resolve much of the enigma regarding the evolution of diverse leaf forms.
Genetic constraints on flower petal and leaf form
One additional developmental consideration is that, as famously described by von Goethe in 1790, flower petals are derivatives of the apical meristem and serial homologues to leaves (von Goethe 2009). Thus, one might hypothesise that although the evolution of leaves substantially predates that of flowers, selection on the form of either of these organs would also result in changes in the other, such that selection on leaf shape and size subsequent to the evolution of floral organs may be constrained by pleiotropic control of floral form. Recent genetic data identifies several genes that have roles in both leaf and flower form (Dinneny et al. 2004; Schmid et al. 2005; Street et al. 2008). For example, genetic changes in both leaf length to width ratio (e.g. rot3) and leaf size (e.g. an3) influence not only leaf proportions, but also that of the floral organs in Arabidopsis (Kim et al. 1999; Horiguchi et al. 2005). The garden pea (Pisum sativum L.) is a good case in point: as discussed above, compound leaf formation depends on LFY/UNI but the same gene is also required for flowering. Thus, if current selection favoured simple leaves in garden pea changes in leaf shape could be constrained by pleiotropic control of flowering (Hofer et al. 1997). Likewise, Shalit et al. (2009) recently reported that the action of florigen – a plant hormone for flowering – influences complexity of leaves by changing numbers of leaflets in tomato. Such relationships suggest that some floral characters as well as other metabolic pathways may now be closely linked with regulation of leaf shape and size. A few ecological studies have also assessed correlations between petal and leaf form (Berg 1960; Armbruster et al. 1999). This question is of particular interest in lineages where animal pollination is associated with increased diversification rates either as a result of direct selection, or as a secondary reinforcing factor (van der Niet et al. 2006; Armbruster and Muchhala 2009; Kay and Sargent 2009).
In situations where leaf and petal form are under similar genetic control and both under selection, one of two things could happen. If leaf shape does not have substantial functional impact then one might expect leaf form to change with selection on petal form. However, given the likely importance of leaf form to photosynthetic function, it is more likely that a breakdown in genetic correlations between leaf and flower form (e.g. by alterations of downstream regulatory pathways) would evolve. Several factors argue against the hypothesis that leaf shape is frequently a product of selection on flower form. First, diverse leaf shapes were in evidence long before the origin of flowers in the angiosperms. Second, the genetic mechanisms underlying leaf shape reveal considerable evolvability with similar genes being co-opted for different functions in different species. Finally, given the potential importance of both petal and leaf shape we suggest that genetic constraints on the independent evolution of both structures are likely to have broken down in those lineages with highly diversified leaf shapes over the many millions of years since petals first evolved.
Ecological correlates and the functional significance of leaf shape
Evolutionary history plays a part in determining leaf morphology, but our consideration of leaf shape evolution and its developmental genetic basis demonstrates that diverse lineages have repeatedly gained and lost many leaf features. Thus, developmental and genetic constraints on the evolution of leaf form, and shape in particular, have been overcome many times, or have led to new evolutionary opportunities. It is of particular interest then to ask: what are the likely ecological drivers and/or functional significance of leaf shape?
Temperature and water
Conventional wisdom would say that thermoregulation is perhaps the main driver of leaf shape evolution. The maintenance of leaf temperatures within certain limits is critical for a plant’s growth and survival and although photosynthetic tissue of some species can withstand temperatures below −6°C (Ball et al. 2006) and above 60°C (Clum 1926; Nobel 1988), irreversible damage to leaves can occur at temperatures well within this range (Levitt 1980; Jones 1992; Ball et al. 2004; Groom et al. 2004; Sharkey 2005). Leaf size and shape potentially have large effects on leaf temperature because the two-dimensional proportions of a flattened leaf determine the rate of heat transfer between across the leaf–air interface by influencing the thickness of its boundary layer.
All else being equal, the thickness of a boundary layer increases with length from the windward edge so that heat convection from small leaves is more rapid than from large leaves (Raschke 1960; Gates 1968; Vogel 1970; Parkhurst and Loucks 1972; Grace et al. 1980; Geller and Smith 1982; Monteith and Unsworth 1990; Schuepp 1993). Likewise, because leaf lobing reduces the distance across the lamina, the rate of heat transfer is predicted to be greater in a lobed leaf than an unlobed leaf with equivalent area (Parkhurst et al. 1968; Vogel 1968; Lewis 1972; Givnish 1978; Gurevitch and Schuepp 1990a).
The rate of heat transfer from lobed metal plates or lobed leaves coated in metal is greater than from shallow-lobed or un-lobed ones (Parkhurst et al. 1968; Thom 1968; Vogel 1970; Gottschlich and Smith 1982; Gurevitch and Schuepp 1990b; Roth-Nebelsick 2001). Artificial or coated leaves have been used because they enable boundary layer resistance to be investigated in the absence of transpiration. However, this approach is biologically flawed because it does not enable an assessment of the relative importance of a range of traits (e.g. leaf water content, absorbance) in determining the actual operating temperatures of leaves in the field.
Actual leaf temperature measurements show that large leaves can operate at below-ambient temperatures even on hot days, as a result of high rates of latent heat loss through transpiration (Drake et al. 1970; Gates 1980; Hegazy and El Amry 1998). Thus, conditions of evaporative demand and the availability of soil water for transpiration are key to understanding leaf shape–temperature relationships under biologically realistic conditions. Because of their much greater transpirational capacities (see above), flowering plants have far greater leeway than other plants regarding the size and shape of their leaves (see ‘A brief history of the angiosperm leaf and shape diversity therein’ section; Boyce et al. 2009).
In addition to reducing heat loads, the morphology of deeply lobed leaves may also reflect direct selection for increased hydraulic efficiency. Water moving through a plant encounters numerous resistances on its path from soil to roots to stems through leaves and out to the external atmosphere. Within the liquid phase of water flow through a plant, leaf hydraulic resistance, Rleaf, accounts for more than 30% of total plant resistance (Sack and Holbrook 2006). The high resistance in leaves is due first to a system of veins of decreasing size, the resistance of which is inversely proportional to the fourth power of the radius of the component conduits in these veins. Therefore, as a proportion of the considerable resistance in the leaf (Zwieniecki et al. 2002), the minor veins provide the greatest resistance to water flow (Sack et al. 2004). Water also encounters high resistance as it exits veins laterally and travels to other cells, mainly via apoplastic pathways through the mesophyll. Accordingly, on the order of half of total Rleaf can occur outside the major veins (Sack and Holbrook 2006). As resistance increases along the pathway towards sites of evaporation, localised water potential (Ψ) becomes progressively more negative (Brodribb et al. 2010). The result of how this phenomenon might affect a leaf has long been observed: regions of leaves lying furthest from the main supply channels are the first to ‘wither’ when exposed to strong winds (Fig. 4; Yapp 1912). Leaf lobing represents an effective removal of this potentially stress-prone tissue and has been suggested as an adaptation to dry conditions (Thoday 1931; Givnish 1979). If deeply lobed leaves have a lower ratio of mesophyll tissue to large, highly conductive veins, they should then have reduced resistance relative to less- or un-lobed leaves (Sack and Tyree 2005).
|
Although leaf lobing might confer adaptive benefits with respect to maintaining stable hydraulic supply, it may also be that the extent of lobing or margin dissection is itself determined by hydraulic limitation (Sack et al. 2003; Zwieniecki et al. 2004b; Boyce 2009). For species with ‘sun’ and ‘shade’ leaves, the morphology of small, deeply lobed sun leaves at the outer canopy is proposed to result from water pressure drop across the leaf lamina caused by reduced water delivery to expanding cells during growth (Fig. 5; Zwieniecki et al. 2004b; Boyce 2009; Leigh et al. 2011). Alternatively, recent studies have suggested that many of the characteristics of ‘sun’ and ‘shade’ leaves are the result of branch autonomy within the plant canopy (Niklas and Cobb 2010).
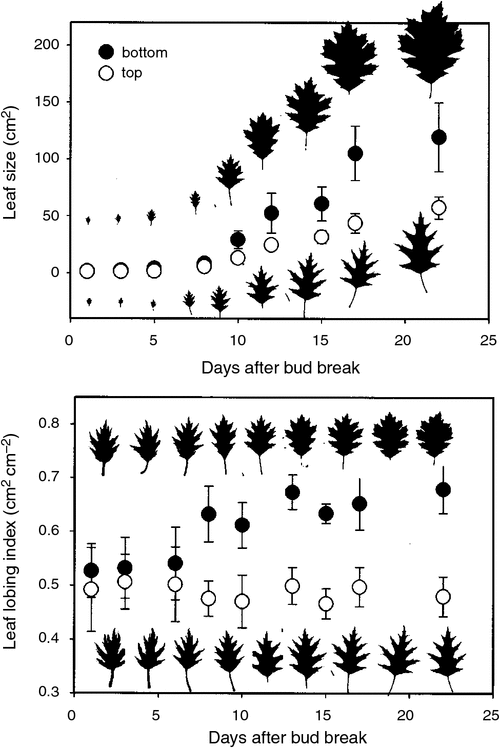
|
Thus, although the effects of leaf shape on leaf thermal regulation hold when tested on model leaves, predictions are less straightforward when hydraulic function is accounted for. Additionally, consideration of the relative contribution of a range of leaf and branch properties to leaf temperature is critical. It is likely that leaf shape is only one among many factors influencing leaf thermal regulation; other factors could include water content, leaf thickness, spectral reflectance, orientation and plant architecture. Further, given the observation of high leaf shape diversity within many plant communities from hot and arid environments, it seems likely that regulating leaf temperature is not the single most important evolutionary influence on leaf shape diversity. The association between leaf shape and hydraulic properties, which is affected by leaf temperature, in contrast, is likely to be of greater importance.
Leaf structure and functional trade-offs
Many foliar leaf traits, including shape, reflect functional trade-offs that have been resolved in various ways by different species depending on their ecological settings (e.g. hydro- vs mesophytes), physiological attributes (e.g. C3 vs C4 metabolism), developmental capabilities (e.g. heterobaric vs non-heterobaric leaves), or evolutionary histories (e.g. micro- vs megaphylls). Life history and optimisation theory show that the number of phenotypic solutions that allow for different equally successful trait combinations increases as the number of trade-offs increases – a conclusion that applies to traits within the leaf (e.g. for shape) as well as to leaf–branch relationships (Niklas 1988).
It is evident that leaves perform several functions simultaneously; that is, they intercept light, transport liquids and solutes, exchange gases with the atmosphere, dissipate heat, cope with externally applied mechanical forces, and defend themselves from herbivores and pathogens. Importantly, many of these functions have conflicting physiological, anatomical, or morphological requirements. For example, orientations of lamina that maximise light interception also minimise the ability to dissipate heat. Further, all of these functions are intrinsically size dependent. For example, the quantity of mechanical tissues required to support laminae depends on the mass of laminae, whereas the ability to intercept light depends on laminae area.
When seen in this light, the trade-offs required for the successful functionality of foliar leaves generate rather than constrain ecological and evolutionary opportunities. This can be illustrated mathematically and empirically in the context of phyllotaxy and its consequences on light interception. Computer simulations of mathematically generated shoots to assess the influence of leaf shape, size, and phyllotactic patterns on the ability to intercept direct solar radiation show that differences in phyllotaxy significantly influence light interception (particularly for shoots with a rosette growth habit). They also show that comparatively small differences in leaf shape can compensate for the negative effects of leaf overlap resulting from virtually any phyllotactic pattern. For example, lobed leaves or pinnifid compound leaves facilitate light penetration through shoots bearing densely packed leaves. Thus, phyllotaxy can be viewed as a developmental limiting factor that can drive compensatory changes in morphological features such as shape, that are not directly controlled by patterns of leaf initiation. In this way, the ‘constraints’ imposed by phyllotaxy can foster evolutionary modifications at the level of the entire shoot (Niklas 1988).
One particularly important trade-off operating at the level of the architecture of a shoot is that between leaf size and number. For any given leaf biomass allocation, an individual shoot can either have a few large leaves or many smaller ones. Across different herbaceous species and juvenile specimens of tree species drawn from diverse lineages and ecological settings, the relationship between total leaf biomass per plant (ML) and total stem mass per plant (MS) falls within a comparatively narrow corridor of variation that obeys a positive isometric scaling relationship (i.e. ML ∝ MS~1.0; Niklas 2004). Accordingly, across these species, any stem mass investment yields a more or less proportional increase in leaf mass. Therefore, it follows mathematically that across these species, the relationship among the average mass of a typical leaf mL, total leaf number NL, and total stem mass complies with the proportionality mL NL ∝ MS~1.0. When individual shoots are sampled across diverse species, the relationship between mL and NL per shoot is negative and isometric (i.e. mL ∝ NL–1; Kleiman and Aarssen 2007), a relationship that appears to be insensitive to how species are grouped according to other functional traits or according to their habitat preference (Yang et al. 2008). That this phenomenology reflects a biomass allocation trade-off is clear. What remains uncertain is whether it is a simple trade-off between leaf mass investment and leaf size, or a more complex set of relationships imposed by twig mass investments and hydraulics or biomechanics.
Another trade-off that appears to hold across many but not all species is the relationship between leaf surface area A and average leaf mass mL. For more than a few species-groupings, this relationship is allometric with a slope that is less than one, which indicates that increases in leaf mass investment do not result in proportional increases in leaf area (i.e. A ∝ mL<1.0; Niklas et al. 2007). This trend of ‘diminishing returns’ has also been reported when total leaf area per plant is plotted against total leaf mass for plants differing in size (Niklas and Cobb 2008, 2010). Several factors may contribute to these trends, e.g. biomechanical analyses show that disproportionately larger investments must be made to mechanically support photosynthetic tissues as leaves increase in their surface area (Niklas et al. 2009). In both the leaf mass to stem mass and leaf mass to area relationships described above, leaf shape variation can provide an extra degree of freedom for selection to act on at both the leaf and branch level.
Case studies of leaf shape variation at different scales
Above, we have discussed evolutionary and proximal drivers of leaf shape, as well as ecological correlates and functional significance thereof. Here, we present case studies at three scales where we can examine the ecological and evolutionary implications of leaf shape. These are the cross community scale case of leaf teeth as a subset of shape modifications, within lineage considerations of the genus Pelargonium and the family Proteaceae, and finally the case of leaf shape variation within a single genetic individual: heteroblasty and heterophylly.
Leaf teeth: leaf shape variation at the community scale
Leaf teeth are conspicuous features in many plants and are notable as a leaf-shape trait that varies in predictable ways across plant communities. Nearly 100 years ago, Bailey and Sinnott (1915, 1916) observed that plant communities from regions with low mean annual temperature (MAT) have a higher site mean proportion of non-monocotyledonous species with toothed leaves. Paleobotanists quickly seized upon this relationship as an index for inferring paleotemperature. Little et al. (2010) recently documented the rich interest in this topic, compiling 351 literature references related to leaf physiognomy and temperature, of which the primary focus is leaf teeth. The link between teeth and temperature is observed on all vegetated continents (Greenwood 2005a; Peppe Royer et al. 2011) and is also seen in related metrics such as tooth size and tooth number (Huff et al. 2003; Royer et al. 2005; Peppe et al. 2011). Further, these tooth variables can display phenotypic plasticity to temperature change (Fig. 6; Royer et al. 2009b).
|
What is known about the functional bases of these leaf–climate relationships? Leaf teeth commonly expand and mature faster than the bulk leaf (e.g. Billings 1905; Feild et al. 2005) and young teeth are disproportionately vigorous in their gas exchange (Baker-Brosh and Peet 1997; Royer and Wilf 2006). From these observations, Royer and Wilf (2006) hypothesised that one role of teeth is to increase sap flow, thereby delivering nutrients and other solutes to young, emerging leaves. In habitats with low temperatures, teeth could play an important role in maximising the potential for carbon gain and growth early in the season. In warmer climates, with longer growing seasons, the carbon benefit conferred by teeth would be outweighed by their unavoidable water cost. Alternatively, Feild et al. (2005) hypothesised that teeth help remove freeze–thaw embolisms through positive root pressure and guttation. Importantly, the transport of fluids is common to both hypotheses. From this, it may be surmised that water-limiting conditions select against teeth (Bailey and Sinnott 1916). Indeed, this pattern is clearly seen across local water availability gradients (Burnham et al. 2001; Kowalski and Dilcher 2003; Greenwood 2005b; Royer et al. 2009a; Peppe et al. 2011) but not globally against mean annual precipitation (e.g. Peppe et al. 2011).
If the hypothesis by Royer and Wilf (2006) is correct, toothed species should have a higher photosynthetic rate, at least at the beginning of the growing season. As such, teeth may be related to the leaf economics spectrum (Wright et al. 2004). Some studies find support for this idea: leaves with a short leaf lifespan (deciduous) (Peppe et al. 2011) and low leaf mass per area (Royer et al. 2005), two leaf economic variables, are more likely to be toothed. It also follows that teeth should be more functionally related to growing season climatic variables like seasonality, growing season length, and growing degree days, not mean annual temperature, however, few studies find support for this idea (Royer et al. 2005; Peppe et al. 2011). Ultimately, it would be valuable to have a better understanding of the genetic processes underlying tooth development, but presently little is known outside model species (see ‘Genetic regulation for patterning of leaf margin: serration and leaflet formation’ section). Further, phylogenetic history has typically been assumed to have only a minor role in these leaf-climate patterns, but rigorous tests demonstrate that this assumption is false (Little et al. 2010). This outcome is of particular concern for paleobotanists because systematic placement of fossils can be difficult, especially for the Cretaceous and Paleogene. Tools to accommodate for this phylogenetic dependency are needed.
Leaf shape variation within lineages
Proteaceae
Proteaceae is an ancient angiosperm family originating in Gondwana over 100 million year ago and possessing 1700 extant species (Weston 2007). The family’s greatest representation is in Australia, followed by South Africa, with smaller numbers across other Gondwanan segments (Cowling and Lamont 1998; Hoot and Douglas 1998; Weston 2007). Within the Proteaceae, there exists an enormous range of leaf sizes and shapes, ranging from a few mm2 to 500 cm2 and including entire, toothed, broadly lobed, deeply dissected, needle-like, simple or compound leaves. Intriguingly, most of this shape diversity is represented in Australia. The majority of taxa outside Australia possess entire leaves; the exception being four South African genera that possess dissected (Serruria and Paranomis) or toothed (Mimites and Leucospermum) leafed species, all of which occur in a single clade (Sauquet et al. 2009).
The reason for the extensive radiation in leaf shape diversity in Australian Proteaceae is unclear. Among South African Proteaceae, leaf size decreases with mean annual temperature and narrow leaves have been shown to overheat less than their wider counterparts (Yates et al. 2010). Given that leaf dissection and small size are functionally equivalent with respect to heat loss (see ‘Temperature and water’ section), it is tempting to suggest that leaf shape variation in Australia represents an adaptive response to increasing hot, dry conditions. Yet, there appears to be no consistent distributional pattern of Proteaceae leaf dimensions in Australia. Many medium or large, un-dissected leaves exist in the hottest habitats; Grevilleas with small, simple leaves can be found across most regions including high rainfall areas; and some of the species found in the shaded rainforest understorey have among the most deeply dissected leaves of all. Leaf shape also varies within a single individual. For example, fern-leaf stenocarpus Stenocarpus davallioides Foreman & B.Hyland has tripinnatisect juvenile leaves but adult leaves can be pinnate or bipinnate (Foreman and Hyland 1995). Other rainforest species, such as Atherton Oak (Athertonia diversifolia (C.T.Whit) L.A.S. Johnson & B.G.Briggs), have very large, lobed intermediate leaves but adult and seedling leaves are small and entire (Weston 1995). Alternatively, leaf lobing within a plant can be random, having no obvious spatial or developmental association, or homogenous across a species. Examining a phylogeny of extant taxa, one finds lobed or dissected leaves alongside entire-leafed species – across most lineages in the group (Weston 2007). Therefore, although suggestions of microclimatic adaptation may be offered at the species level, it seems unlikely that an environmental association with leaf shape could be generalised across Australian Proteaceae. Rather, leaf shape variation in the Proteaceae is likely to reflect the range of ecological solutions to leaf structure/function trade-offs discussed above.
Pelargonium
Similar to the Proteaceae, the genus Pelargonium is distributed predominantly in the southern hemisphere, with nearly 80% of its almost 300 species found in South Africa. Although the age of the genus is estimated to be around 30 million years (Bakker et al. 2005), the highest rate of species accumulation has occurred in the last 10 million years (Martinez-Cabrera 2010). The combination of climatic and edaphic heterogeneity over short distances and limited gene flow (Latimer et al. 2005; Linder 2005) is frequently offered as an explanation for the extraordinary plant species diversity that characterises southern Africa. Pelargonium, the third largest genus in the region, is characterised by remarkable variation in growth form that ranges from annuals and geophytes to shrubs. Variation in leaf shape is no less dramatic, ranging from entire to highly dissected (Fig. 1), often within growth forms and taxonomic sections. Parsing of leaf form categorically revealed that the extent of dissection of the blade is highly evolvable within an overall ovate leaf shape outline that is evolutionarily conserved (Jones et al. 2009).
In addition to high evolutionary lability of leaf dissection, there is variation among major clades in the evolutionary pattern of major veins. One major clade (Clade C) is dominated by palmate venation whereas another (Clade A) is dominated by pinnate venation, although both types are found in both clades. We note that pinnate venation is significantly associated with increased dissection and reductions in functional leaf size characteristic of the highly diverse ‘xerophytic’ clade (Clade A2) of the winter rainfall region.
To examine whether leaf shape translates into functional variation, carbon gain and water use efficiency were measured in pairs of species contrasted for leaf shape and replicated across three subclades of Clade A and one subclade of the third major clade, Clade B (Nicotra et al. 2008). In each case, the species with the more dissected leaves showed higher rates of carbon gain, but also higher rates of water loss in all treatment conditions. That paper argued that the differences in carbon gain were not direct results of leaf shape per se, but rather that the same conditions that result in selection for more dissected leaves also favour the evolution of high photosynthetic rates, high leaf nitrogen content and opportunistic use of water when available. Even in this study, however, there was no correlation between leaf dissection and the climate present in native ranges, suggesting either that climate is selective at a much smaller scale (i.e. the microhabitat), or that for any given set of climate variables, multiple leaf shapes can be adaptive in association with variation in other leaf traits.
Heteroblasty and heterophylly: variation within a single genetic individual
We have so far focussed primarily on cross species variation in leaf shape. As discussed for the Proteaceae genera above (‘The Proteaceae’ section), however, a single genome can produce a remarkable range of leaf shapes during the lifetime of a plant. There are two broad categories of leaf shape variation that can be expressed by a single individual. This variation can arise either in response to different external environments or to ontogenetic signals (some of which may be influenced by environment as well).
Environmentally induced heterophylly
Environmentally induced heterophylly results when leaf shape responds to environmental cues. The classic case is the dramatically different emergent and submergent leaves in aquatic plants. Although many aquatic plants are also heteroblastic (see below, Sculthorpe 1967), environmentally induced heterophylly is not specific to position or plant age and is reversible, depending largely on the environmental signals (mediated by hormonal signals) perceived by cells of the developing leaf primordium. The developmental age of the leaf at the time of signal perception is critical. Very young primordia (L1–L3) generally respond completely to the novel environment whereas primordia that were slightly older at the time of environmental change show intermediate features as mature leaves. Characteristics of specific cells are related to the position of that cell within the differentiation gradient characteristic of that species at the time of environmental change (e.g. Goliber and Feldman 1990; Bruni et al. 1996; Kuwabara and Nagata 2006).
Among terrestrial plants, the ‘sun–shade’ leaf response is frequently cited as the classic example of environmentally induced heterophylly. Historically, differences in leaf form within a canopy have been assumed to be responses to altered light environments (e.g. larger and less-lobed leaves are produced in shade). However, these different leaf shapes also confer distinct hydraulic advantages (see ‘Temperature and water’ section). Whether in response to light or to hydraulic limitation, ‘sun/shade’ heterophylly is accompanied by similar and well-known anatomical differences across a wide range of species (i.e. sun leaves are generally smaller and thicker with smaller cells than shade leaves; Taiz and Zeiger 2002). However, when these anatomical differences become determined developmentally (Dengler 1980; Yano and Terashima 2004) has been less commonly studied. The presence of intermediate anatomical characteristics in leaves transferred among light environments shortly after expansion ceased suggests that leaves of some species have the capacity to respond anatomically to altered light levels until very late in development (Oguchi et al. 2005). In contrast, recent studies suggest that systemic light and humidity signals sent from older to younger leaves allow younger leaves to develop phenotypes suited for their anticipated environments (Lake et al. 2001; Yano and Terashima 2001; Thomas et al. 2004). Exactly when final leaf shape is determined in ‘sun/shade’ leaves remains an open question for most species.
Heteroblasty describes ontogenetically dependent changes in leaf features, most notably shape
In 1900, Goebel originally distinguished differences in ‘juvenile form’ from ‘adult form’ on the basis of dramatic differences in leaf shape and associated traits (Balfour 1969). As an example, he described the shift from compound leaves to phyllodes in Acacia as heteroblastic and reserved the term homoblastic for the less dramatic variation exhibited by Casurina. It is now generally accepted that heteroblasty refers to the full suite of features that change along the shoot during development (Jones 1999).
Heteroblasty results from the interaction of developmental programs expressed at both the leaf and the shoot- or whole-plant level (Kerstetter and Poethig 1998; Tsukaya et al. 2000; Usami et al. 2009; Wu et al. 2009; Chuck and O’Connor 2010). Heteroblasty is generally not reversible unless reiterative growth occurs (i.e. following damage). Morphological differences characteristic of heteroblasty such as differences in lobing are usually expressed relatively early in development, shortly after leaf initiation (Jones 1995).
The sequence of heteroblastic leaf shapes produced along the shoot can be accelerated or delayed in response to different growth environments or architectural manipulations (Ashby 1948; Forster and Bonser 2009; Jaya et al. 2010). Furthermore, both heteroblasty and plasticity in heteroblasty have been shown to be under strong genetic control (Anderson 1989; Climent et al. 2006). Forster et al. (2011) suggested that both heteroblasty and plasticity in heteroblasty are likely to occur in response to ‘which leaf type confers the greatest benefit for a given light level.’ Several studies over the last decade have pointed towards functional consequences of heteroblasty, generally with reference to single environmental variables (e.g. Hansen 1986; Gamage and Jesson 2007; Kubien et al. 2007). It is likely that heteroblasty influences multiple leaf functions because suites of leaf traits change with heteroblasty (Jones 2001). Indeed, this conclusion was supported in a recent study that examined differences in more than a hundred leaf traits in koa (Acacia koa A.Gray; Pasquet-Kok et al. 2010). Heteroblasty has also been proposed as an adaptation to herbivory (Karban and Thaler 1999; Brennan et al. 2001; Fadzly et al. 2009) and it has been associated with differences in frost resistance (Darrow et al. 2001). It is worth noting that studies of the functional significance of heteroblasty have focussed on relatively few plant lineages. Given that heteroblasty occurs widely among the angiosperms, as well as in some ferns and gymosperms (e.g. Wagner 1952; Mueller 1982; Pryer and Hearn 2009), the next questions may focus on the cost of heteroblasty and its fitness consequences: what scale of environmental change selects for ontogenetic response in leaf shape?
Synthesis and next steps
Whether considered at community, lineage or single genome scales, leaf shape is a trait that is highly labile and responsive to a range of biotic and abiotic factors. Although some leaf traits can be explained in terms of particularly strong trade-offs between two or more factors that result in clear scaling relationships (e.g. leaf mass-to-area traits or leaf mass-to-stem mass), leaf shape, in contrast, does not emerge from the above consideration as a factor capable of explaining ecological differentiation between species. Rather, leaf shape emerges as a trait for which there are many quite varied functional trade-offs.
As stated above, these trade-offs for successful functionality of leaves may generate ecological opportunities; leaf shape is perhaps best viewed not as a single major axis, but rather as an option that fine tunes the leaf to its conditions over both short and evolutionary time spans. Numerous elements of this review support this conclusion. Over evolutionary history the extant range of variation in leaf shape has been explored by many lineages, although only the angiosperms have explored this range with the added element of massive increases in vein densities. Likewise, our growing understanding of genetic controls on leaf shape reveal that it is a complex trait determined by many different genes at several different developmental stages. Although some genes have conserved affects on leaf morphology, including shape, the exact nature of their affects can vary widely. Likewise, the function to which a given element of leaf shape ‘is put’ can vary. For example, closer investigation of the internal architecture and developmental pathways associated with different leaf shapes suggests that leaf teeth and leaf lobes often have distinctly different functional foundations, and may play different functions in different environments.
Despite a myriad of functional roles, the one recurrent theme in our review is that leaf shape is affected by and strongly influences leaf water relations. From a functional perspective, the role of leaf shape in leaf thermoregulation has perhaps received the most attention in previous considerations of the functional significance of leaf shape. The relationship between leaf size and temperature being physically determined by basic energy balance principles, this was a logical starting point of enquiry. But, as we have shown, the connection between leaf shape (and size) and leaf temperatures under field conditions is not well supported empirically. Rather, leaf shape variation, as distinct from size, more often will reflect water supply trade-offs. High vein densities support high photosynthetic rates (Brodribb et al. 2010) and dissected leaves maintain a greater proportion of leaf tissue closer to main veins. Leaf temperature plays a role in this story, as higher leaf temperatures lead to greater evaporative water loss, but many factors other than leaf shape influence leaf temperature (e.g. leaf thickness, orientation and reflectivity and branch and plant architecture). Finally, it is also worth noting that although there appears to be an obvious link between shape and water supply, there is a concomitant interaction between water supply and phloem function that remains relatively unexplored.
Thus, our review suggests several angles for further research (Table 1). In particular we advocate those approaches that enable investigation across the scales examined here (gene, development, function and evolutionary ecology) and that focus in particular on the links among leaf shape, development and water relations.

|
Acknowledgements
The authors thank the Botanical Society of America for their support of the symposium from which this review arose. We also thank Lawren Sack and two anonymous reviewers for useful comments on an earlier version of this manuscript.
References
Ackerly DD, Knight CA, Weiss SB, Barton K, Starmer KP (2002) Leaf size, specific leaf area and microhabitat distribution of chaparral woody plants: contrasting patterns in species level and community level analyses. Oecologia 130, 449–457.| Leaf size, specific leaf area and microhabitat distribution of chaparral woody plants: contrasting patterns in species level and community level analyses.Crossref | GoogleScholarGoogle Scholar |
Anderson S (1989) Variation in heteroblastic succession among populations of Crepis tectorum. Nordic Journal of Botany 8, 565–573.
| Variation in heteroblastic succession among populations of Crepis tectorum.Crossref | GoogleScholarGoogle Scholar |
Armbruster WS, Muchhala N (2009) Associations between floral specialization and species diversity: cause, effect, or correlation? Evolutionary Ecology 23, 159–179.
| Associations between floral specialization and species diversity: cause, effect, or correlation?Crossref | GoogleScholarGoogle Scholar |
Armbruster WS, Di Stilio VS, Tuxill JD, Flores TC, Runk JLV (1999) Covariance and decoupling of floral and vegetative traits in nine neotropical plants: a re-evaluation of Berg’s correlation-pleiades concept. American Journal of Botany 86, 39–55.
| Covariance and decoupling of floral and vegetative traits in nine neotropical plants: a re-evaluation of Berg’s correlation-pleiades concept.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MnhtlGhtQ%3D%3D&md5=af4c22d89fe94984cf1f76baa5785617CAS | 21680344PubMed |
Ashby E (1948) Studies in the morphogenesis of leaves I. An essay on leaf shape. New Phytologist 47, 153–176.
| Studies in the morphogenesis of leaves I. An essay on leaf shape.Crossref | GoogleScholarGoogle Scholar |
Aso K, Kato M, Banks JA, Hasebe M (1999) Characterization of homeodomain-leucine zipper genes in the fern Ceratopteris richardii and the evolution of the homeodomain-leucine zipper gene family in vascular plants. Molecular Biology and Evolution 16, 544–552.
Bailey IW, Sinnott EW (1915) A botanical index of Cretaceous and Tertiary climates. Science 41, 831–834.
| A botanical index of Cretaceous and Tertiary climates.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3cvlsleruw%3D%3D&md5=53d3f22f49d3b99f31bbb18217517007CAS | 17835989PubMed |
Bailey IW, Sinnott EW (1916) The climatic distribution of certain types of angiosperm leaves. American Journal of Botany 3, 24–39.
| The climatic distribution of certain types of angiosperm leaves.Crossref | GoogleScholarGoogle Scholar |
Baker-Brosh KF, Peet RK (1997) The ecological significance of lobed and toothed leaves in temperate forest trees. Ecology 78, 1250–1255.
Bakker FT, Culham A, Marais EM, Gibby M (2005) Nested radiation in Cape Pelargonium. In ‘Plant species-level systematics: new perspectives on pattern and process. Vol. 143’. (Eds FT Bakker, LW Chartrou, B Gravendeel, PB Pielser) pp. 75–100. (ARG Ganter Verlag KG: Ruggell, Lichtstein)
Balfour IB (Transl) (1969) ‘Organography of plants. I. General organography.’ by K. Goebel, 1900. (Hafner Publishing Co. Inc.: New York)
Ball MC, Cowan IR, Farquhar GD (1988) Maintenance of leaf temperature and the optimization of carbon gain in relation to water loss in a tropical mangrove forest. Australian Journal of Plant Physiology 15, 263–276.
| Maintenance of leaf temperature and the optimization of carbon gain in relation to water loss in a tropical mangrove forest.Crossref | GoogleScholarGoogle Scholar |
Ball MC, Canny MJ, Huang CX, Heady RD (2004) Structural changes in acclimated and unacclimated leaves during freezing and thawing. Functional Plant Biology 31, 29–40.
| Structural changes in acclimated and unacclimated leaves during freezing and thawing.Crossref | GoogleScholarGoogle Scholar |
Ball MC, Canny MJ, Huang CX, Egerton JJG, Wolfe J (2006) Freeze/thaw-induced embolism depends on nadir temperature: the heterogeneous hydration hypothesis. Plant, Cell & Environment 29, 729–745.
| Freeze/thaw-induced embolism depends on nadir temperature: the heterogeneous hydration hypothesis.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD28nltl2hsw%3D%3D&md5=ff221c7472466d1c56459f28399c938fCAS | 17087458PubMed |
Barkoulas M, Hay A, Kougioumoutzi E, Tsiantis M (2008) A developmental framework for dissected leaf formation in the Arabidopsis relative Cardamine hirsuta. Nature Genetics 40, 1136–1141.
| A developmental framework for dissected leaf formation in the Arabidopsis relative Cardamine hirsuta.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtVGgt77J&md5=52305a7e0e75c671e04032a11d55b712CAS | 19165928PubMed |
Beadle NCW (1966) Soil phosphate and its role in molding segments of the Australian flora and vegetation, with special reference to xeromorphy and sclerophylly. Ecology 47, 992–1007.
| Soil phosphate and its role in molding segments of the Australian flora and vegetation, with special reference to xeromorphy and sclerophylly.Crossref | GoogleScholarGoogle Scholar |
Berg RL (1960) The ecological significance of correlation pleiades. Evolution 14, 171–180.
| The ecological significance of correlation pleiades.Crossref | GoogleScholarGoogle Scholar |
Bharathan G, Goliber TE, Moore C, Kessler S, Pham T, Sinha NR (2002) Homologies in leaf form inferred from KNOXI gene expression during development. Science 296, 1858–1860.
| Homologies in leaf form inferred from KNOXI gene expression during development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XksVSmtrs%3D&md5=59a997e68132c7d99a723fe1bb347139CAS | 12052958PubMed |
Billings FH (1905) Precursory leaf serrations of Ulmus. Botanical Gazette 40, 224–225.
| Precursory leaf serrations of Ulmus.Crossref | GoogleScholarGoogle Scholar |
Blein T, Pulido A, Vialette-Guiraud A, Nikovics K, Morin H, Hay A, Johansen IE, Tsiantis M, Laufs P (2008) A conserved molecular framework for compound leaf development. Science 322, 1835–1839.
| A conserved molecular framework for compound leaf development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsFSmtrnK&md5=d0c0c21c8a41885221467f3f836522daCAS | 19095941PubMed |
Bond WJ (1989) The tortoise and the hare: ecology of angiosperm dominance and gymnosperm persistence. Biological Journal of the Linnean Society. Linnean Society of London 36, 227–249.
| The tortoise and the hare: ecology of angiosperm dominance and gymnosperm persistence.Crossref | GoogleScholarGoogle Scholar |
Boyce CK (2005) Patterns of segregation and convergence in the evolution of fern and seed plant leaf morphologies. Paleobiology 31, 117–140.
| Patterns of segregation and convergence in the evolution of fern and seed plant leaf morphologies.Crossref | GoogleScholarGoogle Scholar |
Boyce CK (2007) Mechanisms of laminar growth in morphologically convergent leaves and flower petals. International Journal of Plant Sciences 168, 1151–1156.
| Mechanisms of laminar growth in morphologically convergent leaves and flower petals.Crossref | GoogleScholarGoogle Scholar |
Boyce CK (2008a) The fossil record of plant physiology and development – what leaves can tell us. Paleontological Society Papers 14, 133–146.
Boyce CK (2008b) How green was Cooksonia? The importance of size in understanding the early evolution of physiology in the vascular plant lineage. Paleobiology 34, 179–194.
| How green was Cooksonia? The importance of size in understanding the early evolution of physiology in the vascular plant lineage.Crossref | GoogleScholarGoogle Scholar |
Boyce CK (2009) Seeing the forest with the leaves – clues to canopy placement from leaf fossil size and venation characteristics. Geobiology 7, 192–199.
| Seeing the forest with the leaves – clues to canopy placement from leaf fossil size and venation characteristics.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1M3ltV2nsw%3D%3D&md5=e39a8b50074009dd647644b4a7832a53CAS | 19207570PubMed |
Boyce CK (2010) The evolution of plant development in a paleontological context. Current Opinion in Plant Biology 13, 102–107.
| The evolution of plant development in a paleontological context.Crossref | GoogleScholarGoogle Scholar | 19897405PubMed |
Boyce CK, Knoll AH (2002) Evolution of developmental potential and the multiple independent origins of leaves in Paleozoic vascular plants. Paleobiology 28, 70–100.
| Evolution of developmental potential and the multiple independent origins of leaves in Paleozoic vascular plants.Crossref | GoogleScholarGoogle Scholar |
Boyce CK, Brodribb TJ, Feild TS, Zwieniecki MA (2009) Angiosperm leaf vein evolution was physiologically and environmentally transformative. Proceedings of the Royal Society B-Biological Sciences 276, 1771–1776.
| Angiosperm leaf vein evolution was physiologically and environmentally transformative.Crossref | GoogleScholarGoogle Scholar |
Bragg JG, Westoby M (2002) Leaf size and foraging for light in a sclerophyll woodland. Functional Ecology 16, 633–639.
| Leaf size and foraging for light in a sclerophyll woodland.Crossref | GoogleScholarGoogle Scholar |
Brennan EB, Weinbaum SA, Rosenheim JA, Karban R (2001) Heteroblasty in Eucalyptus globulus (Myricales: Myricaceae) affects ovipositonal and settling preferences of Ctenarytaina eucalypti and C. spatulata (Homoptera: Psyllidae). Environmental Entomology 30, 1144–1149.
| Heteroblasty in Eucalyptus globulus (Myricales: Myricaceae) affects ovipositonal and settling preferences of Ctenarytaina eucalypti and C. spatulata (Homoptera: Psyllidae).Crossref | GoogleScholarGoogle Scholar |
Brodribb TJ, Feild TS (2010) Leaf hydraulic evolution led a surge in leaf photosynthetic capacity during early angiosperm diversification. Ecology Letters 13, 175–183.
| Leaf hydraulic evolution led a surge in leaf photosynthetic capacity during early angiosperm diversification.Crossref | GoogleScholarGoogle Scholar | 19968696PubMed |
Brodribb TJ, Feild TS, Jordan GJ (2007) Leaf maximum photosynthetic rate and venation are linked by hydraulics. Plant Physiology 144, 1890–1898.
| Leaf maximum photosynthetic rate and venation are linked by hydraulics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXpsVOgs7s%3D&md5=1655bd5a0708a6be957e2e90726baa1eCAS | 17556506PubMed |
Brodribb TJ, Feild TS, Sack L (2010) Viewing leaf structure and evolution from a hydraulic perspective. Functional Plant Biology 37, 488–498.
| Viewing leaf structure and evolution from a hydraulic perspective.Crossref | GoogleScholarGoogle Scholar |
Bruni NC, Young JP, Dengler NG (1996) Leaf developmental plasticity of Ranunculus flabellaris in response to terrestrial and submerged environments. Canadian Journal of Botany 74, 823–837.
| Leaf developmental plasticity of Ranunculus flabellaris in response to terrestrial and submerged environments.Crossref | GoogleScholarGoogle Scholar |
Burnham RJ, Pitman NCA, Johnson KR, Wilf P (2001) Habitat-related error in estimating temperatures from leaf margins in a humid tropical forest. American Journal of Botany 88, 1096–1102.
| Habitat-related error in estimating temperatures from leaf margins in a humid tropical forest.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MrpvV2gtw%3D%3D&md5=633ff21fdc8205c257270061b7f02d79CAS | 11410475PubMed |
Canales C, Barkoulas M, Galinha C, Tsiantis M (2010) Weeds of change: Cardamine hirsuta as a new model system for studying dissected leaf development. Journal of Plant Research 123, 25–33.
| Weeds of change: Cardamine hirsuta as a new model system for studying dissected leaf development.Crossref | GoogleScholarGoogle Scholar | 19821009PubMed |
Chuck G, O’Connor D (2010) Small RNAs going the distance during plant development. Current Opinion in Plant Biology 13, 40–45.
| Small RNAs going the distance during plant development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXpslehuw%3D%3D&md5=c531e559713888e8897b5a74ff0b5d6aCAS | 19796985PubMed |
Climent J, Chambel MR, Lopez R, Mutke S, Alia R, Gil L (2006) Population divergence for heteroblasty in the Canary Island pine (Pinus canariensis, Pinaceae). American Journal of Botany 93, 840–848.
| Population divergence for heteroblasty in the Canary Island pine (Pinus canariensis, Pinaceae).Crossref | GoogleScholarGoogle Scholar | 21642146PubMed |
Clum HH (1926) The effect of transpiration and environmental factors on leaf temperatures 1. Transpiration. American Journal of Botany 13, 194–216.
| The effect of transpiration and environmental factors on leaf temperatures 1. Transpiration.Crossref | GoogleScholarGoogle Scholar |
Cowling RM, Lamont BB (1998) On the nature of Gondwanan species flocks: diversity of Proteaceae in Mediterranean south-western Australia and South Africa. Australian Journal of Botany 46, 335–355.
| On the nature of Gondwanan species flocks: diversity of Proteaceae in Mediterranean south-western Australia and South Africa.Crossref | GoogleScholarGoogle Scholar |
Cunningham SA, Summerhayes B, Westoby M (1999) Evolutionary divergences in leaf structure and chemistry, comparing rainfall and soil nutrient gradients. Ecological Monographs 69, 569–588.
| Evolutionary divergences in leaf structure and chemistry, comparing rainfall and soil nutrient gradients.Crossref | GoogleScholarGoogle Scholar |
Darrow HE, Bannister P, Burritt DJ, Jameson PE (2001) The frost resistance of juvenile and adult forms of some heteroblastic New Zealand plants. New Zealand Journal of Botany 39, 355–363.
| The frost resistance of juvenile and adult forms of some heteroblastic New Zealand plants.Crossref | GoogleScholarGoogle Scholar |
de Reuille PB, Bohn-Courseau I, Ljung K, Morin H, Carraro N, Godin C, Traas J (2006) Computer simulations reveal properties of the cell–cell signaling network at the shoot apex in Arabidopsis. Proceedings of the National Academy of Sciences of the United States of America 103, 1627–1632.
| Computer simulations reveal properties of the cell–cell signaling network at the shoot apex in Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 16432202PubMed |
Dengler N (1980) Comparative histological basis of sun and shade leaf dimorphism in Helianthus annuus. Canadian Journal of Botany 58, 717–730.
| Comparative histological basis of sun and shade leaf dimorphism in Helianthus annuus.Crossref | GoogleScholarGoogle Scholar |
Dinneny JR, Yadegari R, Fischer RL, Yanofsky MF, Weigel D (2004) The role of JAGGED in shaping lateral organs. Development 131, 1101–1110.
| The role of JAGGED in shaping lateral organs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXis1emsrs%3D&md5=611e866a7268e0559f22352a5c74af18CAS | 14973282PubMed |
Doyle J, Hickey L (1976) Pollen and leaves from the mid-Cretaceous potomac group and their bearing on early angiosperm evolution. In ‘Origin and early evolution of Angiosperms’. (Ed. C Beck) pp. 139–206. (Columbia University Press: New York)
Drake BG, Raschke K, Salisbury FB (1970) Temperatures and transpiration resistances of Xanthium leaves as affected by air temperature, humidity, and wind speed. Plant Physiology 46, 324–330.
| Temperatures and transpiration resistances of Xanthium leaves as affected by air temperature, humidity, and wind speed.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3cngvFKnsw%3D%3D&md5=8a22fdf3aa352ca7c5063b189f2f6676CAS | 16657458PubMed |
Efroni I, Blum E, Goldshmidt A, Eshed Y (2008) A protracted and dynamic maturation schedule underlies Arabidopsis leaf development. The Plant Cell 20, 2293–2306.
| A protracted and dynamic maturation schedule underlies Arabidopsis leaf development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtlCnsLvP&md5=bc1e93a187943703ae3706858b0c4495CAS | 18805992PubMed |
Efroni I, Eshed Y, Lifschitz E (2010) Morphogenesis of simple and compound leaves: a critical review. The Plant Cell 22, 1019–1032.
| Morphogenesis of simple and compound leaves: a critical review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXnsV2ju7Y%3D&md5=2b8a4c206817a8e37d875ae58bbc29cfCAS | 20435903PubMed |
Fadzly N, Jack C, Schaefer HM, Burns KC (2009) Ontogenetic colour changes in an insular tree species: signalling to extinct browsing birds? New Phytologist 184, 495–501.
| Ontogenetic colour changes in an insular tree species: signalling to extinct browsing birds?Crossref | GoogleScholarGoogle Scholar | 19674327PubMed |
Feild TS, Sage TL, Czerniak C, Iles WJD (2005) Hydathodal leaf teeth of Chloranthus japonicus (Chloranthaceae) prevent guttation-induced flooding of the mesophyll. Plant, Cell & Environment 28, 1179–1190.
| Hydathodal leaf teeth of Chloranthus japonicus (Chloranthaceae) prevent guttation-induced flooding of the mesophyll.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtVGltrfJ&md5=46038a28efd6a8d7c09d7855554b1723CAS |
Floyd SK, Bowman JL (2006) Distinct developmental mechanisms reflect the independent origins of leaves in vascular plants. Current Biology 16, 1911–1917.
| Distinct developmental mechanisms reflect the independent origins of leaves in vascular plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtVCrt7vJ&md5=2ce1efc6b6b8f7f2768abe677a83caa2CAS | 17027487PubMed |
Floyd SK, Bowman JL (2010) Gene expression patterns in seed plant shoot meristems and leaves: homoplasy or homology? Journal of Plant Research 123, 43–55.
| Gene expression patterns in seed plant shoot meristems and leaves: homoplasy or homology?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsFyit7jE&md5=4c8845e92bacdbd367e00d764104be77CAS | 19784716PubMed |
Fonseca CR, Overton JM, Collins B, Westoby M (2000) Shifts in trait combinations along rainfall and phosphorus gradients. Journal of Ecology 88, 964–977.
| Shifts in trait combinations along rainfall and phosphorus gradients.Crossref | GoogleScholarGoogle Scholar |
Foreman BH, Hyland B (1995) Stenocarpus. In ‘Flora of Australia. Vol. 16’. (Ed. P McCarthy) pp. 364–366. (CSIRO Publishing: Melbourne)
Forster MA, Bonser SP (2009) Heteroblastic development and the optimal partitioning of traits among contrasting environments in Acacia implexa. Annals of Botany 103, 95–105.
| Heteroblastic development and the optimal partitioning of traits among contrasting environments in Acacia implexa.Crossref | GoogleScholarGoogle Scholar | 18978364PubMed |
Forster MA, Ladd B, Bonser SP (2011) Optimal allocation of resources in response to shading and neighbours in the heteroblastic species, Acacia implexa. Annals of Botany 103, 95–105.
| Optimal allocation of resources in response to shading and neighbours in the heteroblastic species, Acacia implexa.Crossref | GoogleScholarGoogle Scholar |
Frohlich MW, Chase MW (2007) After a dozen years of progress the origin of angiosperms is still a great mystery. Nature 450, 1184–1189.
| After a dozen years of progress the origin of angiosperms is still a great mystery.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhsVGjsL%2FP&md5=ef21a2ae26036a92893313bc2ab6b8adCAS | 18097399PubMed |
Galtier J (1981) Structures foliaires de fougères et Ptéridospermales du Carbonifère Inférieur et leur signification évolutive. Palaeontographica Abt. B 180, 1–38.
Gamage HK, Jesson L (2007) Leaf heteroblasty is not an adaptation to shade: seedling anatomical and physiological responses to light. New Zealand Journal of Ecology 31, 245–254.
Gates DM (1968) Transpiration and leaf temperature. Annual Review of Plant Physiology 19, 211–238.
| Transpiration and leaf temperature.Crossref | GoogleScholarGoogle Scholar |
Gates DM (1980) ‘Biophysical ecology.’ (Springer-Verlag: New York)
Gates DM, Alderfer R, Taylor E (1968) Leaf temperatures of desert plants. Science 159, 994–995.
| Leaf temperatures of desert plants.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaF1c7isVSisA%3D%3D&md5=d506f36d57cfadffdf7ef1fcbb68d204CAS | 5636000PubMed |
Geller GN, Smith WK (1982) Influence of leaf size, orientation, and arrangement on temperature and transpiration in three high-elevation, large leafed herbs. Oecologia 53, 227–234.
| Influence of leaf size, orientation, and arrangement on temperature and transpiration in three high-elevation, large leafed herbs.Crossref | GoogleScholarGoogle Scholar |
Givnish TJ (1978) Ecological aspects of plant morphology: leaf form in relation to environment. Acta Biotheoretica 27, 83–142.
Givnish TJ (1979) On the adaptive significance of leaf form. In ‘Topics in plant population biology’. (Eds OT Solbrig, J Subodh, GB Johnson, PH Raven) pp. 375–407. (Columbia University Press: New York)
Goliber TE, Feldman LJ (1990) Developmental analysis of leaf plasticity in the heterophyllous aquatic plant Hippuris vulgaris. American Journal of Botany 77, 399–412.
| Developmental analysis of leaf plasticity in the heterophyllous aquatic plant Hippuris vulgaris.Crossref | GoogleScholarGoogle Scholar |
Gottschlich DE, Smith AP (1982) Convective heat transfer characteristics of toothed leaves. Oecologia 53, 418–420.
| Convective heat transfer characteristics of toothed leaves.Crossref | GoogleScholarGoogle Scholar |
Grace J, Fasehun FE, Dixon M (1980) Boundary layer conductance of the leaves of some tropical timber trees. Plant, Cell & Environment 3, 443–450.
Greenwood DR (2005a) Leaf form and the reconstruction of past climates. New Phytologist 166, 355–357.
| Leaf form and the reconstruction of past climates.Crossref | GoogleScholarGoogle Scholar | 15819898PubMed |
Greenwood DR (2005b) Leaf margin analysis: taphonomic constraints. Palaios 20, 498–505.
| Leaf margin analysis: taphonomic constraints.Crossref | GoogleScholarGoogle Scholar |
Groom PK, Lamont BB, Leighton S, Leighton P, Burrows C (2004) Heat damage in sclerophylls is influenced by their leaf properties and plant environment. Ecoscience 11, 94–101.
Gurevitch J, Schuepp PH (1990a) Boundary layer properties of highly dissected leaves – an investigation using an electrochemical fluid tunnel. Plant, Cell & Environment 13, 783–792.
| Boundary layer properties of highly dissected leaves – an investigation using an electrochemical fluid tunnel.Crossref | GoogleScholarGoogle Scholar |
Gurevitch J, Schuepp PH (1990b) Boundary layer properties of highly dissected leaves: an investigation using an electrochemical fluid tunnel. Plant, Cell & Environment 13, 783–792.
| Boundary layer properties of highly dissected leaves: an investigation using an electrochemical fluid tunnel.Crossref | GoogleScholarGoogle Scholar |
Hansen DH (1986) Water relations of compound leaves and phyllodes in Acacia koa var. latifolia. Plant, Cell & Environment 9, 439–445.
| Water relations of compound leaves and phyllodes in Acacia koa var. latifolia.Crossref | GoogleScholarGoogle Scholar |
Harrison CJ, Corley SB, Moylan EC, Alexander DL, Scotland RW, Langdale JA (2005) Independent recruitment of a conserved developmental mechanism during leaf evolution. Nature 434, 509–514.
| Independent recruitment of a conserved developmental mechanism during leaf evolution.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXisFeit7c%3D&md5=a8a1f646bad87ae67b45f779f04bc54eCAS | 15791256PubMed |
Hegazy AK, El Amry MI (1998) Leaf temperature of desert sand dune plants: perspectives on the adaptability of leaf morphology. African Journal of Ecology 36, 34–43.
| Leaf temperature of desert sand dune plants: perspectives on the adaptability of leaf morphology.Crossref | GoogleScholarGoogle Scholar |
Hilton J, Bateman RM (2006) Pteridosperms are the backbone of seed–plant phylogeny. Journal of the Torrey Botanical Society 133, 119–168.
| Pteridosperms are the backbone of seed–plant phylogeny.Crossref | GoogleScholarGoogle Scholar |
Hofer J, Turner L, Hellens R, Ambrose M, Matthews P, Michael A, Ellis N (1997) UNIFOLIATA regulates leaf and flower morphogenesis in pea. Current Biology 7, 581–587.
| UNIFOLIATA regulates leaf and flower morphogenesis in pea.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXltlakurk%3D&md5=b43beed08ee455012ab3e3c2dacf0302CAS | 9259553PubMed |
Hoot SB, Douglas AW (1998) Phylogeny of the Proteaceae based on atpB and atpB-rbcL intergenic spacer region sequences. Australian Systematic Botany 11, 301–320.
| Phylogeny of the Proteaceae based on atpB and atpB-rbcL intergenic spacer region sequences.Crossref | GoogleScholarGoogle Scholar |
Horiguchi G, Kim GT, Tsukaya H (2005) The transcription factor AtGRF5 and the transcription coactivator AN3 regulate cell proliferation in leaf primordia of Arabidopsis thaliana. The Plant Journal 43, 68–78.
| The transcription factor AtGRF5 and the transcription coactivator AN3 regulate cell proliferation in leaf primordia of Arabidopsis thaliana.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXmsVehsrc%3D&md5=c08755910c14e7bc1f28a14a18a1e57bCAS | 15960617PubMed |
Horiguchi G, Fujikura U, Ferjani A, Ishikawa N, Tsukaya H (2006) Large-scale histological analysis of leaf mutants using two simple leaf observation methods: identification of novel genetic pathways governing the size and shape of leaves. The Plant Journal 48, 638–644.
| Large-scale histological analysis of leaf mutants using two simple leaf observation methods: identification of novel genetic pathways governing the size and shape of leaves.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht1ylurfE&md5=6ed49c820624a2a856df1e2bbd409837CAS | 17076802PubMed |
Huff PM, Wilf P, Azumah EJ (2003) Digital future for paleoclimate estimation from fossil leaves? Preliminary results. Palaios 18, 266–274.
| Digital future for paleoclimate estimation from fossil leaves? Preliminary results.Crossref | GoogleScholarGoogle Scholar |
Husbands AY, Chitwood DH, Plavskin Y, Timmermans MCP (2009) Signals and prepatterns: new insights into organ polarity in plants. Genes & Development 23, 1986–1997.
| Signals and prepatterns: new insights into organ polarity in plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtFagsrzJ&md5=7f8496a3cd6e32929efdb5f80fbcd68bCAS | 19723761PubMed |
Illing N, Klak C, Johnson C, Brito D, Negrao N, Baine F, van Kets V, Ramchurn KR, Seoighe C, Roden L (2009) Duplication of the Asymmetric Leaves1/Rough Sheath 2/Phantastica (ARP) gene precedes the explosive radiation of the Ruschioideae. Development Genes and Evolution 219, 331–338.
| Duplication of the Asymmetric Leaves1/Rough Sheath 2/Phantastica (ARP) gene precedes the explosive radiation of the Ruschioideae.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXotF2nur0%3D&md5=8d60a7a6fe3b80a7c3d8cf9be7b37d83CAS | 19554349PubMed |
Jaya E, Kubien DS, Jameson PE, Clemens J (2010) Vegetative phase change and photosynthesis in Eucalyptus occidentalis: architectural simplification prolongs juvenile traits. Tree Physiology 30, 393–403.
| Vegetative phase change and photosynthesis in Eucalyptus occidentalis: architectural simplification prolongs juvenile traits.Crossref | GoogleScholarGoogle Scholar | 20100699PubMed |
Jones HG (1992) ‘Plants and microclimate: a quantitative approach to environmental plant physiology.’ 2nd edn. (Cambridge University Press: Cambridge)
Jones CS (1995) Does shade prolong juvenile development? A morphological analysis of leaf shape changes in Cucurbita argyrosperma subsp. sororia (Cucurbitaceae). American Journal of Botany 82, 346–359.
| Does shade prolong juvenile development? A morphological analysis of leaf shape changes in Cucurbita argyrosperma subsp. sororia (Cucurbitaceae).Crossref | GoogleScholarGoogle Scholar |
Jones CS (1999) An essay on juvenility, phase change, and heteroblasty in seed plants. International Journal of Plant Sciences 160, S105–S111.
| An essay on juvenility, phase change, and heteroblasty in seed plants.Crossref | GoogleScholarGoogle Scholar | 10572025PubMed |
Jones CS (2001) The functional correlates of heteroblastic variation in leaves: changes in form and ecophysiology with whole plant ontogeny. Bulletin of the Botanical Society of Argentina 36, 171–184.
Jones CS, Bakker FT, Schlichting CD, Nicotra AB (2009) Leaf shape evolution in the South African genus Pelargonium L’ Her. (Geraniaceae). Evolution 63, 479–497.
| Leaf shape evolution in the South African genus Pelargonium L’ Her. (Geraniaceae).Crossref | GoogleScholarGoogle Scholar | 19154370PubMed |
Jonsson H, Heisler MG, Shapiro BE, Meyerowitz EM, Mjolsness E (2006) An auxin-driven polarized transport model for phyllotaxis. Proceedings of the National Academy of Sciences of the United States of America 103, 1633–1638.
| An auxin-driven polarized transport model for phyllotaxis.Crossref | GoogleScholarGoogle Scholar | 16415160PubMed |
Karban R, Thaler JS (1999) Plant phase change and resistance to herbivory. Ecology 80, 510–517.
| Plant phase change and resistance to herbivory.Crossref | GoogleScholarGoogle Scholar |
Kawamura E, Horiguchi G, Tsukaya H (2010) Mechanisms of leaf tooth formation in Arabidopsis. The Plant Journal 62, 429–441.
| Mechanisms of leaf tooth formation in Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXmtFygsLw%3D&md5=b5992a26d3085b76787e9a9f033c3191CAS | 20128880PubMed |
Kay KM, Sargent RD (2009) The role of animal pollination in plant speciation: Integrating ecology, geography, and genetics. Annual Review of Ecology, Evolution, and Systematics 40, 637–656.
| The role of animal pollination in plant speciation: Integrating ecology, geography, and genetics.Crossref | GoogleScholarGoogle Scholar |
Kenrick P, Crane PR (1997) The origin and early evolution of plants on land. Nature 389, 33–39.
| The origin and early evolution of plants on land.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXlvVClt7w%3D&md5=d93e61ecdb74fab1a46826f78c06e377CAS |
Kerstetter RA, Poethig RS (1998) The specification of leaf identity during shoot development. Annual Review of Cell and Developmental Biology 14, 373–398.
| The specification of leaf identity during shoot development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXnvFyisr8%3D&md5=0d3da51ff980875f00e72df935ec4b31CAS | 9891788PubMed |
Kidner CA (2010) The many roles of small RNAs in leaf development. Journal of Genetics and Genomics 37, 13–21.
| The many roles of small RNAs in leaf development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXisVGnsLY%3D&md5=b4e699c145c1c148cd7827579009d0d6CAS | 20171574PubMed |
Kim GT, Tsukaya H, Saito Y, Uchimiya H (1999) Changes in the shapes of leaves and flowers upon overexpression of cytochrome P450 in Arabidopsis. Proceedings of the National Academy of Sciences of the United States of America 96, 9433–9437.
| Changes in the shapes of leaves and flowers upon overexpression of cytochrome P450 in Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXltVCjsLw%3D&md5=3d9c27e2f6f10d43306ab5bf03401118CAS | 10430960PubMed |
Kim M, McCormick S, Timmermans M, Sinha N (2003) The expression domain of PHANTASTICA determines leaflet placement in compound leaves. Nature 424, 438–443.
| The expression domain of PHANTASTICA determines leaflet placement in compound leaves.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXls1aqsbY%3D&md5=106faaf242eca593364775b3cdd4017aCAS | 12879073PubMed |
Kleiman D, Aarssen LW (2007) The leaf size/number trade-off in trees. Journal of Ecology 95, 376–382.
| The leaf size/number trade-off in trees.Crossref | GoogleScholarGoogle Scholar |
Kowalski EA, Dilcher DL (2003) Warmer paleotemperatures for terrestrial ecosystems. Proceedings of the National Academy of Sciences of the United States of America 100, 167–170.
| Warmer paleotemperatures for terrestrial ecosystems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXktlOgsA%3D%3D&md5=5e3d0830c5a47ee05066f4280763d2a7CAS | 12493844PubMed |
Kubien DS, Jaya E, Clemens J (2007) Differences in the structure and gas exchange physiology of juvenile and adult leaves in Metrosideros excelsa. International Journal of Plant Sciences 168, 563–570.
| Differences in the structure and gas exchange physiology of juvenile and adult leaves in Metrosideros excelsa.Crossref | GoogleScholarGoogle Scholar |
Kuwabara A, Nagata T (2006) Cellular basis of developmental plasticity observed in heterophyllous leaf formation of Ludwigia arcuata (Onagraceae). Planta 224, 761–770.
| Cellular basis of developmental plasticity observed in heterophyllous leaf formation of Ludwigia arcuata (Onagraceae).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XosFWktrc%3D&md5=675fa90bf575a6c3a76863813cbe5767CAS | 16557398PubMed |
Lake JA, Quick WP, Beerling DJ, Woodward FI (2001) Plant development: signals from mature to new leaves. Nature 411, 154
| Plant development: signals from mature to new leaves.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXjslGltLg%3D&md5=3938b79dc0bc684acd2251401a22c501CAS | 11346781PubMed |
Latimer AM, Silander JA, Cowling RM (2005) Neutral ecological theory reveals isolation and rapid speciation in a biodiversity hot spot. Science 309, 1722–1725.
| Neutral ecological theory reveals isolation and rapid speciation in a biodiversity hot spot.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXpvFCis78%3D&md5=419bb2a08055e373c3b3e87a7834a8e8CAS | 16151011PubMed |
Lee YK, Kim GT, Kim IJ, Park J, Kwak SS, Choi G, Chung WI (2006) LONGIFOLIA1 and LONGIFOLIA2, two homologous genes, regulate longitudinal cell elongation in Arabidopsis. Development 133, 4305–4314.
| LONGIFOLIA1 and LONGIFOLIA2, two homologous genes, regulate longitudinal cell elongation in Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtlSmt7rL&md5=4b924bf89ade23b626a4c1cda9959b26CAS | 17038516PubMed |
Leigh A, Zwieniecki MA, Rockwell FE, Boyce CK, Nicotra AB, Holbrook NM (2011) Structural and physiological correlates of heterophylly in Ginkgo biloba L. New Phytologist 189, 459–470.
| Structural and physiological correlates of heterophylly in Ginkgo biloba L.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3M%2FpsVSlsA%3D%3D&md5=2caf97c5a691f2647fa7f25ef9b4db26CAS | 20880226PubMed |
Levitt J (1980) ‘Responses of plants to environmental stresses.’ 2nd edn. (Academic Press: New York)
Lewis MC (1972) Physiological significance of variation in leaf structure. Science Progress 60, 25–51.
Linder HP (2005) Evolution of diversity: the Cape flora. Trends in Plant Science 10, 536–541.
| Evolution of diversity: the Cape flora.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtFOmtLzE&md5=b4bcfa764561b32a4e1e3ab608cdf32eCAS | 16213780PubMed |
Little SA, Kembel SW, Wilf P (2010) Paleotemperature proxies from leaf fossils reinterpreted in light of evolutionary history. PLoS ONE 5, e15161
| Paleotemperature proxies from leaf fossils reinterpreted in light of evolutionary history.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXisFWruw%3D%3D&md5=82cfbf502be7573f4406003e94d7613fCAS | 21203554PubMed |
Martinez-Cabrera HI (2010) Influence of climate in functional and species diversification in South African Pelargonium. PhD Thesis, University of Connecticut, Storrs, CT, USA.
McDonald PG, Fonseca CR, Overton JM, Westoby M (2003) Leaf-size divergence along rainfall and soil-nutrient gradients: is the method of size reduction common among clades? Functional Ecology 17, 50–57.
| Leaf-size divergence along rainfall and soil-nutrient gradients: is the method of size reduction common among clades?Crossref | GoogleScholarGoogle Scholar |
Melville R (1969) Leaf venation pattern and origin of angiosperms. Nature 224, 121–125.
| Leaf venation pattern and origin of angiosperms.Crossref | GoogleScholarGoogle Scholar |
Monteith JL, Unsworth MH (1990) ‘Principles of environmental physics.’ 2nd edn. (Edward Arnold: London)
Mueller RJ (1982) Shoot ontogeny and the comparative development of the heteroblastic leaf series in Lygodium japonicum (Thunb.) Sw. Botanical Gazette (Chicago, Ill.) 143, 424–438.
| Shoot ontogeny and the comparative development of the heteroblastic leaf series in Lygodium japonicum (Thunb.) Sw.Crossref | GoogleScholarGoogle Scholar |
Nath U, Crawford BCW, Carpenter R, Coen E (2003) Genetic control of surface curvature. Science 299, 1404–1407.
| Genetic control of surface curvature.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXhsFWrs7k%3D&md5=1e12b98e4a5f9452abdfd55fb8132dceCAS | 12610308PubMed |
Nicotra AB (2010) Leaf size and shape. PrometheusWiki. Available at http://prometheuswiki.publish.csiro.au/tiki-index.php?page=Leaf+size+and+shape [Verified 2 June 2011]
Nicotra AB, Cosgrove MJ, Cowling A, Schlichting CD, Jones CS (2008) Leaf shape linked to photosynthetic rates and temperature optima in South African Pelargonium species. Oecologia 154, 625–635.
| Leaf shape linked to photosynthetic rates and temperature optima in South African Pelargonium species.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2sjhvVGgsw%3D%3D&md5=92d026e5515fe5602c89b6cb1abb9f71CAS | 17943318PubMed |
Niklas KJ (1988) The role of phyllotactic pattern as a developmental constraint on the interception of light by leaf surfaces. Evolution 42, 1–16.
| The role of phyllotactic pattern as a developmental constraint on the interception of light by leaf surfaces.Crossref | GoogleScholarGoogle Scholar |
Niklas KJ (1997) ‘The evolutionary biology of plants.’ (University of Chicago Press: Chicago)
Niklas KJ (2000) The evolution of plant body plans – a biomechanical perspective. Annals of Botany 85, 411–438.
| The evolution of plant body plans – a biomechanical perspective.Crossref | GoogleScholarGoogle Scholar |
Niklas KJ (2004) Plant allometry: is there a grand unifying theory? Biological Reviews of the Cambridge Philosophical Society 79, 871–889.
| Plant allometry: is there a grand unifying theory?Crossref | GoogleScholarGoogle Scholar | 15682874PubMed |
Niklas KJ, Cobb ED (2008) Evidence for ‘diminishing returns’ from the scaling of stem diameter and specific leaf area. American Journal of Botany 95, 549–557.
| Evidence for ‘diminishing returns’ from the scaling of stem diameter and specific leaf area.Crossref | GoogleScholarGoogle Scholar | 21632381PubMed |
Niklas KJ, Cobb ED (2010) Ontogenetic changes in the numbers of short- v. long-shoots account for decreasing specific leaf area in Acer rubrum (Aceraceae) as trees increase in size. American Journal of Botany 97, 27–37.
| Ontogenetic changes in the numbers of short- v. long-shoots account for decreasing specific leaf area in Acer rubrum (Aceraceae) as trees increase in size.Crossref | GoogleScholarGoogle Scholar | 21622364PubMed |
Niklas KJ, Cobb ED, Niinemets U, Reich PB, Sellin A, Shipley B, Wright IJ (2007) ‘Diminishing returns’ in the scaling of functional leaf traits across and within species groups. Proceedings of the National Academy of Sciences of the United States of America 104, 8891–8896.
| ‘Diminishing returns’ in the scaling of functional leaf traits across and within species groups.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXmt1Wgt7g%3D&md5=94ff1e52fdd3642b9cb0b6337e6a55b1CAS | 17502616PubMed |
Niklas KJ, Cobb ED, Spatz H-C (2009) Predicting the allometry of leaf surface area and dry mass. American Journal of Botany 96, 531–536.
| Predicting the allometry of leaf surface area and dry mass.Crossref | GoogleScholarGoogle Scholar | 21628208PubMed |
Nobel PS (1988) ‘Environmental biology of Agaves and Cacti.’ (Cambridge University Press: Cambridge)
Oguchi R, Hikosaka K, Hirose T (2005) Leaf anatomy as a constraint for photosynthetic acclimation: differential responses in leaf anatomy to increasing growth irradiance among three deciduous trees. Plant, Cell & Environment 28, 916–927.
| Leaf anatomy as a constraint for photosynthetic acclimation: differential responses in leaf anatomy to increasing growth irradiance among three deciduous trees.Crossref | GoogleScholarGoogle Scholar |
Okada K, Ueda J, Komaki MK, Bell CJ, Shimura Y (1991) Requirement of the auxin polar transport-system in early stages of Arabidopsis floral bud formation. The Plant Cell 3, 677–684.
Parkhurst DF, Loucks OL (1972) Optimal leaf size in relation to environment. Journal of Ecology 60, 505–537.
| Optimal leaf size in relation to environment.Crossref | GoogleScholarGoogle Scholar |
Parkhurst DF, Duncan PR, Gates DM, Kreith F (1968) Wind-tunnel modelling of convection of heat between air and broad leaves of plants. Agricultural Meteorology 5, 33–47.
| Wind-tunnel modelling of convection of heat between air and broad leaves of plants.Crossref | GoogleScholarGoogle Scholar |
Pasquet-Kok J, Creese C, Sack L (2010) Turning over a new ‘leaf’: multiple functional significances of leaves versus phyllodes in Hawaiian Acacia koa. Plant, Cell & Environment 33, 2084–2100.
| Turning over a new ‘leaf’: multiple functional significances of leaves versus phyllodes in Hawaiian Acacia koa.Crossref | GoogleScholarGoogle Scholar | 20636491PubMed |
Peppe DJ, Royer DL, Cariglino B, Oliver SY, Newman S, et al (2011) Sensitivity of leaf size and shape to climate: global patterns and paleoclimatic applications. New Phytologist 190, 724–739.
| Sensitivity of leaf size and shape to climate: global patterns and paleoclimatic applications.Crossref | GoogleScholarGoogle Scholar | 21294735PubMed |
Perez-Perez JM, Serrano-Cartagena J, Micol JL (2002) Genetic analysis of natural variations in the architecture of Arabidopsis thaliana vegetative leaves. Genetics 162, 893–915.
Perez-Perez JM, Candela H, Robles P, Quesada V, Ponce MR, Micol JL (2009) Lessons from a search for leaf mutants in Arabidopsis thaliana. The International Journal of Developmental Biology 53, 1623–1634.
| Lessons from a search for leaf mutants in Arabidopsis thaliana.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXjtVyks7o%3D&md5=8a34ebd111ea99fafd4dc3ed1c572ed1CAS | 19247929PubMed |
Poethig RS, Sussex IM (1985a) The cellular-parameters of leaf development in tobacco – a clonal analysis. Planta 165, 170–184.
| The cellular-parameters of leaf development in tobacco – a clonal analysis.Crossref | GoogleScholarGoogle Scholar |
Poethig RS, Sussex IM (1985b) The developmental morphology and growth dynamics of the tobacco leaf. Planta 165, 158–169.
| The developmental morphology and growth dynamics of the tobacco leaf.Crossref | GoogleScholarGoogle Scholar |
Pray TR (1955) Foliar venation of angiosperms. 2. Histogenesis of the venation of Liriodendron. American Journal of Botany 42, 18–27.
| Foliar venation of angiosperms. 2. Histogenesis of the venation of Liriodendron.Crossref | GoogleScholarGoogle Scholar |
Pray TR (1960) Ontogeny of the open dichotomous venation in the pinna of the fern Nephrolepis. American Journal of Botany 47, 319–328.
| Ontogeny of the open dichotomous venation in the pinna of the fern Nephrolepis.Crossref | GoogleScholarGoogle Scholar |
Pryer KM, Hearn DJ (2009) Evolution of leaf form in Marsileaceous ferns: evidence for heterochrony. Evolution 63, 498–513.
| Evolution of leaf form in Marsileaceous ferns: evidence for heterochrony.Crossref | GoogleScholarGoogle Scholar | 19154361PubMed |
Raschke K (1960) Heat transfer between the plant and the environment. Annual Review of Plant Physiology and Plant Molecular Biology 11, 111–126.
Raunkiaer C (1934) ‘The life forms of plants and statistical plant geography.’ (Clarendon Press: Oxford)
Raven JA (1996) Into the voids: the distribution, function, development and maintenance of gas spaces in plants. Annals of Botany 78, 137–142.
| Into the voids: the distribution, function, development and maintenance of gas spaces in plants.Crossref | GoogleScholarGoogle Scholar |
Roth-Nebelsick A (2001) Computer-based analysis of steady-state and transient heat transfer of small-sized leaves by free and mixed convection. Plant, Cell & Environment 24, 631–640.
| Computer-based analysis of steady-state and transient heat transfer of small-sized leaves by free and mixed convection.Crossref | GoogleScholarGoogle Scholar |
Royer DL, Wilf P (2006) Why do toothed leaves correlate with cold climates? Gas exchange at leaf margins provides new insights into a classic paleotemperature proxy. International Journal of Plant Sciences 167, 11–18.
| Why do toothed leaves correlate with cold climates? Gas exchange at leaf margins provides new insights into a classic paleotemperature proxy.Crossref | GoogleScholarGoogle Scholar |
Royer DL, Wilf P, Janesko DA, Kowalski EA, Dilcher DL (2005) Correlations of climate and plant ecology to leaf size and shape: potential proxies for the fossil record. American Journal of Botany 92, 1141–1151.
| Correlations of climate and plant ecology to leaf size and shape: potential proxies for the fossil record.Crossref | GoogleScholarGoogle Scholar | 21646136PubMed |
Royer DL, Kooyman RM, Wilf P (2009a) Ecology of leaf teeth: a multi-site analysis from an Australian subtropical rainforest. American Journal of Botany 96, 738–750.
| Ecology of leaf teeth: a multi-site analysis from an Australian subtropical rainforest.Crossref | GoogleScholarGoogle Scholar | 21628229PubMed |
Royer DL, Meyerson LA, Robertson KM, Adams JM (2009b) Phenotypic plasticity of leaf shape along a temperature gradient in Acer rubrum. PLoS ONE 4, e7653
| Phenotypic plasticity of leaf shape along a temperature gradient in Acer rubrum.Crossref | GoogleScholarGoogle Scholar | 19893620PubMed |
Sack L, Frole K (2006) Leaf structural diversity is related to hydraulic capacity in tropical rain forest trees. Ecology 87, 483–491.
| Leaf structural diversity is related to hydraulic capacity in tropical rain forest trees.Crossref | GoogleScholarGoogle Scholar | 16637372PubMed |
Sack L, Holbrook NM (2006) Leaf hydraulics. Annual Review of Plant Biology 57, 361–381.
| Leaf hydraulics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XosVKhtrs%3D&md5=a29ff2260a6d0acbec987a640491d44cCAS | 16669766PubMed |
Sack L, Tyree MT (2005) Leaf hydraulics and its implications in plant structure and function. In ‘Vascular transport in plants.’ (Eds NM Holbrook, MA Zwieniecki) pp. 93–114. (Elsevier Academic Press: Burlington, MA, USA)
Sack L, Cowan PD, Jaikumar N, Holbrook NM (2003) The ‘hydrology’ of leaves: co-ordination of structure and function in temperate woody species. Plant, Cell & Environment 26, 1343–1356.
| The ‘hydrology’ of leaves: co-ordination of structure and function in temperate woody species.Crossref | GoogleScholarGoogle Scholar |
Sack L, Streeter CM, Holbrook NM (2004) Hydraulic analysis of water flow through leaves of sugar maple and red oak. Plant Physiology 134, 1824–1833.
| Hydraulic analysis of water flow through leaves of sugar maple and red oak.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXjsFKmtrY%3D&md5=a1852439547a36d1cfe1d6ceabf4d384CAS | 15064368PubMed |
Sakakibara K, Nishiyama T, Kato M, Hasebe M (2001) Isolation of homeodomain-leucine zipper genes from the moss Physcomitrella patens and the evolution of homeodomain-leucine zipper genes in land plants. Molecular Biology and Evolution 18, 491–502.
Sanders H, Rothwell GW, Wyatt S (2007) Paleontological context for the developmental mechanisms of evolution. International Journal of Plant Sciences 168, 719–728.
| Paleontological context for the developmental mechanisms of evolution.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXntVCmtr4%3D&md5=6d5940153a7a2d1882e30cf5050f35cdCAS |
Sarojam R, Sappl PG, Goldshmidt A, Efroni I, Floyd SK, Eshed Y, Bowman JL (2010) Differentiating Arabidopsis shoots from leaves by combined YABBY activities. The Plant Cell 22, 2113–2130.
| Differentiating Arabidopsis shoots from leaves by combined YABBY activities.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsVKrtLbL&md5=81f15ad8fbd4a03a4d6e9e3b979fbcbeCAS | 20628155PubMed |
Sauquet H, Weston PH, Anderson CL, Barker NP, Cantrill DJ, Mast AR, Savolainen V (2009) Contrasted patterns of hyperdiversification in Mediterranean hotspots. Proceedings of the National Academy of Sciences of the United States of America 106, 221–225.
| Contrasted patterns of hyperdiversification in Mediterranean hotspots.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXltF2ksw%3D%3D&md5=f0a63ba67d3b6e8778afe24c0b9bf5c3CAS | 19116275PubMed |
Schmid M, Davison TS, Henz SR, Pape UJ, Demar M, Vingron M, Scholkopf B, Weigel D, Lohmann JU (2005) A gene expression map of Arabidopsis thaliana development. Nature Genetics 37, 501–506.
| A gene expression map of Arabidopsis thaliana development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXjsF2ksrg%3D&md5=693c8a9c22e41f46bebb9288a7a6ff87CAS | 15806101PubMed |
Schuepp PH (1993) Tansley Review No. 59. Leaf boundary layers. New Phytologist 125, 477–507.
| Tansley Review No. 59. Leaf boundary layers.Crossref | GoogleScholarGoogle Scholar |
Sculthorpe CD (1967) Vegetative polymorphism and the problem of heterophylly. In ‘The biology of aquatic vascular plants’. pp. 218–247. (Koeltz Scientific Books: Konigstein, West Germany)
Shalit A, Rozman A, Goldshmidt A, Alvarez JP, Bowman JL, Eshed Y, Lifschitz E (2009) The flowering hormone florigen functions as a general systemic regulator of growth and termination. Proceedings of the National Academy of Sciences of the United States of America 106, 8392–8397.
| The flowering hormone florigen functions as a general systemic regulator of growth and termination.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXmsl2nsLo%3D&md5=f846465347784c495535da86541a8e2fCAS | 19416824PubMed |
Sharkey TD (2005) Effects of moderate heat stress on photosynthesis: importance of thylakoid reactions, rubisco deactivation, reactive oxygen species, and thermotolerance provided by isoprene. Plant, Cell & Environment 28, 269–277.
| Effects of moderate heat stress on photosynthesis: importance of thylakoid reactions, rubisco deactivation, reactive oxygen species, and thermotolerance provided by isoprene.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXislKqs78%3D&md5=e59e1da7e0ac4b2cadca3f2ca99ac2aeCAS |
Smith WK, Nobel PS (1977) Temperature and water relations for sun and shade leaves of a desert broadleaf, Hyptis emoryi. Journal of Experimental Botany 28, 169–183.
| Temperature and water relations for sun and shade leaves of a desert broadleaf, Hyptis emoryi.Crossref | GoogleScholarGoogle Scholar |
Specht RL, Specht A (1999) ‘Australian plant communities. Dynamics of structure, growth and biodiversity.’ (Oxford University Press: Oxford)
Street NR, Sjödin A, Bylesjö M, Gustafsson P, Trygg J, Jansson S (2008) A cross-species transcriptomics approach to identify genes involved in leaf development. BMC Genomics 9, 589
| A cross-species transcriptomics approach to identify genes involved in leaf development.Crossref | GoogleScholarGoogle Scholar | 19061504PubMed |
Taiz L, Zeiger E (2002) ‘Plant physiology.’ 3rd edn. (Sinauer Associates: Sunderland, MA, USA)
Thoday D (1931) The significance of reduction in the size of leaves. Journal of Ecology 19, 297–303.
| The significance of reduction in the size of leaves.Crossref | GoogleScholarGoogle Scholar |
Thom AS (1968) Exchange of momentum mass and heat between an artificial leaf and airflow in a wind tunnel. Quarterly Journal of the Royal Meteorological Society 94, 44–55.
| Exchange of momentum mass and heat between an artificial leaf and airflow in a wind tunnel.Crossref | GoogleScholarGoogle Scholar |
Thomas PW, Woodward FI, Quick WP (2004) Systemic irradiance signalling in tobacco. New Phytologist 161, 193–198.
| Systemic irradiance signalling in tobacco.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXmsVWksw%3D%3D&md5=8b18c34e4a22e4484fea85d1e5f1cdc3CAS |
Tomescu AMF (2009) Megaphylls, microphylls and the evolution of leaf development. Trends in Plant Science 14, 5–12.
| Megaphylls, microphylls and the evolution of leaf development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXntVygsw%3D%3D&md5=ab3b8290bb5c8f48e51ab66b31ef2abaCAS | 19070531PubMed |
Tsukaya H (2006) Mechanism of leaf-shape determination. Annual Review of Plant Biology 57, 477–496.
| Mechanism of leaf-shape determination.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XosVKht74%3D&md5=e6be7c1f30c979cf9b1bd1e2f213a3fbCAS | 16669771PubMed |
Tsukaya H (2010) Leaf development and evolution. Journal of Plant Research 123, 3–6.
| Leaf development and evolution.Crossref | GoogleScholarGoogle Scholar | 19946725PubMed |
Tsukaya H, Shoda K, Kim GT, Uchimiya H (2000) Heteroblasty in Arabidopsis thaliana (L.) Heynh. Planta 210, 536–542.
| Heteroblasty in Arabidopsis thaliana (L.) Heynh.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXhsFenuro%3D&md5=1511ac744521ab67c486e4fbaf0c5ec0CAS | 10787046PubMed |
Usami T, Horiguchi G, Yano S, Tsukaya H (2009) The more and smaller cells mutants of Arabidopsis thaliana identify novel roles for SQUAMOSA PROMOTER BINDING PROTEIN-LIKE genes in the control of heteroblasty. Development 136, 955–964.
| The more and smaller cells mutants of Arabidopsis thaliana identify novel roles for SQUAMOSA PROMOTER BINDING PROTEIN-LIKE genes in the control of heteroblasty.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXksFWrurc%3D&md5=40e1af96f24a56998eefb42d68352f1eCAS | 19211679PubMed |
van der Niet T, Johnson SD, Linder HP (2006) Macroevolutionary data suggest a role for reinforcement in pollination system shifts. Evolution 60, 1596–1601.
| Macroevolutionary data suggest a role for reinforcement in pollination system shifts.Crossref | GoogleScholarGoogle Scholar | 17017060PubMed |
Vogel S (1968) ‘Sun leaves’ and ‘shade leaves’: differences in convective heat dissipation. Ecology 49, 1203–1204.
| ‘Sun leaves’ and ‘shade leaves’: differences in convective heat dissipation.Crossref | GoogleScholarGoogle Scholar |
Vogel S (1970) Convective cooling at low airspeeds and the shapes of broad leaves. Journal of Experimental Botany 21, 91–101.
| Convective cooling at low airspeeds and the shapes of broad leaves.Crossref | GoogleScholarGoogle Scholar |
von Goethe JW (2009) ‘The metamorphosis of plants’. (Reprinted by MIT Press: Cambridge, MA, USA) Originally published in 1790.
Wagner WH (1952) Types of foliar dichotomy in living ferns. American Journal of Botany 39, 578–592.
| Types of foliar dichotomy in living ferns.Crossref | GoogleScholarGoogle Scholar |
Waites R, Hudson A (1995) Phantastica – a gene required for dorsoventrality of leaves in Antirrhinum majus. Development 121, 2143–2154.
Weston PH (1995) Athertonia. In ‘Flora of Australia. Vol. 16’. Ed. P McCarthy) pp. 413–415. (CSIRO Publishing: Melbourne)
Weston PH (2007) ‘Proteaceae in the families and genera of vascular plants. Vol. 9. Flowering plants – Eudicots’. (Volume Eds C Jeffrey, JW Kadereit, Series Ed. K Kubitzki) (Springer: Berlin)
Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, et al (2004) The worldwide leaf economics spectrum. Nature 428, 821–827.
| The worldwide leaf economics spectrum.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXjt1Crt74%3D&md5=82cfce166589ece51b55eaf3f99e661cCAS | 15103368PubMed |
Wu G, Park MY, Conway SR, Wang JW, Weigel D, Poethig RS (2009) The sequential action of miR156 and miR172 regulates developmental timing in Arabidopsis. Cell 138, 750–759.
| The sequential action of miR156 and miR172 regulates developmental timing in Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsVCjs7rK&md5=001c7a6dd2681bc475722177e8e10088CAS | 19703400PubMed |
Yamaguchi T, Tsukaya H (2010) Evolutionary and developmental studies of unifacial leaves in monocots: Juncus as a model system. Journal of Plant Research 123, 35–41.
| Evolutionary and developmental studies of unifacial leaves in monocots: Juncus as a model system.Crossref | GoogleScholarGoogle Scholar | 19693435PubMed |
Yamaguchi T, Yano S, Tsukaya H (2010) Genetic framework for flattened leaf blade formation in unifacial leaves of Juncus prismatocarpus. The Plant Cell 22, 2141–2155.
| Genetic framework for flattened leaf blade formation in unifacial leaves of Juncus prismatocarpus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsVKrtLbF&md5=71f4a338480258884431b6c6fc310828CAS | 20647346PubMed |
Yang D, Li G, Sun S (2008) The generality of leaf size versus number trade-off in temperate woody species. Annals of Botany 102, 623–629.
| The generality of leaf size versus number trade-off in temperate woody species.Crossref | GoogleScholarGoogle Scholar | 18682438PubMed |
Yano S, Terashima I (2001) Separate localization of light signal perception for sun or shade type chloroplast and palisade tissue differentiation in Chenopodium album. Plant & Cell Physiology 42, 1303–1310.
| Separate localization of light signal perception for sun or shade type chloroplast and palisade tissue differentiation in Chenopodium album.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XkslOq&md5=d160b4a5d747cbe61d761c05d33548aaCAS | 11773522PubMed |
Yano S, Terashima I (2004) Developmental process of sun and shade leaves in Chenopodium album L. Plant, Cell & Environment 27, 781–793.
| Developmental process of sun and shade leaves in Chenopodium album L.Crossref | GoogleScholarGoogle Scholar |
Yapp RH (1912) Spirea ulmaria, L., and its bearing on the problem of xeromorphy in marsh plants. Annals of Botany 26, 815–870.
Yates MJ, Verboom GA, Rebelo AG, Cramer MD (2010) Ecophysiological significance of leaf size variation in Proteaceae from the Cape Floristic Region. Functional Ecology 24, 485–492.
| Ecophysiological significance of leaf size variation in Proteaceae from the Cape Floristic Region.Crossref | GoogleScholarGoogle Scholar |
Zhong RQ, Ye ZH (1999) IFL1, a gene regulating interfascicular fiber differentiation in Arabidopsis, encodes a homeodomain-leucine zipper protein. The Plant Cell 11, 2139–2152.
Zurakowski KA, Gifford EM (1988) Quantitative studies of pinnule development in the ferns Adiantum raddianum and Cheilanthes viridis. American Journal of Botany 75, 1559–1570.
| Quantitative studies of pinnule development in the ferns Adiantum raddianum and Cheilanthes viridis.Crossref | GoogleScholarGoogle Scholar |
Zwieniecki MA, Melcher PJ, Boyce CK, Sack L, Holbrook NM (2002) Hydraulic architecture of leaf venation in Laurus nobilis L. Plant, Cell & Environment 25, 1445–1450.
| Hydraulic architecture of leaf venation in Laurus nobilis L.Crossref | GoogleScholarGoogle Scholar |
Zwieniecki MA, Boyce CK, Holbrook NM (2004a) Functional design space of single-veined leaves: role of tissue hydraulic properties in constraining leaf size and shape. Annals of Botany 94, 507–513.
| Functional design space of single-veined leaves: role of tissue hydraulic properties in constraining leaf size and shape.Crossref | GoogleScholarGoogle Scholar | 15319225PubMed |
Zwieniecki MA, Boyce CK, Holbrook NM (2004b) Hydraulic limitations imposed by crown placement determine final size and shape of Quercus rubra L. leaves. Plant, Cell & Environment 27, 357–365.
| Hydraulic limitations imposed by crown placement determine final size and shape of Quercus rubra L. leaves.Crossref | GoogleScholarGoogle Scholar |
Zwieniecki MA, Stone HA, Leigh A, Boyce CK, Holbrook NM (2006) Hydraulic design of pine needles: one-dimensional optimization for single-vein leaves. Plant, Cell & Environment 29, 803–809.
| Hydraulic design of pine needles: one-dimensional optimization for single-vein leaves.Crossref | GoogleScholarGoogle Scholar | 17087464PubMed |


