First report of pathogenicity of Pantoea ananatis in sorghum (Sorghum bicolor) in Brazil
L. V. Cota A D , R. V. Costa A , D. D. Silva B , D. F. Parreira C , U. G. P. Lana A and C. R. Casela AA Embrapa Milho e Sorgo, 35701-970 Sete Lagoas, Minas Gerais, Brazil.
B FAPEMIG, Embrapa Milho e Sorgo, 35701-970 Sete Lagoas, Minas Gerais, Brazil.
C Departamento de Fitopatologia, Universidade Federal de Viçosa, 36570-000 Viçosa, Minas Gerais, Brazil.
D Corresponding author. Email: lvcota@cnpms.embrapa.br
Australasian Plant Disease Notes 5(1) 120-122 https://doi.org/10.1071/DN10044
Submitted: 6 August 2010 Accepted: 28 October 2010 Published: 17 November 2010
Abstract
Bacterial isolates from sorghum plants showing leaf spot symptoms were identified through molecular and phenotypic traits, showing that the isolates belong to Pantoea ananatis. Sorghum plants inoculated with those isolates showed a pathogenic reaction. The causal agent was reisolated and Koch’s postulates were fulfilled. This is the first report of P. ananatis causing leaf spots on sorghum plants in Brazil.
The bacteria Pantoea ananatis is reported to survive in nature on different hosts as an epiphyte, a saprophyte and a pathogen. Natural infections occur in pineapple, corn, onion, Sudan grass (Sorghum sudanense), eucalyptus, rice, tomato and melon (Azad et al. 2000; Coutinho and Venter 2009). Artificial inoculations have shown this organism to be pathogenic to sugarcane, oats, cotton and sorghum (Sorghum bicolor) (Azad et al. 2000; Coutinho and Venter 2009). On maize P. ananatis was identified as the causal agent of white spot (Paccola-Meirelles et al. 2001; Bomfeti et al. 2008) where it caused more than 60% yield losses on susceptible genotypes (Pinto 1999). Sawazaki et al. (1997) has also shown a correlation between the incidence of white spot on maize and a reduction in corn kernel weight. In Mexico, maize is an important cereal for human nutrition and P. ananatis has been detected causing leaf spots on corn (Pérez-y-Terrón et al. 2009). This disease has also been reported in two other South American maize-producing countries: Argentina (Alippi and López 2010) and Brazil (Paccola-Meirelles et al. 2001).
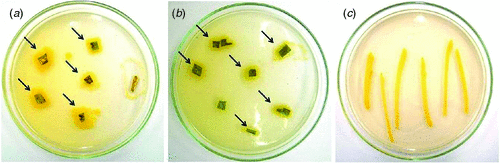
In April 2010, near Embrapa, at the Maize and Sorghum Research Center Experiment Station in Sete Lagoas, Minas Gerais, Brazil, sorghum plants were observed with leaf spot symptoms, which were ellipsoid to irregular in shape, had reddish brown borders and chlorotic centers (Fig. 1). Infected tissue, collected from these plants, was examined under a stereomicroscope and revealed no fungal structures on the lesions. Tissue segments from the lesion borders were excised, surface sterilised, and plated out on TSA (soybeans casein digest agar – tryptone soya agar) media. Two days later, bacterial colonies were observed (Fig. 2), from which one yellow colony was subcultured and purified for pathogenicity testing and DNA extraction.

|
|
For the pathogenicity test, yellow colonies were transferred to 100 mL TSB (tryptone soya broth – soybean casein digest) media, and grown on a shaker for 16 h at room temperature (25–27°C). Subsequently, 1 mL of this suspension was transferred to fresh 100 mL TSB media and shaken for 4 h. The bacterial concentration of this suspension was measured in a spectrophotometer at OD600 and adjusted to a final concentration of 108–109 cells/mL (Lelliot and Stead 1987; Romeiro 2001), using 0.85% saline in a 1 : 1 ratio. Healthy leaves were inoculated by spraying both leaf surfaces of 30-day-old sorghum plants with this suspension. After inoculation, plants were kept for 18 h in a dew chamber at a temperature of 28°C and 80% humidity. After the incubation period, plants were transferred to a growth chamber at a temperature of 28°C and 40% humidity, where they remained until evaluation. First symptoms were observed 24 h after inoculation and were characterised by the presence of red spots scattered on the leaf surface. After 3–4 days, these spots had progressed to necrotic lesions similar to the symptoms observed on sorghum plants in the field (Fig. 3). Tissue segments of infected leaves were collected and used for reisolation of the bacterium, following the above described procedures. Typical yellow colonies similar to the original colony obtained from infected material collected in the field were produced on TSA (Fig. 2).

|
Molecular identification of the bacteria isolated both from the original material and the greenhouse inoculations were conducted. Genomic DNA was extracted from bacterial colonies according to Sambrook et al. (1989). The recombinant DNA (rDNA) fragments were amplified using primers F968 and R1401, targeting the 16S rDNA gene (Nübel et al. 1996). The polymerase chain reaction consisted of 20 ng DNA, 50 mmol/L of each deoxynucleotide triphosphate (dNTP), 2.5 mmol/L MgCl2, 2 mmol/L TRIS-HCl (pH 8.4), 50 mmol/L KCl, 0.2 mmol/L of each primer and 1 unit of Taq DNA polymerase (Invitrogen, Carslbad, CA, US) in a final volume of 50 mL. The samples were denatured at 94°C for 2 min, followed by 30 amplification cycles (94°C for 1 min, 55°C for 1 min, 72°C for 2 min) and a final extension (72°C for 10 min). The amplification products were analysed by electrophoresis on 1.5% agarose gel using TAE buffer (40 mmol/L TRIS-acetate, 1 mmol/L ethylenediaminetetraacetic acid, pH 8.0). The gel was stained with ethidium bromide (0.5 mg/mL), viewed under ultraviolet light and images were captured and stored in a photo documentation system (Gel Logic 200 Kodak, Rochester, NY, US). The amplification products were removed from the gel, purified using the kit ‘QIAquick Gel Extraction’, according to the manufacturer’s instructions (Qiagen, Hilden, Germany) and sequenced using ‘Big Dye Terminator v3.1.Cycle Sequencing’ (Applied Biosystems, Foster City, CA, US). The samples were analysed in an automatic sequencer (ABI Prism 3100, Applied Biosystems) and nucleotide sequences were compared with sequences deposited in the GenBank database (http://www.ncbi.nlm.nih.gov/) using the program BlastN (Altschul et al. 1997). Both colonies most closely matched P. ananatis, showing greater than 99% similarity to an isolate deposited by Kido et al. (2008). The partial nucleotide sequence of 16S rRNA, used in the present work, was deposited in GenBank with the accession no. HQ333482, and a culture of the bacterial strain was deposited in the Collection of Plant Pathogens of Culture of Maize and Sorghum of Embrapa Milho e Sorgo, with the accession no. PA 033.10
This is the first reported occurrence of P. ananatis as a sorghum pathogen in Brazil, and the first report of natural infection of sorgham plants by P. ananatis. In the case of sorghum, there are no studies that show the extent or severity of occurrence of this pathogen in Brazil. However, given that sorghum is grown in areas that are adjacent to or used for maize production, identification of this pathogen on sorghum is important as this host may provide an alternative inoculum source for maize crops. White spot is currently a major disease of maize in Brazil and causes losses in virtually all maize-producing regions.
Acknowledgements
The authors thanks Coordenação de Aperfeiçoamento de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Cientifico e Teológico (CNPQ), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) and Embrapa Milho e Sorgo for providing financial support.
References
Alippi AM, López AC (2010) First report of leaf spot disease of maize caused by Pantoea ananatis in Argentina. Plant Disease 94, 487| First report of leaf spot disease of maize caused by Pantoea ananatis in Argentina.Crossref | GoogleScholarGoogle Scholar |
Altschul SF, Madden TL, Shaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Research 25, 3389–3402.
| Gapped BLAST and PSI-BLAST: a new generation of protein database search programs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXlvFyhu7w%3D&md5=11a95367f22ad72602f0b2d2d7c2c6ceCAS | 9254694PubMed |
Azad HR, Holmes GJ, Cooksey DA (2000) A new leaf blotch disease of sudangrass caused by Pantoea ananas and Pantoea stewartii. Plant Disease 84, 973–979.
| A new leaf blotch disease of sudangrass caused by Pantoea ananas and Pantoea stewartii.Crossref | GoogleScholarGoogle Scholar |
Bomfeti CA, Souza-Pacolla EA, Massola Júnior NS, Marriel IE, Meirelles WF, Casela CR, Paccola-Meirelles LD (2008) Localization of Pantoea ananatis inside lesions of maize white spot diseases using transmission electron microscopy and molecular techniques. Tropical Plant Pathology 33, 63–66.
| Localization of Pantoea ananatis inside lesions of maize white spot diseases using transmission electron microscopy and molecular techniques.Crossref | GoogleScholarGoogle Scholar |
Coutinho TA, Venter SN (2009) Pantoea ananatis: an unconventional plant pathogen. Molecular Plant Pathology 10, 325–335.
| Pantoea ananatis: an unconventional plant pathogen.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXmtVOkt7g%3D&md5=afe191744d6e5ea96dbecdbd0f822096CAS | 19400836PubMed |
Kido K, Adachi R, Hasegawa M, Yano K, Hikichi Y, Takeuchi S, Atsuchi T, Takikawa Y (2008) Internal fruit rot of netted melon caused by Pantoea ananatis (=Erwinia ananas) in Japan. Journal of General Plant Pathology 74, 302–312.
| Internal fruit rot of netted melon caused by Pantoea ananatis (=Erwinia ananas) in Japan.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtFOmt7jO&md5=aa7cbf1f72ea60a06f164ba724e82ec5CAS |
Lelliot RA, Stead DE (1987) ‘Methods for diagnosis of bacterial plant disease’. (Blakwell Scientific Publications: Oxford)
Nübel U, Engelen B, Felske A, Snaidr J, Wieshuber A, Amann RI, Ludwig W, Backhaus H (1996) Sequence heterogeneities of genes encoding 16S rRNAs in Paenibacillus polymyxa detected by temperature gradient gel electrophoresis. Journal of Bacteriology 178, 5636–5643.
Paccola-Meirelles LD, Ferreira AS, Meirelles WF, Marriel IE, Casela CR (2001) Detection of a bacterium associated with a leaf spot disease of maize in Brazil. Journal of Phytopathology 149, 275–279.
| Detection of a bacterium associated with a leaf spot disease of maize in Brazil.Crossref | GoogleScholarGoogle Scholar |
Pérez-y-Terrón R, Villegas MC, Cuellar A, Muñoz-Rojas J, Castañeda-Lucio M, Hernández-Lucas I, Bustillos-Cristales R, Bautista-Sosa L, Munive JA, Caicedo-Rivas R, Fuentes-Ramírez LE (2009) Detection of Pantoea ananatis, causal agent of leaf spot disease of maize, in Mexico. Australasian Plant Disease Notes 4, 96–99.
Pinto NFJA (1999) Eficiência de doses e intervalos de aplicação no controle da mancha foliar provocada por Phaeosphaeria maydis Rene, Payak & Renfro. Ciência e Agrotecnologia 23, 1006–1009.
Romeiro RD (2001) ‘Métodos em Bacteriologia de Plantas’. (Editora UFV: Imprensa Universitária, Viçosa, MG, Brasil)
Sambrook J, Fritsch EF, Maniatis T (1989) ‘Molecular cloning: a laboratory manual’. (Cold Spring Harbor Laboratory: Cold Spring Harbor, N.Y.)
Sawazaki E, Dudienas C, Paterniani MEAGZ, Galvão JCC, Castro JL, Pereira J (1997) Reação de cultivares de milho à Phaeosphaeria no estado de São Paulo. Pesquisa Agropecuaria Brasileira 32, 585–589.


