The central role of the VERNALIZATION1 gene in the vernalization response of cereals
Ben TrevaskisA CSIRO Division of Plant Industry, GPO Box 1600, Canberra, ACT 2601, Australia. Email: ben.trevaskis@csiro.au
This paper originates from the Peter Goldacre Award 2009 of the Australian Society of Plant Scientists that was received by the author.
Functional Plant Biology 37(6) 479-487 https://doi.org/10.1071/FP10056
Submitted: 12 March 2010 Accepted: 18 April 2010 Published: 20 May 2010
Abstract
Many varieties of wheat (Triticum spp.) and barley (Hordeum vulgare L.) require prolonged exposure to cold during winter in order to flower (vernalization). In these cereals, vernalization-induced flowering is controlled by the VERNALIZATION1 (VRN1) gene. VRN1 is a promoter of flowering that is activated by low temperatures. VRN1 transcript levels increase gradually during vernalization, with longer cold treatments inducing higher expression levels. Elevated VRN1 expression is maintained in the shoot apex and leaves after vernalization, and the level of VRN1 expression in these organs determines how rapidly vernalized plants flower. Some alleles of VRN1 are expressed without vernalization due to deletions or insertions within the promoter or first intron of the VRN1 gene. Varieties of wheat and barley with these alleles flower without vernalization and are grown where vernalization does not occur. The first intron of the VRN1 locus has histone modifications typically associated with the maintenance of an inactive chromatin state, suggesting this region is targeted by epigenetic mechanisms that contribute to repression of VRN1 before winter. Other mechanisms are likely to act elsewhere in the VRN1 gene to mediate low-temperature induction. This review examines how understanding the mechanisms that regulate VRN1 provides insights into the biology of vernalization-induced flowering in cereals and how this will contribute to future cereal breeding strategies.
Additional keywords: barley, flowering, wheat.
Vernalization-induced flowering
Plants growing in temperate regions time flowering to coincide with favourable seasonal conditions. Winter frost can damage cold-sensitive floral organs, whereas heat and water stress during summer can reduce fertility, so flowering often occurs in spring when conditions are optimal (see King and Heide 2009). One cue that promotes spring flowering is prolonged exposure to cold during winter, or vernalization. Vernalization occurs in many plants (see Chouard 1960; Amasino 2004; King and Heide 2009) but the focus of this review is the molecular mechanisms that control vernalization-induced flowering in economically important cereal crops such as wheat (Triticum aestivum L.) and barley (Hordeum vulgare L.).
The vernalization response in cereals
In temperate regions, wheat and barley are sown in autumn then over-winter before flowering in spring. When sown in spring, these same cereal varieties typically show delayed flowering or fail to flower altogether. Several researchers recognised that the cold of winter is a critical factor required to trigger flowering of these plants and that this is lacking when plants are sown in spring (see McKinney 1940; Chouard 1960). For example, Gassner showed that germinating wheat or rye (Secale cereale M. Bieb.) seeds at normal growth temperatures can cause a strong delay of flowering, whereas germination at low temperatures can stimulate flowering (Gassner 1918). He concluded that many cereals have a requirement for cold, or “Kaltbedürfnis”, which must be satisfied to allow flowering (Gassner 1918). This phenomenon later came to be referred to as vernalization (vernalis meaning “pertaining to spring”; see Chouard 1960).
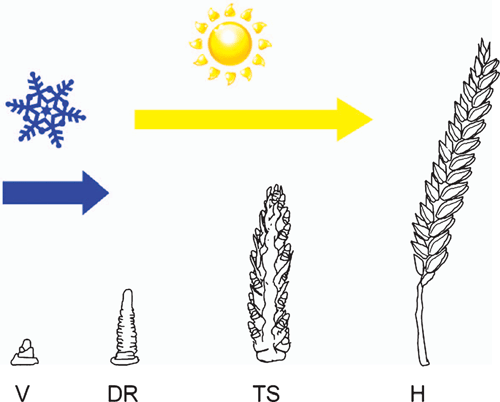
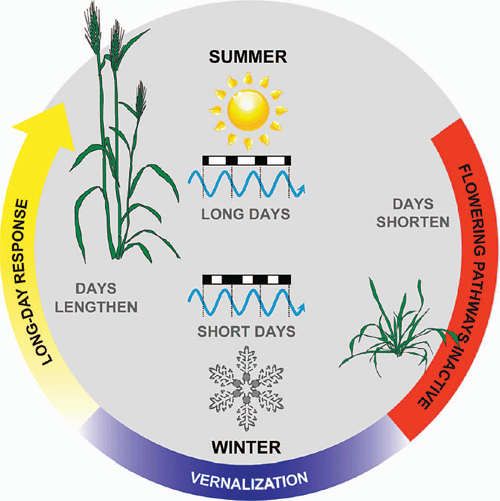
Vernalization promotes the transition to reproductive development at the shoot apex (Flood and Halloran 1984). Thus, after vernalization, the production of leaf primordia ceases and floral primordial appear at the shoot apex (Bonnet 1935, 1936; Zadoks et al. 1974). These develop into inflorescence branches that bear the florets (flowers) (Bonnet 1935, 1936; Zadoks et al. 1974). Vernalization is sufficient to trigger the transition to reproductive development, but long days are then required for rapid inflorescence development and stem elongation (Purvis 1934; Gott et al. 1955) (Fig. 1). Critically, vernalization is a prerequisite for the acceleration of flowering by long days, so long days cause rapid flowering only after plants have been vernalized (Purvis 1934). This combination of vernalization requirement and daylength sensitivity ensures that flowering is delayed until after winter to avoid frost damage but then occurs rapidly as daylength increases during spring, thereby avoiding heat and water stress during summer (Fig. 2). A similar combination of vernalization requirement and daylength sensitivity is found in many plants from temperate zones (see Thomas and Vince-Prue 1997; King and Heide 2009).
|
|
The effect of vernalization is cumulative, so increasing durations of cold accelerate flowering to greater extents until a point when the vernalization response is saturated (Gott et al. 1955). Acceleration of flowering by vernalization is also temperature dependent and, typically, there is an optimal temperature for vernalization between 0 and 10°C that will saturate the vernalization response more rapidly than warmer or colder temperatures (Gassner 1918; Chouard 1960). Thus, vernalization triggers a quantitative flowering response in cereals, and the effectiveness of vernalization is both time and temperature dependent.
Vernalization is remembered
When cells from vernalized wheat are used to regenerate plants through tissue culture, the resulting plants do not require vernalization to flower (Marcińska et al. 1995). Similarly, wheat cells can be vernalized during tissue culture to give rise to plants that flower rapidly with no further requirement for vernalization (Whelan and Schaalje 1992). Furthermore, maturing seeds on the spike (inflorescence) can be exposed to prolonged cold and then allowed to undergo the normal process of seed drying and germination to give rise to seedlings that flower without vernalization (Gregory and Purvis 1936). These observations suggest that cereals retain a cellular memory of vernalization. The idea that plants retain a memory of vernalization is consistent with the way vernalization promotes flowering: when germinating seeds are exposed to prolonged cold, there are no visible signs of floral development at the end of vernalization, but plants undergo rapid floral development when placed at normal growth temperatures after vernalization (Purvis 1934; Flood and Halloran 1984; Sasani et al. 2009). So exposure to cold is remembered and this exerts an after-effect during subsequent development (Chouard 1960). After plants flower, the memory of vernalization is presumably reset in seeds to allow the vernalization response to reoccur in the next generation.
The molecular basis of vernalization-induced flowering in cereals
The VERNALIZATION1 gene (VRN1) controls vernalization-induced flowering in cereals (see Trevaskis et al. 2007a; Distelfeld et al. 2009). VRN1 encodes a MADS box transcription factor (a class of transcription factor named after the archetypal genes MCM1, AGAMOUS, DEFICIENS, SRF1) related to genes that promote flowering in other plant species (Danyluk et al. 2003; Trevaskis et al. 2003; Yan et al. 2003). VRN1 transcripts are present at low basal levels but increase during prolonged cold treatment (Danyluk et al. 2003; Trevaskis et al. 2003; Yan et al. 2003). This response is quantitative, with longer cold treatments inducing higher transcript levels (Danyluk et al. 2003; Yan et al. 2003; von Zitzewitz et al. 2005; Sasani et al. 2009). This parallels the degree to which flowering is accelerated by increasing lengths of cold treatment (Sasani et al. 2009). Thus, it seems likely that VRN1 is a cold-activated promoter of flowering that mediates vernalization-induced flowering in cereals. The activity of VRN1 might be essential for flowering in cereals, since a mutant of Einkorn wheat (Triticum monococcum) that lacks VRN1 is unable to flower (Shitsukawa et al. 2007). However, a recent study shows that the non-flowering phenotype of this mutant might also be partly due to deletion of genes flanking VRN1 (Distelfeld and Dubcovsky 2010).
VRN1 acts at the shoot apex and in leaves to promote flowering
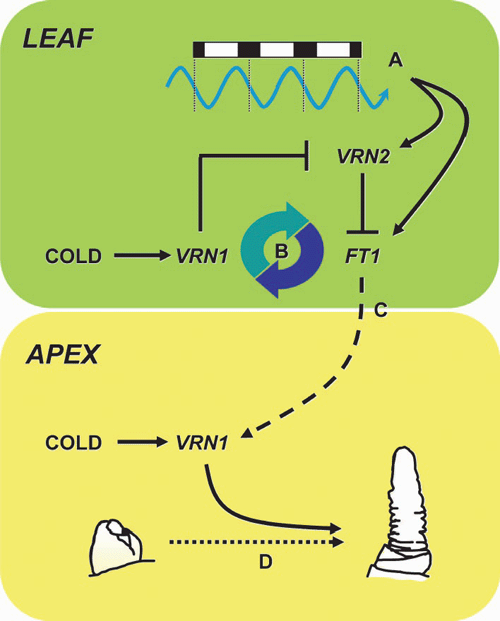
Vernalization activates expression of VRN1 in the leaf and shoot apex, and elevated expression of VRN1 is maintained in these tissues following vernalization (Yan et al. 2003; Sasani et al. 2009). VRN1 is likely to have distinct functions in each of these organs. At the shoot apex, expression of VRN1 is likely to promote the transition to reproductive development, so the production of leaves ceases and inflorescence development begins (Gocal et al. 2001; Loukoianov et al. 2005; Hemming et al. 2008; Preston and Kellogg 2008; Sasani et al. 2009) (Fig. 3). VRN1-like genes play similar roles in promoting phase transition in other plants, including Arabidopsis thaliana (L.) Heynh (Ferrándiz et al. 2000). In the leaves, expression of VRN1 unlocks the long-day flowering response, allowing increasing daylength to accelerate flowering in spring (Trevaskis et al. 2007a; Hemming et al. 2008; Preston and Kellogg 2008) (Fig. 3).
|
Studies in A. thaliana have shown that FLOWERING LOCUS T (FT) mediates the long-day flowering response (Kardailsky et al. 1999; Kobayashi et al. 1999). Expression of FT is controlled by interactions between the circadian clock and light receptors, such that the FT protein is produced in leaves when daylight extends into the late afternoon and exceeds a critical length (Suárez-López et al. 2001; An et al. 2004; Valverde et al. 2004). The FT protein is then transported to the shoot apex where it promotes floral development (Corbesier et al. 2007; Tamaki et al. 2007). Thus, FT is proposed to be the main component of the ‘florigen’, the leaf-derived signal that triggers flowering at the shoot apex (Zeevaart 2008).
In temperate cereals, the FT-like 1 (FT1) gene (also known as VRN3) seems to play a role similar to that of FT in A. thaliana, and is induced by long days to accelerate flowering (Turner et al. 2005; Yan et al. 2006; Faure et al. 2007). In vernalization-responsive cereals, FT1 is expressed only after plants are vernalized (Yan et al. 2006; Hemming et al. 2008). It seems that expression of VRN1 is required for long-day induction of FT1 (Hemming et al. 2008). VRN1 might promote expression of FT1 via regulatory interactions with VRN2 (Trevaskis et al. 2007a; Hemming et al. 2008). VRN2 is a repressor of flowering that is expressed in leaves in long days (Yan et al. 2004a; Dubcovsky et al. 2006; Trevaskis et al. 2006) and it seems likely that VRN2 delays flowering by suppressing long-day induction of FT1 (Hemming et al. 2008). VRN1 downregulates VRN2 (Loukoianov et al. 2005; Trevaskis et al. 2006), so downregulation of VRN2 by VRN1 provides a mechanism to allow long-day induction of FT1 in the leaves of vernalized plants (Hemming et al. 2008; Sasani et al. 2009) (Fig. 3). It is not known whether the VRN1 protein interacts directly with the VRN2 gene or whether other genes mediate this regulatory interaction.
Active alleles of VRN1 reduce vernalization requirement and adapt crops to different climates
Wheat and barley crops are often grown in regions with mild winter conditions where vernalization does not occur. In other regions, crops are sown in spring to avoid harsh winter conditions and so do not experience vernalization. To adapt crops to these regions, breeders have produced varieties that flower without vernalization (see Chouard 1960). Varieties of wheat and barley that flower without vernalization are referred to as spring types, whereas those that require vernalization to flower are termed winter types.
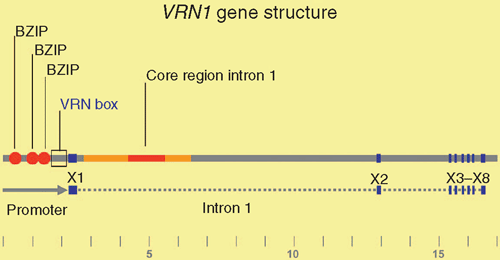
Genetic analyses have shown that VRN1 is a major determinant of the vernalization requirement in wheat and barley (Pugsley 1971; Takahashi and Yasuda 1971). Some alleles of VRN1 are expressed without prior cold treatment and these allow flowering without vernalization (Danyluk et al. 2003; Trevaskis et al. 2003; Yan et al. 2003). Increased expression of VRN1 from these ‘active alleles’ is associated either with insertions and deletions in the promoter (wheat) or with deletions in the first intron (wheat and barley) of the VRN1 gene (Yan et al. 2004b; Fu et al. 2005) (Fig. 4). Presumably, these regions contain sequences required to maintain low levels of VRN1 expression until plants are vernalized.
|
Comparison of the promoter regions from wild-type versus active alleles of VRN1 has identified a critical region near the transcriptional start site that is altered in some active alleles of VRN1 (Pidal et al. 2009). This region might contain a binding site for a repressor protein that is required to maintain VRN1 in an inactive state until plants are vernalized. Alternatively, the conformation of DNA in this region might limit the rate of transcriptional initiation, and mutations might remove this limit.
Studies in barley have shown that the first intron of VRN1 contains a broad region that is required for repression of VRN1 before winter. Around a dozen different alleles of VRN1 have been identified in barley (Fu et al. 2005; von Zitzewitz et al. 2005; Cockram et al. 2007; Szucs et al. 2007; Hemming et al. 2009). Some lack large sections of the ~11 kb first intron, while others lack smaller sections (Fu et al. 2005; von Zitzewitz et al. 2005; Cockram et al. 2007; Szucs et al. 2007; Hemming et al. 2009). Typically, alleles lacking larger sections are more active and are associated with earlier flowering without vernalization, whereas alleles lacking smaller segments are associated with only a moderate increase in VRN1 activity and weaker promotion of flowering (Szucs et al. 2007; Hemming et al. 2009). Comparison of the regions deleted in these different active alleles has identified a core region within the first intron that seems to be important for repression of VRN1 (Hemming et al. 2009). In addition to this core region, sections at the 5′ end of the intron (towards the promoter) also seem to play an important role in maintaining repression of VRN1, and alleles with large deletions that remove both the core region and the 5′ end of the intron typically have the highest activity (Szucs et al. 2007; Hemming et al. 2009). An insertion of a mobile genetic element in the 5′ end of the first intron is also associated with active expression of VRN1 without vernalization (Stockinger et al. 2007; Hemming et al. 2009).
Repressive histone modifications occur within the first intron of VRN1
The state of chromatin (the packaging of DNA and associated histones into higher order structures) appears to be an important determinant of VRN1 activity (Oliver et al. 2009). Analysis of histone modifications at the VRN1 locus in barley plants that have not been vernalized has shown that the chromatin at VRN1 contains a specific histone modification typically associated with an inactive chromatin state and long-term transcriptional repression: histone-3-lysine-27-trimethylation (H3K27Me3) (Oliver et al. 2009). This modification is found within the first intron of VRN1 and also at the promoter (Oliver et al. 2009). Deposition of H3K27Me3 at these sites might contribute to repression of VRN1 before winter. In other organisms, H3K27Me3 is deposited by the polycomb repressor complex 2 (PRC2) (Cao et al. 2002). The intron of VRN1 might contain sequences targeted by such a complex, explaining why this region is required to maintain repression of VRN1; however, the sequence motifs targeted by plant PRC2 complexes are not known.
When plants are vernalized, the level of H3K27Me3 at the VRN1 locus decreases and histone modifications typically associated with an active chromatin state appear (H3K4Me3), suggesting that vernalization induces a change in the state of chromatin at the VRN1 locus (Oliver et al. 2009). Chromatin modifications can be inherited through cell divisions, so these epigenetic modifications, and the associated changes in chromatin state, might allow VRN1 to remain active after vernalization. This could contribute to a cellular memory of vernalization in cereals (Fig. 5).
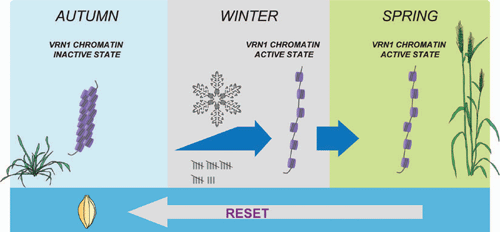
|
Mechanisms that activate expression of VRN1 in response to low temperatures
Prolonged exposure to cold activates expression of VRN1, but how this occurs is not known. The regulatory regions in the first intron are not required for cold induction of VRN1, since exposure to low temperatures can further activate expression of VRN1 alleles that lack most of the first intron (Trevaskis et al. 2007b; Hemming et al. 2009). So while the first intron is important to maintain low levels of VRN1 transcripts before winter, cold activation of VRN1 is probably mediated by other parts of the gene. Transient transformation experiments using tobacco leaves (Nicotiana benthamiana L.) suggest that fusion of a 1 kb region of the VRN1 promoter to a reporter gene (Green Fluorescent Protein) can mediate cold induction of reporter gene activity (Kane et al. 2007). This suggests that cold activation of VRN1 is controlled by regulatory elements in the promoter. Presumably these elements are targeted by a temperature response mechanism that is conserved in cereals (monocots: Poaceae) and tobacco (dicot: Solanaceae). Unfortunately the study of Kane et al. (2007) did not examine whether the increase in reporter gene activity seen after cold treatment resulted from increased transcription or simply from increased stability of the reporter protein, so the results of this experiment are inconclusive.
Genes that control the daylength flowering response can influence the vernalization requirement
Some wheats and barleys carry alleles of FT1 (VRN3) that are expressed at high levels and cause rapid flowering without vernalization (Takahashi and Yasuda 1971; Yan et al. 2006). Other wheats and barleys lack a functional VRN2 gene and so flower rapidly in long day conditions, without any requirement for vernalization (Takahashi and Yasuda 1971; Dubcovsky et al. 1998; Yan et al. 2004a). In these genotypes, activation of the long-day flowering response without prior cold treatment overcomes the normal requirement for vernalization.
VRN2 and FT1 (VRN3) have different effects on flowering behaviour. Active alleles of FT1 cause rapid inflorescence initiation and development, irrespective of daylength or vernalization (Yan et al. 2006). Loss of VRN2 also causes rapid inflorescence initiation and development without vernalization, but only in long-day conditions (Karsai et al. 2005; Hemming et al. 2008). By comparison, active alleles of VRN1 mimic the effects of vernalization by promoting inflorescence initiation and allowing long days to accelerate subsequent stages of inflorescence development. The way each gene influences overall flowering behaviour determines how these genes are utilised to produce wheats and barleys that flower without vernalization. For example, active alleles of FT1 seem to be useful in regions with extreme winters and short summer growing seasons, where rapid flowering is beneficial regardless of growing conditions (Takahashi and Yasuda 1971).
The daylength–flowering response pathway influences VRN1 expression
VRN1 is expressed without prior vernalization in varieties that carry active alleles of FT1 (Yan et al. 2006). In A. thaliana, FT interacts with a ‘basic leucine zipper’ transcription factor, FD, to activate target genes that include MADS box transcription factors that are related to VRN1; FRUITFUL and APETALLA1 (Abe et al. 2005; Teper-Bamnolker and Samach 2005; Wigge et al. 2005; Corbesier et al. 2007). The wheat FT1 protein can interact with the FD-like protein 2 (TaFDL2), which can, in turn, bind to the promoter of VRN1 (Li and Dubcovsky 2008). Thus, in cereals, FT1 might activate expression of VRN1 in leaves and the shoot apex through interactions with FD-like proteins (Li and Dubcovsky 2008). Activation of VRN1 by FT1 might also occur in varieties that lack VRN2, where rapid induction of both FT1 and VRN1 occurs when plants are exposed to long days (Yan et al. 2006; Trevaskis et al. 2007b).
Although activation of FT1 can induce expression of VRN1 in some genotypes, the relevance of this to vernalization-induced flowering is unclear. In autumn-sown (vernalization-responsive) varieties, VRN1 is induced by cold during winter when days are short (Danyluk et al. 2003; von Zitzewitz et al. 2005; Trevaskis et al. 2006) and FT1 is not expressed (Hemming et al. 2008). Similarly, vernalization induces expression of VRN1 in seeds germinated in darkness where FT1 is not expressed (Sasani et al. 2009). So FT1 is unlikely to mediate induction of VRN1 by cold. Instead, long-day induction of VRN1 might occur in spring if plants are partially vernalized – after mild winters, for example – and this could compensate for incomplete vernalization. Similarly, long-day activation of VRN1 might allow some varieties to flower eventually even if plants do not experience vernalization. Activation of VRN1 by long days might also be important after the transition to reproductive development, since long days can enhance expression of VRN1 in leaves after the initiation inflorescence (Sasani et al. 2009). This might occur through a positive feedback mechanism whereby FT1 enhances expression of VRN1, which further activates FT1 (Fig. 3). This might strengthen the long-day flowering response once inflorescence development begins.
Utilising genetic diversity in VRN1 for crop improvement
Different alleles of VRN1 can generate a wide spectrum of vernalization requirements and have been used to adapt varieties to a wide range of growing regions and sowing times (Pugsley 1971; Takahashi and Yasuda 1971). Preliminary surveys of genetic diversity at the VRN1 locus have shown how breeders have utilised particular alleles of VRN1 to breed cultivars adapted to specific regions. For example, an active allele of VRN1 on the D genome is common in wheats from some growing regions and might offer an adaptive benefit relative to other alleles (Zhang et al. 2008). Similarly, some alleles of VRN1 gene are prevalent in European barleys (Cockram et al. 2007; Hemming et al. 2009).
The prevalence of an allele within a breeding program might indicate an adaptive benefit of this allele in a particular region, but might also reflect breeding history, since a founding variety or recurrent parent can bias breeding material towards a particular VRN1 genotype. Therefore, genetic surveys alone might not identify the optimal VRN1 genotype for a particular region. To determine which VRN1 alleles are most suitable for use in Australian wheat and barley varieties, we used DNA diagnostic techniques to identify different alleles of VRN1 amongst diverse cultivars, breeding lines and landraces of wheat (3000 accessions) and barley (4400 accessions) (Hemming et al. 2009; S. Fieg and B. Trevaskis, unpubl. data). The different alleles thus identified have been introgressed into elite wheat and barley varieties, and are undergoing recurrent backcrossing to develop near-isogenic lines that vary for VRN1 genotype but otherwise have similar genetic backgrounds. These can be tested for performance in different regions with different sowing times, and will provide valuable information to Australian cereal breeders.
An alternative approach being used to identify alleles of VRN1 that are suited to different Australian growing regions is to identify VRN1 alleles that are common among Australian cereal breeding programs and then analyse the association of these alleles with crop performance among diverse materials grown in different regions over multiple years. This approach makes good use of existing pedigree and phenotype data, and is a powerful way to identify favourable alleles of VRN1 within existing breeding material. This strategy is currently being used to identify useful alleles of VRN1 among Australian wheat breeding material (see Eagles et al. 2009).
The relationship between VRN1, vernalization requirement and frost tolerance
Autumn-sown wheats and barleys, which require vernalization to flower, can acclimatise to low temperatures and are subsequently able to survive freezing conditions during winter. This process is known as cold acclimation (reviewed in Galiba et al. 2009). The longer plants are exposed to low temperatures, the more frost tolerance increases. This continues until the point when further cold treatment has no additional impact on flowering time (the vernalization saturation point) then frost tolerance begins to decrease (Vasiljev 1934 cited by McKinney 1940; Roberts 1979; Mahfoozi et al. 2001; Prasil et al. 2004). Thus, it seems that cold acclimation and the vernalization response are interconnected.
Activation of VRN1 might cause the decrease in frost tolerance that begins when plants are fully vernalized. This hypothesis is supported by genetic studies that show active alleles of VRN1 reduce frost tolerance (Roberts 1990; Hayes et al. 1993; Fowler et al. 1996; Koemel et al. 2004; Limin and Fowler 2006). For example, a comparison of near-isogenic lines that differ in VRN1 genotype has shown that varieties with active alleles of VRN1, which flower without vernalization, have a greatly reduced capacity to acclimate to cold compared with lines with wild-type alleles of VRN1, which require vernalization to flower (Koemel et al. 2004; Limin and Fowler 2006). At present, it is not clear whether this is a direct consequence of induction of VRN1 or an indirect consequence, caused by the effect that VRN1 has on other genes or on plant development per se. Regardless of the mechanism, this relationship between VRN1 activity and frost tolerance is important and has implications for cereal breeders, since altering vernalization requirement can also affect winter survival.
The evolution of vernalization-induced flowering
Vernalization occurs in a wide range of plants. Genes controlling vernalization-induced flowering have now been isolated from temperate cereals (monocots: Poaceae) and the model plant A. thaliana (dicot: Brassicaceae). In A. thaliana, the vernalization response is mediated by FLOWERING LOCUS C (FLC) (Michaels and Amasino 1999; Sheldon et al. 1999). FLC encodes a MADS box floral repressor that is downregulated by vernalization (Michaels and Amasino 1999; Sheldon et al. 1999). This contrasts with the situation in temperate cereals, where no FLC-like genes occur and instead VRN1 is activated by vernalization to promote flowering (see Trevaskis et al. 2007a; Greenup et al. 2009). It seems that the vernalization response has evolved independently in these distantly related angiosperms.
Although different genes control the vernalization response in cereals and A. thaliana, there are parallels between the pathways controlling vernalization-induced flowering in these plants. For example, vernalization is a prerequisite for long-day induction of both FT and FT1 (Michaels and Amasino 1999; Sheldon et al. 1999; Michaels et al. 2005; Yan et al. 2006; Hemming et al. 2008). In cereals, VRN2 downregulates FT1 before winter, directly or indirectly (Yan et al. 2006; Hemming et al. 2008), and in A. thaliana, FLC interacts directly with the FT gene sequence to repress transcription (Helliwell et al. 2006). Furthermore, in both cereals and in A. thaliana, the state of chromatin at key MADS box transcription factors plays an important role in regulating the vernalization response. The repressive chromatin mark H3K27Me3 is required for repression of VRN1 before winter in cereals and for repression of FLC after winter in A. thaliana (Schubert et al. 2006; Sung et al. 2006; Wood et al. 2006; Finnegan and Dennis 2007; De Lucia et al. 2008; Oliver et al. 2009). These parallels are probably the result of convergent evolution.
Directions for future research
One key area for further research is in understanding how plants sense cold and how increasing durations of cold cause increased expression of VRN1. Another key question is how VRN1 promotes flowering. More specifically, what are the direct targets of VRN1? By using molecular tools such as reporter gene fusions, microarrays and targeted mutagenesis, it should be possible to address these questions. In this regard, barley is a useful genetic model, since it is transformable and large collections of genetically diverse barley accessions exist in cereal seed banks. There are also many mapping populations, including recombinant inbred lines and doubled haploids, which can be used to identify potential regulators of VRN1.
Conclusions
The activity of VRN1 underlies many of the physiological features of vernalization-induced flowering in cereals, so understanding how VRN1 is regulated will provide further insights into the biology of the vernalization response in these plants. A better understanding of the regulation and molecular functions of VRN1 will also have important implications for cereal breeding programs. It should eventually be possible to predict with precision how different alleles of VRN1 will influence flowering behaviour in different genetic backgrounds or different environments, and to tailor breeding strategies accordingly. Knowledge developed in temperate cereals will also be relevant to related grasses, including a range of economically important pasture grasses. For these reasons, the vernalization response of cereals, the plants where vernalization-induced flowering was first recognised, will be an important area for ongoing research.
Acknowledgements
I thank my friends and colleagues Steve Swain, Aaron Greenup, Sarah Fieg, Sandra Oliver and Megan Hemming for reading drafts of this manuscript and providing constructive suggestions. I also gratefully acknowledge both the Commonwealth Scientific and Industrial Research Organisation, and the Grains Research and development Corporation for long-term support of this research.
Abe M,
Kobayashi Y,
Yamamoto S,
Daimon Y,
Yamaguchi A,
Ikeda Y,
Ichinoki H,
Notaguchi M,
Goto K, Araki T
(2005) FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 309, 1052–1056.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Amasino RM
(2004) Vernalization, competence, and the epigenetic memory of winter. The Plant Cell 16, 2553–2559.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

An H,
Roussot C,
Suarez-Lopez P,
Corbesier L, Vincent C ,
et al
.
(2004)
CONSTANS acts in the phloem to regulate a systemic signal that induces photoperiodic flowering of Arabidopsis. Development 131, 3615–3626.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Bonnet OT
(1935) The development of the barley spike. Journal of Agricultural Research 51, 451–457.

Bonnet OT
(1936) The development of the wheat spike. Journal of Agricultural Research 53, 445–451.

Cao R,
Wang L,
Wang H,
Xia L,
Erdjument-Bromage H,
Tempst P,
Jones RS, Zhang Y
(2002) Role of histone H3 lysine 27 methylation in polycomb-group silencing. Science 298, 1039–1043.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Chouard P
(1960) Vernalization and its relation to dormancy. Annual Review of Plant Physiology 11, 191–238.
| Crossref | GoogleScholarGoogle Scholar |

Cockram J,
Chiapparino E,
Taylor S,
Stamati K,
Donini P,
Laurie D, O’Sullivan D
(2007) Haplotype analysis of vernalization loci in European barley germplasm reveals novel VRN-H1 alleles and a predominant winter VRN-H1/VRN-H2 multi-locus haplotype. Theoretical and Applied Genetics 115, 993–1001.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Corbesier L,
Vincent C,
Jang S,
Fornara F, Fan Q ,
et al
.
(2007) FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 316, 1030–1033.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Danyluk J,
Kane NA,
Breton G,
Limin AE,
Fowler DB, Sarhan F
(2003) TaVRT-1, a putative transcription factor associated with vegetative to reproductive transition in cereals. Plant Physiology 132, 1849–1860.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

De Lucia F,
Crevillen P,
Jones AME,
Greb T, Dean C
(2008) A PHD-polycomb repressive complex 2 triggers the epigenetic silencing of FLC during vernalization. Proceedings of the National Academy of Sciences of the United States of America 105, 16 831–16 836.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Distelfeld A, Dubcovsky J
(2010) Characterization of the maintained vegetative phase deletions from diploid wheat and their effect on VRN2 and FT transcript levels. Molecular Genetics and Genomics in press. ,
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Distelfeld A,
Li C, Dubcovsky J
(2009) Regulation of flowering in temperate cereals. Current Opinion in Plant Biology 12, 178–184.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Dubcovsky J,
Lijavetzky D,
Appendino L, Tranquilli G
(1998) Comparative RFLP mapping of Triticum monococcum genes controlling vernalization requirement. Theoretical and Applied Genetics 97, 968–975.
| Crossref | GoogleScholarGoogle Scholar |

Dubcovsky J,
Loukoianov A,
Fu D,
Valarik M,
Sanchez A, Yan L
(2006) Effect of photoperiod on the regulation of wheat vernalization genes VRN1 and VRN2. Plant Molecular Biology 60, 469–480.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Eagles HA,
Cane K, Neil V
(2009) The flow of alleles of important photoperiod and vernalisation genes through Australian wheat. Crop and Pasture Science 60, 646–657.
| Crossref | GoogleScholarGoogle Scholar |

Faure S,
Higgins J,
Turner A, Laurie DA
(2007) The FLOWERING LOCUS T-like gene family in Barley (Hordeum vulgare). Genetics 176, 599–609.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Ferrándiz C,
Gu Q,
Martienssen R, Yanofsky MF
(2000) Redundant regulation of meristem identity and plant architecture by FRUITFULL, APETALA1 and CAULIFLOWER. Development 127, 725–734.
| PubMed |

Finnegan EJ, Dennis ES
(2007) Vernalization-induced trimethylation of histone H3 lysine 27 at FLC is not maintained in mitotically quiescent cells. Current Biology 17, 1978–1983.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Flood RG, Halloran GM
(1984) The nature and duration of gene action for vernalization response in wheat. Annals of Botany 53, 363–368.

Fowler DB,
Chauvin LP,
Limin AE, Sarhan F
(1996) The regulatory role of vernalization in the expression of low-temperature-induced genes in wheat and rye. Theoretical and Applied Genetics 93, 554–559.
| Crossref | GoogleScholarGoogle Scholar |

Fu DL,
Szucs P,
Yan LL,
Helguera M,
Skinner JS,
von Zitzewitz J,
Hayes PM, Dubcovsky J
(2005) Large deletions within the first intron in VRN-1 are associated with spring growth habit in barley and wheat. Molecular Genetics and Genomics 273, 54–65.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Galiba G,
Vagujfalvi A,
Li C,
Soltesz A, Dubcovsky J
(2009) Regulatory genes involved in the determination of frost tolerance in temperate cereals. Plant Science 176, 12–19.
| Crossref | GoogleScholarGoogle Scholar |

Gassner G
(1918) Beitraege zur physiologischen charakteristik sommer- und winterannueller gewaechse in besondere der getreidepflanzen. Zeitschrift für Botanik 10, 417–480.

Gocal GFW,
King RW,
Blundell CA,
Schwartz OM,
Andersen CH, Weigel D
(2001) Evolution of floral meristem identity genes: analyses of Lolium temulentum genes related to APETALA1 and LEAFY of Arabidopsis. Plant Physiology 125, 1788–1801.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Gott MB,
Gregory FG, Purvis ON
(1955) Studies in vernalization of cereals VIII. Photoperiodic control of stage in flowering between initiation and ear formation in vernalised and unvernalised petkus winter rye. Annals of Botany 21, 87–126.

Greenup A,
Peacock WJ,
Dennis ES, Trevaskis B
(2009) The molecular biology of seasonal flowering-responses in Arabidopsis and the cereals. Annals of Botany 103, 1165–1172.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Gregory FG, Purvis ON
(1936) Vernalisation of winter rye during ripening. Nature [Abstract] 138, 973.
| Crossref | GoogleScholarGoogle Scholar |

Hayes PM,
Blake T,
Chen THH,
Tragoonrung S,
Chen F,
Pan A, Liu B
(1993) Quantitative trait loci on barley (Hordeum vulgare L.) chromosome 7 associated with components of winter hardiness. Genome 36, 66–71.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Helliwell CA,
Wood CC,
Robertson M,
Peacock WJ, Dennis ES
(2006) The Arabidopsis FLC protein interacts directly in vivo with SOC1 and FT chromatin and is part of a high-molecular-weight protein complex. The Plant Journal 46, 183–192.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Hemming MN,
Peacock WJ,
Dennis ES, Trevaskis B
(2008) Low-temperature and daylength cues are integrated to regulate FLOWERING LOCUS T in barley. Plant Physiology 147, 355–366.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Hemming MN,
Fieg S,
Peacock WJ,
Dennis ES, Trevaskis B
(2009) Regions associated with repression of the barley (Hordeum vulgare) VERNALIZATION1 gene are not required for cold induction. Molecular Genetics and Genomics 282, 107–117.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Kane NA,
Agharbaoui Z,
Diallo AO,
Adam H,
Tominaga Y,
Ouellet F, Sarhan F
(2007) TaVRT2 represses transcription of the wheat vernalization gene TaVRN1. The Plant Journal 51, 670–680.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Kardailsky I,
Shukla VK,
Ahn JH,
Dagenais N,
Christensen SK,
Nguyen JT,
Chory J,
Harrison MJ, Weigel D
(1999) Activation tagging of the floral inducer FT. Science 286, 1962–1965.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Karsai I,
Szucs P,
Meszaros K,
Filichkina T,
Hayes PM,
Skinner JS,
Lang L, Bedo Z
(2005) The Vrn-H2 locus is a major determinant of flowering time in a facultative × winter growth habit barley (Hordeum vulgare L.) mapping population. Theoretical and Applied Genetics 110, 1458–1466.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

King RW, Heide OM
(2009) Seasonal flowering and evolution: the heritage from Charles Darwin. Functional Plant Biology 36, 1027–1036.
| Crossref | GoogleScholarGoogle Scholar |

Kobayashi Y,
Kaya H,
Goto K,
Iwabuchi M, Araki T
(1999) A pair of related genes with antagonistic roles in mediating flowering signals. Science 286, 1960–1962.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Koemel JE,
Guenzi AC,
Anderson JA, Smith EL
(2004) Cold hardiness of wheat near-isogenic lines differing in vernalization alleles. Theoretical and Applied Genetics 109, 839–846.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Li CX, Dubcovsky J
(2008) Wheat FT protein regulates VRN1 transcription through interactions with FDL2. The Plant Journal 55, 543–554.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Limin AE, Fowler DB
(2006) Low-temperature tolerance and genetic potential in wheat (Triticum aestivum L.): response to photoperiod, vernalization, and plant development. Planta 224, 360–366.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Loukoianov A,
Yan L,
Blechl A,
Sanchez A, Dubcovksy J
(2005) Regulation of VRN-1 vernalization genes in normal and transgenic polyploid wheat. Plant Physiology 138, 2364–2373.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Mahfoozi S,
Limin AE, Fowler DB
(2001) Influence of vernalization and photoperiod responses on cold hardiness in winter cereals. Crop Science 41, 1006–1011.

Marcińska I,
Dubert F, Biesaga-Koscielniak J
(1995) Transfer of the ability to flower in winter wheat via callus tissue regenerated from immature inflorescences. Plant Cell, Tissue and Organ Culture 41, 285–288.
| Crossref | GoogleScholarGoogle Scholar |

McKinney HH
(1940) Vernalization and the growth phase concept. Botanical Review 6, 25–47.
| Crossref | GoogleScholarGoogle Scholar |

Michaels SD, Amasino RM
(1999)
FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. The Plant Cell 11, 949–956.
| Crossref |
PubMed |

Michaels SD,
Himelblau E,
Kim SY,
Schomburg FM, Amasino RM
(2005) Integration of flowering signals in winter-annual Arabidopsis. Plant Physiology 137, 149–156.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Oliver SN,
Finnegan EJ,
Dennis ES,
Peacock WJ, Trevaskis B
(2009) Vernalization-induced flowering in cereals is associated with changes in histone methylation at the VERNALIZATION1 gene. Proceedings of the National Academy of Sciences of the United States of America 106, 8386–8391.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Pidal B,
Yan L,
Fu D,
Zhang F,
Tranquilli G, Dubcovsky J
(2009) The CARG-box located upstream from the transcriptional start of wheat vernalization gene VRN1 is not necessary for the vernalization response. Heredity 100, 355–364.
| Crossref | GoogleScholarGoogle Scholar |

Prasil IT,
Prasilova P, Pankova K
(2004) Relationships among vernalization, shoot apex development and frost tolerance in wheat. Annals of Botany 94, 413–418.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Preston JC, Kellogg EA
(2008) Discrete developmental roles for temperate cereal grass VERNALIZATION1/FRUITFULL-like genes in flowering competency and the transition to flowering. Plant Physiology 146, 265–276.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Pugsley AT
(1971) A genetic analysis of the spring–winter habit of growth in wheat. Australian Journal of Agricultural Research 22, 21–31.
| Crossref | GoogleScholarGoogle Scholar |

Purvis ON
(1934) An analysis of the influence of temperature during germination on the subsequent development of certain winter cereals and its relation to the effect of length of day. Annals of Botany 48, 919–955.

Roberts DWA
(1979) Duration of hardening and cold hardiness in winter wheat. Canadian Journal of Botany 57, 1511–1517.
| Crossref | GoogleScholarGoogle Scholar |

Roberts DWA
(1990) Identification of loci on chromosome 5A of wheat involved in control of cold hardiness, vernalization, leaf length, rosette growth habit, and height of hardened plants. Genome 33, 247–259.

Sasani S,
Hemming MN,
Oliver S,
Greenup A, Tavakkol-Afshari R ,
et al
.
(2009) The influence of vernalization and daylength cues on the expression of flowering-time genes in the leaves and shoot apex of barley (Hordeum vulgare). Journal of Experimental Botany 60, 2169–2178.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Schubert D,
Primavesi L,
Bishopp A,
Roberts G,
Doonan J,
Jenuwein T, Goodrich J
(2006) Silencing by plant polycomb-group genes requires dispersed trimethylation of histone H3 at lysine 27. The EMBO Journal 25, 4638–4649.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Sheldon CC,
Burn JE,
Perez PP,
Metzger J,
Edwards JA,
Peacock WJ, Dennis ES
(1999) The FLF MADS box gene, a repressor of flowering in Arabidopsis regulated by vernalization and methylation. The Plant Cell 11, 445–458.
| Crossref |
PubMed |

Shitsukawa N,
Ikari C,
Shimada S,
Kitagawa S, Sakamoto K ,
et al
.
(2007) The einkorn wheat (Triticum monococcum) mutant, maintained vegetative phase, is caused by a deletion in the VRN1 gene. Genes & Genetic Systems 82, 167–170.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Stockinger EJ,
Skinner JS,
Gardner KG,
Francia E, Pecchioni N
(2007) Expression levels of barley Cbf genes at the frost resistance-H2 locus are dependent upon alleles at Fr-H1 and Fr-H2. The Plant Journal 51, 308–321.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Suárez-López P,
Wheatley K,
Robson F,
Onouchi H,
Valverde F, Coupland G
(2001)
CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis. Nature 410, 1116–1120.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Sung SB,
Schmitz RJ, Amasino RM
(2006) A PHD finger protein involved in both the vernalization and photoperiod pathways in Arabidopsis. Genes & Development 20, 3244–3248.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Szucs P,
Skinner JS,
Karsai I,
Cuesta-Marcos A,
Haggard KG,
Corey AE,
Chen TH, Hayes PM
(2007) Validation of the VRN-H2/VRN-H1 epistatic model in barley reveals that intron length variation in VRN-H1 may account for a continuum of vernalization sensitivity. Molecular Genetics and Genomics 277, 249–261.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Tamaki S,
Matsuo S,
Wong HL,
Yokoi S, Shimamoto K
(2007) Hd3a protein is a mobile flowering signal in rice. Science 316, 1033–1036.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Teper-Bamnolker P, Samach A
(2005) The flowering integrator FT regulates SEPALLATA3 and FRUITFULL accumulation in Arabidopsis leaves. The Plant Cell 17, 2661–2675.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Trevaskis B,
Bagnall DJ,
Ellis MH,
Peacock WJ, Dennis ES
(2003) MADS box genes control vernalization-induced flowering in cereals. Proceedings of the National Academy of Sciences of the United States of America 100, 13 099–13 104.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Trevaskis B,
Hemming MN,
Peacock WJ, Dennis ES
(2006)
HvVRN2 responds to daylength, whereas HvVRN1 is regulated by vernalization and developmental status. Plant Physiology 140, 1397–1405.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Trevaskis B,
Hemming MN,
Dennis ES, Peacock WJ
(2007a) The molecular basis of vernalization-induced flowering in cereals. Trends in Plant Science 12, 352–357.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Trevaskis B,
Tadege M,
Hemming MN,
Peacock WJ,
Dennis ES, Sheldon C
(2007b)
Short Vegetative Phase-like MADS-box genes inhibit floral meristem identity in barley. Plant Physiology 143, 225–235.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Turner A,
Beales J,
Faure S,
Dunford RP, Laurie DA
(2005) The pseudo-response regulator Ppd-H1 provides adaptation to photoperiod in barley. Science 310, 1031–1034.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Valverde F,
Mouradov A,
Soppe W,
Ravenscroft D,
Samach A, Coupland G
(2004) Photoreceptor regulation of CONSTANS protein in photoperiodic flowering. Science 303, 1003–1006.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

von Zitzewitz J,
Szucs P,
Dubcovsky J,
Yan LL,
Francia E,
Pecchioni N,
Casas A,
Chen THH,
Hayes PM, Skinner JS
(2005) Molecular and structural characterization of barley vernalization genes. Plant Molecular Biology 59, 449–467.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Whelan EDP, Schaalje GB
(1992) Vernalization of embryogenic callus from immature embryos of winter wheat. Crop Science 32, 78–80.

Wigge PA,
Kim MC,
Jaeger KE,
Busch W,
Schmid M,
Lohmann JU, Weigel D
(2005) Integration of spatial and temporal information during floral induction in Arabidopsis. Science 309, 1056–1059.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Wood CC,
Robertson M,
Tanner G,
Peacock WJ,
Dennis ES, Helliwell CA
(2006) The Arabidopsis thaliana vernalization response requires a polycomb-like protein complex that also includes VERNALIZATION INSENSITIVE 3. Proceedings of the National Academy of Sciences of the United States of America 103, 14 631–14 636.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Yan L,
Loukoianov A,
Tranquilli G,
Helguera M,
Fahima T, Dubcovsky J
(2003) Positional cloning of the wheat vernalization gene VRN1. Proceedings of the National Academy of Sciences of the United States of America 100, 6263–6268.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Yan L,
Loukoianov A,
Blechl A,
Tranquilli G,
Ramakrishna W,
SanMiguel P,
Bennetzen JL,
Echenique V, Dubcovsky J
(2004a) The wheat VRN2 gene is a flowering repressor down-regulated by vernalization. Science 303, 1640–1644.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Yan L,
Helguera M,
Kato K,
Fukuyama S,
Sherman J, Dubcovsky J
(2004b) Allelic variation at the VRN-1 promoter region in polyploid wheat. Theoretical and Applied Genetics 109, 1677–1686.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Yan L,
Fu D,
Li C,
Blechl A,
Tranquilli G,
Bonafede M,
Sanchez A,
Valarik M,
Yasuda S, Dubcovsky J
(2006) The wheat and barley vernalization gene VRN3 is an orthologue of FT. Proceedings of the National Academy of Sciences of the United States of America 103, 19 581–19 586.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Zadoks JC,
Chang TT, Konzak CF
(1974) A decimal code for the growth stages of cereals. Weed Research 14, 415–421.
| Crossref | GoogleScholarGoogle Scholar |

Zeevaart JA
(2008) Leaf-produced floral signals. Current Opinion in Plant Biology 11, 541–547.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Zhang XK,
Xiao TG,
Zhang Y,
Xia XC,
Dubcovsky J, He ZH
(2008) Allelic variation at the vernalization gene Vrn-A1, Vrn-B1, VRN-D1 and Vrn-B3 in Chinese wheat cultivars and their association with growth habit. Crop Science 48, 458–470.
| Crossref | GoogleScholarGoogle Scholar |



