Sugarcane genotypes differ in internal nitrogen use efficiency
Nicole Robinson A B E , Andrew Fletcher A B , Alex Whan A B , Christa Critchley A B , Nicolaus von Wirén C , Prakash Lakshmanan B D and Susanne Schmidt A BA School of Integrative Biology, The University of Queensland, Brisbane, Qld 4072, Australia.
B CRC for Sugar Industry Innovation through Biotechnology, The University of Queensland, Qld 4072, Australia.
C Institute for Plant Nutrition, University of Hohenheim, D-70593 Stuttgart, Germany.
D BSES Ltd, 50 Meiers Road, Indooroopilly, Qld 4068, Australia.
E Corresponding author. Email: Nicole.Robinson@uq.edu.au
Functional Plant Biology 34(12) 1122-1129 https://doi.org/10.1071/FP07183
Submitted: 26 July 2007 Accepted: 10 October 2007 Published: 27 November 2007
Abstract
The large amounts of nitrogen (N) fertiliser applied to most cropping systems support high yields but cause N pollution. More efficient use of N in cropping systems can be achieved through improved N management practices combined with genetic improvement of the crop. The magnitude of genetic variation in sugarcane (Saccharum officinarum L.) for internal nitrogen use efficiency (iNUE, biomass produced per unit tissue N) was investigated as this could provide a basis for breeding varieties with reduced N demand. Genotypes of a mapping population were examined for biomass production and physiological variables under low or high N supply in controlled conditions. Key findings were: (i) genotypic variation for biomass production and iNUE was up to 3-fold greater under low than high N supply, (ii) elite parent Q165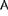 was among the best performing genotypes for biomass and iNUE at high N but not at low N supply, and (iii) several genotypes had high iNUE at both N supplies. While glutamine synthetase (GS; EC 6.3.1.2) activity has been linked with grain yield in other crops, no direct relationship was observed between whole tissue GS activity and vegetative biomass or iNUE in sugarcane genotypes. Soluble protein content was negatively correlated with iNUE and biomass production. This study demonstrates that there is considerable genetic variation for iNUE in sugarcane, which can be exploited for breeding. It is proposed that breeding programs should assess genotypes not only at high N, but also at low N supply rates to select genotypes that produce high biomass with low and high N supply.
was among the best performing genotypes for biomass and iNUE at high N but not at low N supply, and (iii) several genotypes had high iNUE at both N supplies. While glutamine synthetase (GS; EC 6.3.1.2) activity has been linked with grain yield in other crops, no direct relationship was observed between whole tissue GS activity and vegetative biomass or iNUE in sugarcane genotypes. Soluble protein content was negatively correlated with iNUE and biomass production. This study demonstrates that there is considerable genetic variation for iNUE in sugarcane, which can be exploited for breeding. It is proposed that breeding programs should assess genotypes not only at high N, but also at low N supply rates to select genotypes that produce high biomass with low and high N supply.
Additional keywords: biomass crop, genetic variation, glutamine synthetase, plant breeding.
Acknowledgements
This research was funded by the Cooperative Research Centre for Sugar Industry Innovation Through Biotechnology (CRC SIIB). We thank CRC SIIB internship students Richard Brackin, Heather Vikstrom, Jenny Vo, Thi Hoang, Michael Christie, Nicole Schmid and Harshi Gamage for help with glasshouse and laboratory work. We thank Terry Morgan from CSR Kalamia for providing the sett material, and Jo Stringer from BSES for statistical consultation. We also thank Dr Bertrand Hirel from INRA for his help with development of high through put enzyme assay techniques and insightful discussions on NUE.
Aitken K,
Jackson P, McIntyre C
(2005) A combination of AFLP and SSR markers provides extensive map coverage and identification of homo(eo)logous linkage groups in a sugarcane cultivar. Theoretical and Applied Genetics 110, 789–801.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Ameziane R,
Bernhard K, Lightfoot D
(2000) Expression of the bacterial gdhA gene encoding a NADPH glutamate dehydrogenase in tobacco affects plant growth and development. Plant and Soil 221, 47–57.
| Crossref | GoogleScholarGoogle Scholar |

Baldani JI,
Reis VM,
Baldani VLD, Dobereiner J
(2002) A brief story of nitrogen fixation in sugarcane – reasons for success in Brazil. Functional Plant Biology 29, 417–423.
| Crossref | GoogleScholarGoogle Scholar |

Bänziger M,
Betran FJ, Lafitte HR
(1997) Efficiency of high-N selection environments for improving maize for low-N target environments. Crop Science 37, 1103–1109.

Bertin P, Gallais A
(2000) Genetic variation for nitrogen use efficiency in a set of recombinant maize inbred lines. I. Agrophysiological results. Maydica 45, 53–66.

Boddey RM,
Oliveira OC,
Urquiaga S,
Reis VM,
Olivares FL,
Baldani VLD, Döbereiner J
(1995) Biological nitrogen fixation associated with sugar cane and rice: contributions and prospects for improvement. Plant and Soil 174, 195–209.
| Crossref | GoogleScholarGoogle Scholar |

Bransby DI,
McLaughlin SB, Parrish DJ
(1998) A review of carbon and nitrogen balances in switchgrass grown for energy. Biomass and Bioenergy 14, 379–384.
| Crossref | GoogleScholarGoogle Scholar |

Cassman KG
(1999) Ecological intensification of cereal production systems: yield potential, soil quality, and precision agriculture. Proceedings of the National Academy of Sciences of the United States of America 96, 5952–5959.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Fuentes S,
Allen DJ,
Ortiz-Lopez A, Hernández G
(2001) Over-expression of cytosolic glutamine synthetase increases photosynthesis and growth at low nitrogen concentrations. Journal of Experimental Botany 52, 1071–1081.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Gallais A, Hirel B
(2004) An approach to the genetics of nitrogen use efficiency in maize. Journal of Experimental Botany 55, 295–306.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Good A,
Shrawat AK, Muench DG
(2004) Can less yield more? Is reducing nitrogen input into the environment compatible with maintaining crop production? Trends in Plant Science 9, 597–605.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Good A,
Johnson S,
De Pauw M,
Carroll R,
Savidov N,
Vidmar J,
Lu Z,
Taylor G, Stroeher V
(2007) Engineering nitrogen use efficiency with alanine aminotransferase. Canadian Journal of Botany 85, 252–262.
| Crossref | GoogleScholarGoogle Scholar |

Habash DZ,
Massiah AJ,
Rong HL,
Wallsgrove RM, Leigh RA
(2001) The role of cytosolic glutamine synthetase in wheat. The Annals of Applied Biology 138, 83–89.
| Crossref | GoogleScholarGoogle Scholar |

Hirel B,
Bertin P,
Quilleré I,
Bourdoncle W, Attagnant C , et al..
(2001) Towards a better understanding of the genetic and physiological basis for nitrogen use efficiency in maize. Plant Physiology 125, 1258–1270.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Hirel B,
Martin A,
Tercé-Laforguea T,
Gonzalez-Morob M, Estavillo J
(2005a) Physiology of maize. I. A comprehensive and integrated view of nitrogen metabolism in a C4 plant. Physiologia Plantarum 124, 167–177.
| Crossref | GoogleScholarGoogle Scholar |

Hirel B,
Andrieu B,
Valadier MH,
Renard S,
Quilleré I,
Chelle M,
Pommel B,
Fournier C, Drouet JL
(2005b) Physiology of maize. II. Identification of physiological markers representative of the nitrogen status of maize (Zea mays) leaves during grain filling. Physiologia Plantarum 124, 178–188.
| Crossref | GoogleScholarGoogle Scholar |

Hirel B,
Le Gouis J,
Ney B, Gallais A
(2007) The challenge of improving nitrogen use efficiency in crop plants: towards a more central role for genetic variability and quantitative genetics within integrated approaches. Journal of Experimental Botany 58, 2369–2387.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Hoefsloot G,
Termorshuizen AJ,
Watt DA, Cramer MD
(2005) Biological nitrogen fixation is not a major contributor to the nitrogen demand of a commercially grown South African sugarcane cultivar. Plant and Soil 277, 85–96.
| Crossref | GoogleScholarGoogle Scholar |

Kamprath EJ,
Moll RH, Rodriquez N
(1982) Effects of N fertilization and recurrent selection on performance of hybrid populations of corn. Agronomy Journal 74, 955–958.

Kichey T,
Heumez E,
Pocholle D,
Pageau K,
Vanacker H,
Dubois F,
Le Gouis J, Hirel B
(2006) Combined agronomic and physiological aspects of nitrogen management in wheat highlight a central role for glutamine synthetase. The New Phytologist 169, 265–278.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Lam HM,
Wong P,
Chan H-K,
Yam K-M,
Chow C-M, Coruzzi GM
(2003) Overexpression of the ASN1 gene enhances nitrogen status in seeds of Arabidopsis. Plant Physiology 132, 926–935.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Le Gouis J,
Béghin B,
Heumez E, Pluchard P
(2000) Genetic differences for nitrogen uptake and nitrogen utilisation efficiencies in winter wheat. European Journal of Agronomy 12, 163–173.
| Crossref | GoogleScholarGoogle Scholar |

Limami A,
Phillipson B,
Ameziane R,
Pernollet N,
Jiang Q,
Roy R,
Deleens E,
Chaumont-Bonnet M,
Gresshoff P, Hirel B
(1999) Does root glutamine synthetase control plant biomass production in Lotus japonicus L.? Planta 209, 495–502.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Man H,
Boriel R,
El-Khatib R, Kirby EG
(2005) Characterization of transgenic poplar with ectopic expression of pine cytosolic glutamine synthetase under conditions of varying nitrogen availability. The New Phytologist 167, 31–39.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Martin A,
Lee J,
Kichey T,
Gerentes D, Zivy M , et al..
(2006) Two cytosolic glutamine synthetase isoforms of maize are specifically involved in the control of grain production. The Plant Cell 18, 3252–3274.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Masclaux C,
Valadier MH,
Bruiére N,
Morot-Gaudry JF, Hirel B
(2000) Characterisation of the sink/source transition in tobacco (Nicotiana tabacum L.) shoots in relation to nitrogen management and leaf senescence. Planta 211, 510–518.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Masclaux C,
Quillere I,
Gallais A, Hirel B
(2001) The challenge of remobilisation in plant nitrogen economy. A survey of physioagronomic and molecular approaches. The Annals of Applied Biology 138, 69–81.
| Crossref | GoogleScholarGoogle Scholar |

de Matos Nogueria E,
Olivares FL,
Japiassu JC,
Vilar C,
Vinagre F,
Baldani JI, Hemerly AS
(2005) Characterization of glutamine synthetase genes in sugarcane genotypes with different rates of biological nitrogen fixation. Plant Science 169, 819–832.
| Crossref | GoogleScholarGoogle Scholar |

Meier EA,
Thorburn PJ,
Wegener MK, Basford KE
(2006) The availability of nitrogen from sugarcane trash on contrasting soils in the wet tropics of north Queensland. Nutrient Cycling In Agroecosystems 75, 101–114.
| Crossref | GoogleScholarGoogle Scholar |

Miflin BJ, Habash DZ
(2002) The role of glutamine synthetase and glutamate dehydrogenase in nitrogen assimilation and possibilities for improvement in the nitrogen utilization of crops. Journal of Experimental Botany 53, 979–987.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Moll RH,
Kamprath EJ, Jackson WA
(1982) Analysis and interpretation of factors which contribute to efficiency of nitrogen utilization. Agronomy Journal 74, 562–564.

Oaks A
(1994) Efficiency of nitrogen utilization in C3 and C4 cereals. Plant Physiology 106, 407–414.
| PubMed |

O’Neal D, Joy KW
(1973) Glutamine synthetase of pea leaves. 1. Purification, stabilization, and pH optima. Archives of Biochemistry and Biophysics 159, 113–122.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Presterl T,
Groh S,
Landbeck M,
Seitz G,
Schmidt W, Geiger HH
(2002) Nitrogen uptake and utilization efficiency of European maize hybrids developed under conditions of low and high nitrogen input. Plant Breeding 121, 480–486.
| Crossref | GoogleScholarGoogle Scholar |

Quillère I,
Dufosse C,
Roux Y,
Foyer CH,
Caboche M, Morot-Gaudry JF
(1994) The effects of deregulation of NR gene expression on growth and nitrogen metabolism of Nicotiana plumbaginifolia plants. Journal of Experimental Botany 45, 1205–1211.
| Crossref | GoogleScholarGoogle Scholar |

Raun WR, Johnson VG
(1999) Improving nitrogen use efficiency for cereal production. Agronomy Journal 91, 357–363.

Samonte SOPB,
Samonte PB,
Wilson LT,
Medley JC,
Pinson S,
McClung AM, Lales JS
(2006) Nitrogen utilization efficiency: relationships with grain yield, grain protein, and yield-related traits in rice. Agronomy Journal 98, 168–176.
| Crossref | GoogleScholarGoogle Scholar |

Scheible WR,
González-Fontes A,
Morcuende R,
Lauerer M,
Geiger M,
Glaab J,
Gojon A,
Schulze ED, Stitt M
(1997) Tobacco mutants with a decreased number of functional nia genes compensate by modifying the diurnal regulation of transcription, post-translational modification and turnover of nitrate reductase. Planta 203, 304–319.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Sinebo W,
Gretzmacherb R, Edelbauer A
(2004) Genotypic variation for nitrogen use efficiency in Ethiopian barley. Field Crops Research 85, 43–60.
| Crossref | GoogleScholarGoogle Scholar |

Svečnjak Z, Rengel Z
(2006) Canola cultivars differ in nitrogen utilization efficiency in vegetative stage. Field Crops Research 97, 221–226.
| Crossref | GoogleScholarGoogle Scholar |

Thorburn PJ,
Meier EA, Probert ME
(2005) Modelling nitrogen dynamics in sugarcane systems: recent advances and applications. Field Crops Research 92, 337–351.
| Crossref | GoogleScholarGoogle Scholar |

Tilman D,
Cassman KG,
Matson PA,
Naylor R, Polasky S
(2002) Agricultural sustainability and intensive production practices. Nature 418, 671–677.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Vallis I,
Catchpoole VR,
Hughes RM,
Myers RJK,
Ridge DR, Weier KL
(1996) Recovery in plants and soils of 15N applied as subsurface bands of urea to sugarcane. Australian Journal of Agricultural Research 47, 355–370.
| Crossref | GoogleScholarGoogle Scholar |

Weier KL
(1998) Sugarcane fields: sources or sinks for greenhouse gas emissions? Australian Journal of Agricultural Research 49, 1–9.
| Crossref | GoogleScholarGoogle Scholar |

Worku M,
Bänziger M,
Schulte G,
Friesen D,
Diallo AO, Horst WJ
(2007) Nitrogen uptake and utilization in contrasting nitrogen efficient tropical maize hybrids. Crop Science 47, 519–528.



