Proteinaceous necrotrophic effectors in fungal virulence
Kar-Chun Tan A B D , Richard P. Oliver B , Peter S. Solomon C and Caroline S. Moffat AA Australian Centre for Necrotrophic Fungal Pathogens, Murdoch University, SABC, Murdoch, WA 6150, Australia.
B Australian Centre for Necrotrophic Fungal Pathogens, Curtin University, Bentley, WA 6845, Australia.
C Division of Plant Sciences, Research School of Biology, The Australian National University, Canberra, ACT 0200, Australia.
D Corresponding author. Email: kar-chun.tan@curtin.edu.au
Functional Plant Biology 37(10) 907-912 https://doi.org/10.1071/FP10067
Submitted: 29 March 2010 Accepted: 10 August 2010 Published: 23 September 2010
Abstract
The host–pathogen interface can be considered as a biological battlefront. Molecules produced by both the pathogen and the host are critical factors determining the outcome of the interaction. Recent studies have revealed that an increasing number of necrotrophic fungal pathogens produce small proteinaceous effectors that are able to function as virulence factors. These molecules can cause tissue death in host plants that possess dominant sensitivity genes, leading to subsequent pathogen colonisation. Such effectors are only found in necrotrophic fungi, yet their roles in virulence are poorly understood. However, several recent key studies of necrotrophic effectors from two wheat (Triticum aestivum L.) pathogens, Pyrenophora tritici-repentis (Died.) Drechs. and Stagonospora nodorum (Berk.) Castell. & Germano, have shed light upon how these effector proteins serve to disable the host from the inside out.
Additional keywords: host-selective toxin, net blotch, Pyrenophora, septoria, Stagonospora, tan spot.
Introduction
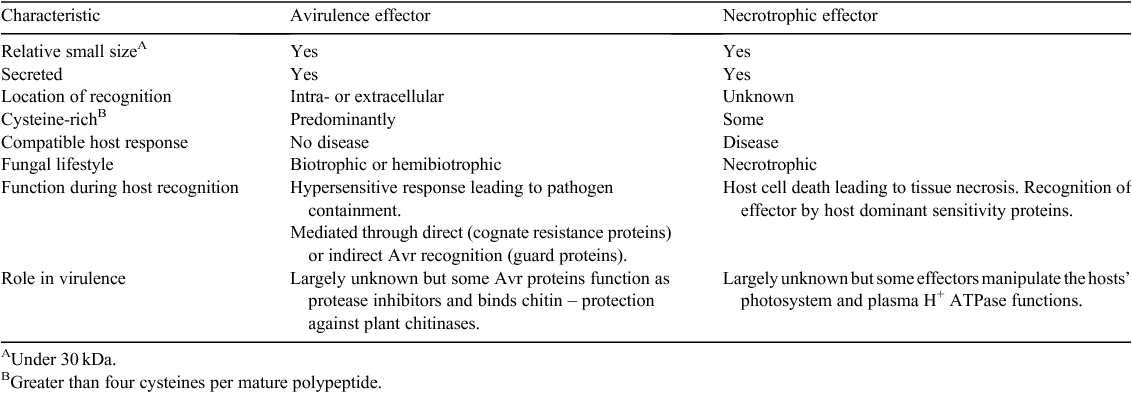
Necrotrophic fungi were traditionally considered as non-host-specific pathogens that use a large array of cell wall-degrading enzymes and non-specific toxins for pathogenicity (Hammond-Kosack and Rudd 2008). It is now known that some of these necrotrophs possess an arsenal of effectors used to disable susceptible hosts ahead of colonisation. Necrotrophic effectors share some common properties with the avirulence (Avr) effectors of biotrophic fungal pathogens (reviewed elsewhere in this issue) (Table 1). However, in contrast to the classical gene-for-gene hypothesis, where the interaction of avirulence effectors with host resistance (R)-gene complexes leads to resistance, necrotrophic effectors function in an ‘inverse’ manner. An interaction between a necrotrophic effector and the product of a host dominant sensitivity gene leads instead to disease (Fig. 1).
|

|
Necrotrophic effectors are a diverse group of molecules that induce tissue death in host plants possessing the appropriate genotype. They are typically small and can be proteinaceous in nature or secondary metabolites. Examples of metabolite-based effectors include victorin from Cochliobolus victoriae Nelson and AAL-toxin from Alternaria alternate (Fr. : Fr.) Keissl., and these have been extensively reviewed elsewhere (Walton 1996; Wolpert et al. 2002). The purpose of this review will focus on discussing recently discovered proteinaceous necrotrophic effectors and their roles in virulence.
Effectors of Pyrenophora tritici f. sp. repentis
Pyrenophora tritici-repentis (Died.) Drechs. is the causal agent of tan spot (previously called yellow spot or yellow leaf spot), a devastating disease of wheat (Triticum aestivum L.). To date, two proteinaceous effectors of this necrotroph have been identified, PtrToxA and PtrToxB, which are encoded by the genes PtrToxA and PtrToxB.
PtrToxA was the first effector to be isolated and is the best characterised. The PtrToxA protein is the product of a single copy gene that is present in ~80% of a worldwide collection of isolates (Friesen et al. 2006). PtrToxA is a small (13.2 kDa) secreted protein that causes necrosis in sensitive wheat genotypes (Ballance et al. 1989; Tomas et al. 1990; Tuori et al. 1995). Ciuffetti et al. (1997) demonstrated that the PtrToxA gene is both necessary and sufficient for the pathogenicity of P. tritici-repentis, since transformation of a non-pathogenic P. tritici-repentis isolate with the PtrToxA gene was sufficient to render that isolate pathogenic on PtrToxA-sensitive wheat lines.
Wheat sensitivity to the PtrToxA effector is conditioned by Tsn1, a single gene present on the long arm of chromosome 5B (Faris et al. 1996; Anderson et al. 1999). The Tsn1 gene has very recently been cloned and found to possess nucleotide-binding site (NBS), leucine-rich repeat (LRR) and serine/threonine protein kinase domains, all of which are necessary for PtrToxA sensitivity (Faris et al. 2010). Paradoxically, these domains are common features of plant disease R genes involved in defence against biotrophic pathogens (Martin et al. 2003).
Parallels can be drawn with the victorin effector of C. victoriae, the causal agent of Victoria blight disease in oats (Avena sativa L.) (Meehan and Murphy 1946). The C. victoriae susceptibility gene has been identified in Arabidopsis thaliana (L.) Heynh. as LOV1, which, like Tsn1, has a NBS-LRR structure and mediates responses associated with biotrophic disease resistance (Lorang et al. 2007). However, victorin rapidly induces resistance-like physiology in oats, including a respiratory burst and apoptotic-like cell death (Wolpert et al. 2002). Yet how can the elicitation of a ‘resistance’ response lead to disease susceptibility? What seems at first to be a contradiction in terms could in fact be accounted for by the lifestyle of the pathogen. Thus, an environment that would be unfavourable to biotrophic pathogens (such as programmed cell death generated by the plant host) would actually be favourable to pathogens with a necrotrophic lifestyle. This suggests that R genes could paradoxically play a role in disease susceptibility by serving as targets for necrotrophic effectors such as PtrToxA.
Several recent studies have helped to unravel the mode of action of PtrToxA. Cytological analyses have revealed that PtrToxA is rapidly internalised into the mesophyll cells of sensitive wheat cultivars (Manning and Ciuffetti 2005; Manning et al. 2008). However, the protein or proteins with which PtrToxA interacts at the cell membrane remain unidentified. Although Tsn1 is necessary to mediate PtrToxA recognition, yeast two-hybrid experiments suggest that Tsn1 does not interact directly with PtrToxA, nor does it possess any apparent transmembrane domains (Faris et al. 2010).
Analysis of the mature PtrToxA protein sequence has demonstrated the presence of an arginyl-glycyl-aspartic (RGD) motif present at the surface of the PtrToxA protein (Zhang et al. 1997), which is required for PtrToxA internalisation (Manning and Ciuffetti 2005; Manning et al. 2008). This sequence is located on a solvent-exposed loop and is easily accessible for protein–protein interactions (Sarma et al. 2005b). In animals, the RGD motif is involved in the binding of extracellular matrix proteins to transmembrane integrin proteins (Ruoslahti and Pierschbacher 1986; D’Souza et al. 1991). These integrins have been utilised by many mammalian pathogens as adhesion sites and as binding sites for effectors (Isberg and Tran Van Nhieu 1994). Thus, it is it conceivable that PtrToxA internalisation relies on recognition of the RGD motif by a plant integrin-like protein receptor. Indeed, integrin-like proteins have been identified in plants (Faik et al. 1998; Labouré et al. 1999; Nagpal and Quatrano 1999; Swatzell et al. 1999; Sun et al. 2000; Baluska et al. 2003) and may provide candidates for mediating PtrToxA internalisation.
Much of what occurs once PtrToxA is internalised is still unknown. However, there is evidence to suggest that the action of PtrToxA is associated with photosynthetic pathways (Manning et al. 2009). For example, once inside the cell, the chloroplast appears to be a target for PtrToxA. In vitro experiments suggest that PtrToxA is able to interact with the chloroplast-localised protein called ToxA-binding protein 1 (ToxABP1), homologues of which have been found across several plant species (Sarma et al. 2005a; Manning et al. 2007). Although the precise function of ToxABP1 is unknown, it has been suggested that it plays a part in photosystem function or thylakoid formation (Wang et al. 2004; Keren et al. 2005). Indeed, PtrToxA treatment has been demonstrated to induce changes in PSI and PSII, leading to light-dependent accumulation of reactive oxygen species (ROS) in the chloroplast (Manning et al. 2009). The link between PtrToxA and photosynthesis is further supported by the light-dependent nature of PtrToxA-induced necrosis and the tight regulation of Tsn1 transcription by both the circadian clock and light (Manning and Ciuffetti 2005; Faris et al. 2010).
Two independent studies have examined the global transcriptional changes induced by PtrToxA on sensitive wheat cultivars (Adhikari et al. 2009; Pandelova et al. 2009). Both studies illustrate that considerable transcriptional reprogramming occurs following PtrToxA treatment. Numerous defence-related host genes were upregulated at both early and late time points, including those associated with the phenylpropanoid pathway, lignification and ROS production, as well as genes functioning in signal transduction. Taken together, these studies suggest that PtrToxA disrupts photosynthetic electron transport, leading to ROS accumulation and plant cell death upon light exposure, thus creating an environment in which necrotrophic pathogens may thrive.
Another effector from P. tritici-repentis that has been characterised is PtrToxB. Like PtrToxA, PtrToxB is also a small secreted protein (6.6 kDa) which causes chlorosis on sensitive wheat genotypes and is encoded by a multicopy gene, PtrToxB (Orolaza et al. 1995; Strelkov et al. 1999; Martinez et al. 2001). Although not as prevalent as PtrToxA, PtrToxB has been found to be produced by several isolates around the world (Ali and Francl 2003; Friesen et al. 2005; Lamari et al. 2005). Wheat sensitivity is conditioned by the dominant Tsc2 gene, which has been mapped to the short arm of chromosome 2B (Strelkov et al. 1999; Friesen and Faris 2004). Unlike most effectors, PtrToxB homologues have been found across a broad range of plant pathogenic ascomycetes, suggesting that it may have arisen in an early ancestor of the Ascomycota (Andrie et al. 2008). However, whether PtrToxB and its homologues play a role in plant–microbe interactions is yet to be elucidated.
Effectors of Stagonospora nodorum
Stagonospora nodorum (Berk.) Castell & Germano is the causal agent of stagonospora (previously septoria) nodorum blotch (SNB) in wheat (Solomon et al. 2006). Evidence of necrotrophic effectors produced by S. nodorum was first reported by Keller et al. (1994) using wheat embryos. Genes encoding effector proteins have only been identified and characterised recently (Friesen et al. 2006; Liu et al. 2009).
SnToxA was the first reported necrotrophic effector gene identified in S. nodorum (Friesen et al. 2006). A BLAST search of the S. nodorum genome sequence with PtrToxA identified an almost identical gene. Further genome exploration has revealed that SnToxA is located within a highly conserved genomic region of 11 kb that is present in both organisms. This ‘transfercon’ was hypothesised to be acquired by P. tritici-repentis from S. nodorum through lateral gene transfer, a biological process previously thought to be uniquely prokaryotic. This hypothesis is supported by several key pieces of evidence. Firstly, SNB has been known since the 1800s whilst tan spot was described as recently as 1941. Prior to this, P. tritici-repentis was described as a saprophyte. Secondly, ToxA has only been found in these two organisms to date. Finally, the nucleotide sequence of SnToxA exhibits greater diversity in its polypeptide sequence than that of PtrToxA. Taken together, this strongly suggests that ToxA was acquired by P. tritici-repentis before 1941 (Friesen et al. 2006; Stukenbrock and McDonald 2007). The identification of SnToxA in S. nodorum highlights the importance of genome sequencing in effector discovery (Hane et al. 2007). SnToxA and PtrToxA possess the same mode of action. Both effectors cause necrosis on wheat carrying Tsn1 in a light-dependent manner (Manning and Ciuffetti 2005; Friesen et al. 2006).
The identification of SnToxA provided an opportunity to study the role of this gene in fungal virulence, as unlike P. tritici-repentis, S. nodorum is genetically tractable. Several lines of evidence have been published confirming that SnToxA interacts (directly or indirectly) with Tsn1. Firstly, S. nodorum strains lacking SnToxA were non-pathogenic on Tsn1 wheat varieties (Friesen et al. 2006). Secondly, protein extracts from SnToxA-expressing S. nodorum strains induced necrosis on Tsn1 wheat, whilst extracts from SntoxA lines did not (Friesen et al. 2006). Lastly, transformation of an avirulent ToxA-deficient wild-type strain of S. nodorum with PtrToxA allowed the fungus to become virulent and cause necrosis on Tsn1 wheat lines (Friesen et al. 2006).
SnTox3 was the second necrotrophic effector gene identified in S. nodorum. SnTox3 was first reported as a partially purified protein that caused necrosis on wheat carrying the Snn3 dominant sensitivity gene, which is located on the short arm of chromosome 5 (Friesen et al. 2008b; Liu et al. 2009). Gene knockout analysis of SnTox3 indicated it to be a critical component in S. nodorum virulence on Snn3 wheat. The introduction of SnTox3 into an avirulent SnTox3-deficient S. nodorum wild-type strain allowed it to infect and cause necrosis on Snn3 wheat varieties. Whilst detailed mechanistic studies have yet to be undertaken, SnTox3 appears to be functionally different to SnToxA. SnTox3 is cysteine-rich, a characteristic typically associated with several described biotrophic avirulence effectors (Van den Ackerveken et al. 1993; Catanzariti et al. 2006). Also, unlike SnToxA, SnTox3 does not require light to induce necrosis on Snn3 wheat. This suggests a different mode of function compared with SnToxA. Gene expression analysis indicates that SnTox3 is upregulated during the early stage of infection, coinciding with host penetration (Liu et al. 2009). SnToxA also showed a similar expression profile during infection (Ipcho and Oliver, unpubl. data).
These studies imply that these effectors function to disable host cells during the early stage of infection. Thus, the invading fungus will have a readily accessible nutrient supply during infection (Solomon et al. 2003).
S. nodorum also possesses at least three other proteinaceous necrotrophic effectors. These are SnTox1, SnTox2 and SnTox4. However, genes that code for these proteins have yet to be identified and therefore, the extent of their involvement in fungal virulence cannot be fully gauged (Liu et al. 2004; Friesen et al. 2007; Reddy et al. 2008; Abeysekara et al. 2009). The wheat genes that confer sensitivity to these effectors are Snn1, Snn2 and Snn4, respectively. The use of molecular marker-based quantitative trait locus (QTL) analysis of various mapping populations of wheat has led to the identification of major QTLs in wheat chromosome arms 1BS (Snn1), 2DS (Snn2) and 1AS (Snn4) that accounted for up to 58%, 47% and 41% in disease variations, respectively (Friesen et al. 2008a; Abeysekara et al. 2009).
Effectors of other necrotrophic fungi
Proteinaceous effectors from other prominent necrotrophic fungi have also been recently identified. Alternaria brassicae (Berk.) Sacc. is a pathogen of the Brassicaceae. Evidence that this pathogen produces necrotrophic effectors was reported by Parada et al. (2008). Semi-purified protein fractions were shown to contain a 27.5 kDa protein, Abr-toxin, which is able to cause necrosis on cabbage (Brassica oleracea L.) and oilseed (Brassica napus L.). Abr-toxin caused no necrosis on the non-brassica tomato (Lycopersicon esculentum Mill.). Coinoculation of the Abr-toxin and an avirulent isolate of A. alternata resulted in infectious symptoms on the host leaf similar to A. brassicae. Partial protein sequencing revealed that the Abr-toxin possesses amino acid sequence similarities to the protease trypsin.
Pyrenophora teres f. sp. teres and f. sp. maculata Drechs. cause net-form net blotch and spot-form net blotch in barley (Hordeum vulgare L.), respectively. Using protein chromatographic techniques, Sarpeleh et al. (2007) demonstrated that both pathogens produce proteinaceous effectors that are between 20 and 100 kDa. These semi-purified effectors induce strong necrosis on a barley variety that is susceptible to both fungi, but caused a weak reaction in a resistant line of barley. Like PtrToxA and SnToxA, these effectors require light to cause necrosis on the host plant (Sarpeleh et al. 2008). The identity of these effectors from both P. teres subspecies is currently unknown.
Corynespora cassiicola (Berk. & Curtis) Wei, a serious pathogen of rubber trees (Hevea brasiliensis Müll. Arg.), produces a cysteine-rich necrotrophic effector called cassiicolin. The effector is able to cause necrosis on detached rubber tree leaves and on other host plants such as tobacco (Nicotiana tabacum L.) and soy (Glycine max (L.) Merr. (Barthe et al. 2007; de Lamotte et al. 2007). Although the deduced effector amino acid sequence did not show significant homology with other proteins, structural analysis indicates that the protein structure resembles trypsin-like inhibitors (Barthe et al. 2007).
The fungus Rhynchosporium secalis (Oudem.) Davis is the causal agent of barley scald. Several small cysteine-rich proteins designated as Nip1, -2 and -3 were identified in R. secalis, and these are capable of causing necrosis on a broad range of plants. Nip1 and 3 has been shown to stimulate barley plasma H+ ATPase, which may be the likely cause of host tissue necrosis (Wevelsiep et al. 1991, 1993). Nip1 has recently been shown to bind to a single unidentified receptor that triggers the plant’s defence response (van’t Slot et al. 2007). In addition, Nip1 also functions as an avirulence effector on barley varieties that possess the uncloned Rrs1 gene (Rohe et al. 1995). A total of 14 Nip1 forms were identified, three of which are associated with a gain in virulence on Rrs1 barley (Schürch et al. 2004).
Conclusion
Necrotrophic fungi were, up until recently, considered as simplistic pathogens that rely on a plethora of non-host-specific mechanisms to storm the host. Recent seminal discoveries of host-selective necrotrophic effectors have revealed a new level of pathogenic complexity. These breakthroughs highlight that necrotrophic fungi possess the ability to disable their host selectively from within before an effective defence response can be mounted. The mode of action of these effectors is largely unknown, although studies on both ToxA proteins clearly demonstrate that the host metabolism is disabled before cell death. Hence, host-selective effectors are paramount for these fungi to live a necrotrophic lifestyle.
Abeysekara NS,
Friesen TL,
Keller B, Faris JD
(2009) Identification and characterization of a novel host-toxin interaction in the wheat–Stagonospora nodorum pathosystem. Theoretical and Applied Genetics 120, 117–126.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Adhikari TB,
Bai J,
Meinhardt SW,
Gurung S,
Myrfield M,
Patel J,
Ali S,
Gudmestad NC, Rasmussen JB
(2009)
Tsn1-mediated host responses to ToxA from Pyrenophora tritici-repentis. Molecular Plant—Microbe Interactions 22, 1056–1068.
| Crossref | GoogleScholarGoogle Scholar |

Ali S, Francl LJ
(2003) Population race structure of Pyrenophora tritici-repentis prevalent on wheat and noncereal grasses in the Great Plains. Plant Disease 87, 418–422.
| Crossref | GoogleScholarGoogle Scholar |

Anderson JA,
Effertz RJ,
Faris JD,
Francl LJ,
Meinhardt SW, Gill BS
(1999) Genetic analysis of sensitivity to a Pyrenophora tritici-repentis necrosis-inducing toxin in durum and common wheat. Phytopathology 89, 293–297.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Andrie RM,
Schoch CL,
Hedges R,
Spatafora JW, Ciuffetti LM
(2008) Homologs of ToxB, a host-selective toxin gene from Pyrenophora tritici-repentis, are present in the genome of sister-species Pyrenophora bromi and other members of the Ascomycota. Fungal Genetics and Biology 45, 363–377.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Ballance GM,
Lamari L, Bernier CC
(1989) Purification and characterization of a host-selective necrosis toxin from Pyrenophora tritici-repentis. Physiological and Molecular Plant Pathology 35, 203–213.
| Crossref | GoogleScholarGoogle Scholar |

Baluska F,
Samaj J,
Wojtaszek P,
Volkmann D, Menzel D
(2003) Cytoskeleton-plasma membrane-cell wall continuum in plants. Emerging links revisited. Plant Physiology 133, 482–491.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Barthe P,
Pujade-Renaud V,
Breton F,
Gargani D,
Thai R,
Roumestand C, de Lamotte F
(2007) Structural analysis of cassiicolin, a host-selective protein toxin from Corynespora cassiicola. Journal of Molecular Biology 367, 89–101.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Catanzariti AM,
Dodds PN,
Lawrence GJ,
Ayliffe MA, Ellis JG
(2006) Haustorially expressed secreted proteins from flax rust are highly enriched for avirulence elicitors. The Plant Cell 18, 243–256.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Ciuffetti LM,
Tuori RP, Gaventa JM
(1997) A single gene encodes a selective toxin causal to the development of tan spot of wheat. The Plant Cell 9, 135–144.
| Crossref |
PubMed |

D’Souza SE,
Ginsberg MH, Plow EF
(1991) Arginyl-glycyl-aspartic acid (RGD): a cell adhesion motif. Trends in Biochemical Sciences 16, 246–250.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

de Lamotte F,
Duviau MP,
Sanier C,
Thai R,
Poncet J,
Bieysse D,
Breton F, Pujade-Renaud V
(2007) Purification and characterization of cassiicolin, the toxin produced by Corynespora cassiicola, causal agent of the leaf fall disease of rubber tree. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences 849, 357–362.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Faik A,
Laboure AM,
Gulino D,
Mandaron P, Falconet D
(1998) A plant surface protein sharing structural properties with animal integrins. European Journal of Biochemistry 253, 552–559.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Faris JD,
Anderson JA,
Francl LJ, Jordahl JG
(1996) Chromosomal location of a gene conditioning insensitivity in wheat to a necrosis-inducing culture filtrate from Pyrenophora tritici-repentis. Phytopathology 86, 459–463.
| Crossref | GoogleScholarGoogle Scholar |

Faris JD,
Zhang Z,
Lu H,
Lu S, Reddy L ,
et al
.
(2010) A unique wheat disease resistance-like gene governs effector-triggered susceptibility to necrotrophic pathogens. Proceedings of the National Academy of Sciences of the United States of America 107, 13544–13549.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Friesen TL, Faris JD
(2004) Molecular mapping of resistance to Pyrenophora tritici-repentis race 5 and sensitivity to Ptr ToxB in wheat. Theoretical and Applied Genetics 109, 464–471.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Friesen TL,
Ali S,
Klein KK, Rasmussen JB
(2005) Population genetic analysis of a global collection of Pyrenophora tritici-repentis, a causal agent of tan spot of wheat. Phytopathology 95, 1144–1150.
| Crossref |
PubMed |

Friesen TL,
Stukenbrock EH,
Liu ZH,
Meinhardt S, Ling H ,
et al
.
(2006) Emergence of a new disease as a result of interspecific virulence gene transfer. Nature Genetics 38, 953–956.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Friesen TL,
Meinhardt SW, Faris JD
(2007) The Stagonospora nodorum–wheat pathosystem involves multiple proteinaceous host-selective toxins and corresponding host sensitivity genes that interact in an inverse gene-for-gene manner. The Plant Journal 51, 681–692.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Friesen TL,
Faris JD,
Solomon PS, Oliver RP
(2008a) Host-specific toxins: effectors of necrotrophic pathogenicity. Cellular Microbiology 10, 1421–1428.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Friesen TL,
Zhang Z,
Solomon PS,
Oliver RP, Faris JD
(2008b) Characterization of the interaction of a novel Stagonospora nodorum host-selective toxin with a wheat susceptibility gene. Plant Physiology 146, 682–693.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Hammond-Kosack KE, Rudd JJ
(2008) Plant resistance signalling hijacked by a necrotrophic fungal pathogen. Plant Signaling & Behavior 3, 993–995.
| PubMed |

Hane JK,
Lowe RG,
Solomon PS,
Tan KC, Schoch CL ,
et al
.
(2007) Dothideomycete plant interactions illuminated by genome sequencing and EST analysis of the wheat pathogen Stagonospora nodorum. The Plant Cell 19, 3347–3368.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Isberg RR, Tran Van Nhieu G
(1994) Binding and internalisation of micro-organisms by integrin receptors. Trends in Microbiology 2, 10–14.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Keller B,
Winzeler H,
Winzeler M, Fried PM
(1994) Differential sensitivity of wheat embryos against extracts containing toxins of Septoria nodorum: first steps towards in vitro selection. Journal of Phytopathology 141, 233–240.
| Crossref | GoogleScholarGoogle Scholar |

Keren N,
Ohkawa H,
Welsh EA,
Liberton M, Pakrasi HB
(2005) Psb29, a conserved 22-kD protein, functions in the biogenesis of Photosystem II complexes in Synechocystis and Arabidopsis. The Plant Cell 17, 2768–2781.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Labouré AM,
Faik A,
Mandaron P, Falconet D
(1999) RGD-dependent growth of maize calluses and immunodetection of an integrin-like protein. FEBS Letters 442, 123–128.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Lamari L,
Strelkov SE,
Yahyaoui A,
Amedov M,
Saidov M,
Djunusova M, Koichibayev M
(2005) Virulence of Pyrenophora tritici-repentis in the countries of the Silk Road. Canadian Journal of Plant Pathology 27, 383–388.
| Crossref |

Liu ZH,
Faris JD,
Meinhardt SW,
Ali S,
Rasmussen JB, Friesen TL
(2004) Genetic and physical mapping of a gene conditioning sensitivity in wheat to a partially purified host-selective toxin produced by Stagonospora nodorum. Phytopathology 94, 1056–1060.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Liu Z,
Faris JD,
Oliver RP,
Tan KC, Solomon PS ,
et al
.
(2009) SnTox3 acts in effector triggered susceptibility to induce disease on wheat carrying the Snn3 gene. PLoS Pathogens 5, e1000581.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Lorang JM,
Sweat TA, Wolpert TJ
(2007) Plant disease susceptibility conferred by a “resistance” gene. Proceedings of the National Academy of Sciences of the United States of America 104, 14861–14866.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Manning VA, Ciuffetti LM
(2005) Localization of Ptr ToxA produced by Pyrenophora tritici-repentis reveals protein import into wheat mesophyll cells. The Plant Cell 17, 3203–3212.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Manning VA,
Hardison LK, Ciuffetti LM
(2007) Ptr ToxA interacts with a chloroplast-localized protein. Molecular Plant—Microbe Interactions 20, 168–177.
| Crossref | GoogleScholarGoogle Scholar |

Manning VA,
Hamilton SM,
Karplus PA, Ciuffetti LM
(2008) The Arg-Gly-Asp-containing, solvent-exposed loop of Ptr ToxA is required for internalization. Molecular Plant—Microbe Interactions 21, 315–325.
| Crossref | GoogleScholarGoogle Scholar |

Manning VA,
Chu AL,
Steeves JE,
Wolpert TJ, Ciuffetti LM
(2009) A host-selective toxin of Pyrenophora tritici-repentis, Ptr ToxA, induces photosystem changes and reactive oxygen species accumulation in sensitive wheat. Molecular Plant—Microbe Interactions 22, 665–676.
| Crossref | GoogleScholarGoogle Scholar |

Martin GB,
Bogdanove AJ, Sessa G
(2003) Understanding the functions of plant disease resistance proteins. Annual Review of Plant Biology 54, 23–61.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Martinez JP,
Ottum SA,
Ali S,
Franci LJ, Ciuffetti LM
(2001) Characterization of the ToxB gene from Pyrenophora tritici-repentis. Molecular Plant—Microbe Interactions 14, 675–677.
| Crossref | GoogleScholarGoogle Scholar |

Meehan F, Murphy HC
(1946) A new Helminthosporium blight of oats. Science 104, 413–414.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Nagpal P, Quatrano RS
(1999) Isolation and characterization of a cDNA clone from Arabidopsis thaliana with partial sequence similarity to integrins. Gene 230, 33–40.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Orolaza NP,
Lamari L, Ballance GM
(1995) Evidence of a host-specific chlorosis toxin from Pyrenophora tritici-repentis, the causal agent of tan spot of wheat. Phytopathology 85, 1282–1287.
| Crossref | GoogleScholarGoogle Scholar |

Pandelova I,
Betts MF,
Manning VA,
Wilhelm LJ,
Mockler TC, Ciuffetti LM
(2009) Analysis of transcriptome changes induced by Ptr ToxA in wheat provides insights into the mechanisms of plant susceptibility. Molecular Plant 2, 1067–1083.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Parada RY,
Sakuno E,
Mori N,
Oka K,
Egusa M,
Kodama M, Otani H
(2008)
Alternaria brassicae produces a host-specific protein toxin from germinating spores on host leaves. Phytopathology 98, 458–463.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Reddy L,
Friesen TL,
Meinhardt SW,
Chao S, Faris JD
(2008) Genomic analysis of the Snn1 locus on wheat chromosome arm 1BS and the identification of candidate genes. The Plant Genome 1, 55–66.
| Crossref | GoogleScholarGoogle Scholar |

Rohe M,
Gierlich A,
Hermann H,
Hahn M,
Schmidt B,
Rosahl S, Knogge W
(1995) The race-specific elicitor, NIP1, from the barley pathogen, Rhynchosporium secalis, determines avirulence on host plants of the Rrs1 resistance genotype. The EMBO Journal 14, 4168–4177.
| PubMed |

Ruoslahti E, Pierschbacher MD
(1986) Arg-gly-asp: a versatile cell recognition signal. Cell 44, 517–518.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Sarma GN,
Manning VA,
Ciuffetti LM, Karplus PA
(2005a) Structure of Ptr ToxA: an RGD-containing host-selective toxin from Pyrenophora tritici-repentis. The Plant Cell 17, 3190–3202.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Sarma GN,
Manning VA,
Ciuffetti LM, Karplus PA
(2005b) Structure of Ptr ToxA: an RGD-containing host-selective toxin from Pyrenophora tritici-repentis. The Plant Cell 17, 3190–3202.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Sarpeleh A,
Wallwork H,
Catcheside DE,
Tate ME, Able AJ
(2007) Proteinaceous metabolites from Pyrenophora teres contribute to symptom development of barley net blotch. Phytopathology 97, 907–915.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Sarpeleh A,
Wallwork H,
Tate ME,
Catcheside DEA, Able AJ
(2008) Initial characterization of phytotoxic proteins isolated from Pyrenophora teres. Physiological and Molecular Plant Pathology 72, 73–79.
| Crossref | GoogleScholarGoogle Scholar |

Schürch S,
Linde CC,
Knogge W,
Jackson LF, McDonald BA
(2004) Molecular population genetic analysis differentiates two virulence mechanisms of the fungal avirulence gene NIP1. Molecular Plant—Microbe Interactions 17, 1114–1125.
| Crossref | GoogleScholarGoogle Scholar |

Solomon PS,
Tan K-C, Oliver RP
(2003) The nutrient supply of pathogenic fungi; a fertile field for study. Molecular Plant Pathology 4, 203–210.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Solomon PS,
Lowe RGT,
Tan K-C,
Waters ODC, Oliver RP
(2006)
Stagonospora nodorum: cause of stagonospora nodorum blotch of wheat. Molecular Plant Pathology 7, 147–156.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Strelkov SE,
Lamari L, Ballance GM
(1999) Characterization of a host-specific protein toxin (Ptr ToxB) from Pyrenophora tritici-repentis. Molecular Plant—Microbe Interactions 12, 728–732.
| Crossref | GoogleScholarGoogle Scholar |

Stukenbrock EH, McDonald BA
(2007) Geographical variation and positive diversifying selection in the host specific toxin SnToxA. Molecular Plant Pathology 8, 321–332.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Sun Y,
Qian H,
Xu XD,
Han Y,
Yen LF, Sun DY
(2000) Integrin-like proteins in the pollen tube: detection, localization and function. Plant & Cell Physiology 41, 1136–1142.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Swatzell LJ,
Edelmann RE,
Makaroff CA, Kiss JZ
(1999) Integrin-like proteins are localized to plasma membrane fractions, not plastids, in Arabidopsis. Plant & Cell Physiology 40, 173–183.
| PubMed |

Tomas A,
Feng GH,
Reeck GR,
Bockus WW, Leach JE
(1990) Purification of a cultivar-specific toxin from Pyrenophora tritici-repentis, causal agent of tan spot of wheat. Molecular Plant—Microbe Interactions 3, 221–224.

Tuori RP,
Wolpert TJ, Ciuffetti LM
(1995) Purification and immunological characterization of toxic components from cultures of Pyrenophora tritici-repentis. Molecular Plant—Microbe Interactions 8, 41–48.

Van den Ackerveken GF,
Van Kan JA,
Joosten MH,
Muisers JM,
Verbakel HM, De Wit PJ
(1993) Characterization of two putative pathogenicity genes of the fungal tomato pathogen Cladosporium fulvum. Molecular Plant—Microbe Interactions 6, 210–215.

van’t Slot KA,
Gierlich A, Knogge W
(2007) A single binding site mediates resistance- and disease-associated activities of the effector protein NIP1 from the barley pathogen Rhynchosporium secalis. Plant Physiology 144, 1654–1666.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Walton JD
(1996) Host-selective toxins: agents of compatibility. The Plant Cell 8, 1723–1733.
| Crossref |
PubMed |

Wang Q,
Sullivan RW,
Kight A,
Henry RL,
Huang J,
Jones AM, Korth KL
(2004) Deletion of the chloroplast-localized Thylakoid formation1 gene product in Arabidopsis leads to deficient thylakoid formation and variegated leaves. Plant Physiology 136, 3594–3604.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Wevelsiep L,
Kogel KH, Knogge W
(1991) Purification and characterization of peptides from Rhynchosporium secalis inducing necrosis in barley. Physiological and Molecular Plant Pathology 39, 471–482.
| Crossref | GoogleScholarGoogle Scholar |

Wevelsiep L,
Rupping E, Knogge W
(1993) Stimulation of barley plasmalemma H+-ATPase by phytotoxic peptides from the fungal pathogen Rhynchosporium secalis. Plant Physiology 101, 297–301.
| PubMed |

Wolpert TJ,
Dunkle LD, Ciuffetti LM
(2002) Host-selective toxins and avirulence determinants: what’s in a name? Annual Review of Phytopathology 40, 251–285.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Zhang HF,
Francl LJ,
Jordahl JG, Meinhardt SW
(1997) Structural and physical properties of a necrosis-inducing toxin from Pyrenophora tritici-repentis. Phytopathology 87, 154–160.
| Crossref | GoogleScholarGoogle Scholar | PubMed |



