The Eucalyptus canker pathogen Holocryphia eucalypti on Eucalyptus in New Zealand
M. Gryzenhout A D , M. Vermeulen B , M. Dick C and M. J. Wingfield AA Forestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, Pretoria 0002, South Africa.
B Department of Microbiology and Plant Pathology, Forestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, Pretoria 0002, South Africa.
C Scion Research, New Zealand Forest Institute Ltd, Rotorua, New Zealand.
D Corresponding author. Email: Marieka.Gryzenhout@fabi.up.ac.za
Australasian Plant Disease Notes 5(1) 5-8 https://doi.org/10.1071/DN10003
Submitted: 26 October 2009 Accepted: 7 December 2009 Published: 1 February 2010
Abstract
Holocryphia eucalypti is an opportunistic canker pathogen of Eucalyptus and Corymbia spp. (Myrtaceae, Myrtales) in Australia and South Africa. It is also known in Australia on Tibouchina trees (Melastomataceae, Myrtales). Using DNA sequence comparisons and morphological characterisation, we show for the first time that H. eucalypti is present in New Zealand on Eucalyptus spp.
Holocryphia eucalypti (=Cryphonectria eucalypti) causes die-back, and stem and branch cankers of several Eucalyptus and Corymbia spp. in natural forests and commercial plantations in Australia and South Africa (Walker et al. 1985; Van der Westhuizen et al. 1993; Carnegie 2007). The pathogen has also recently been reported on Tibouchina urvilleana (Lassiandra) planted as ornamentals in Australia (Heath et al. 2007). The canker disease caused by H. eucalypti is closely associated with stress conditions such as drought and cankers can be relatively mild or lethal where host trees are susceptible and stress is severe (Old et al. 1986; Wardlaw 1999; Gryzenhout et al. 2003). Because predisposition plays such an important role in disease development, H. eucalypti is not considered as severe (Yuan and Mohammed 2000) as other closely related Eucalyptus pathogens such as species of Chrysoporthe (Gryzenhout et al. 2009).
Canker caused by H. eucalypti is common on many Eucalyptus and Corymbia species in forests and plantations of eastern Australia including Tasmania, and it also occurs in Western Australia (Old et al. 1986; Davison and Coates 1991; Wardlaw 1999; Yuan and Mohammed 2000; Carnegie 2007). In South Africa, it occurs on commercially propagated, non-native Eucalyptus spp. in plantations (Van der Westhuizen et al. 1993; Gryzenhout et al. 2003). Population genetic studies employing microsatellite data have shown that H. eucalypti was most likely introduced into South Africa, although evidence for its origin in Western Australia was less convincing (Nakabonge et al. 2008).
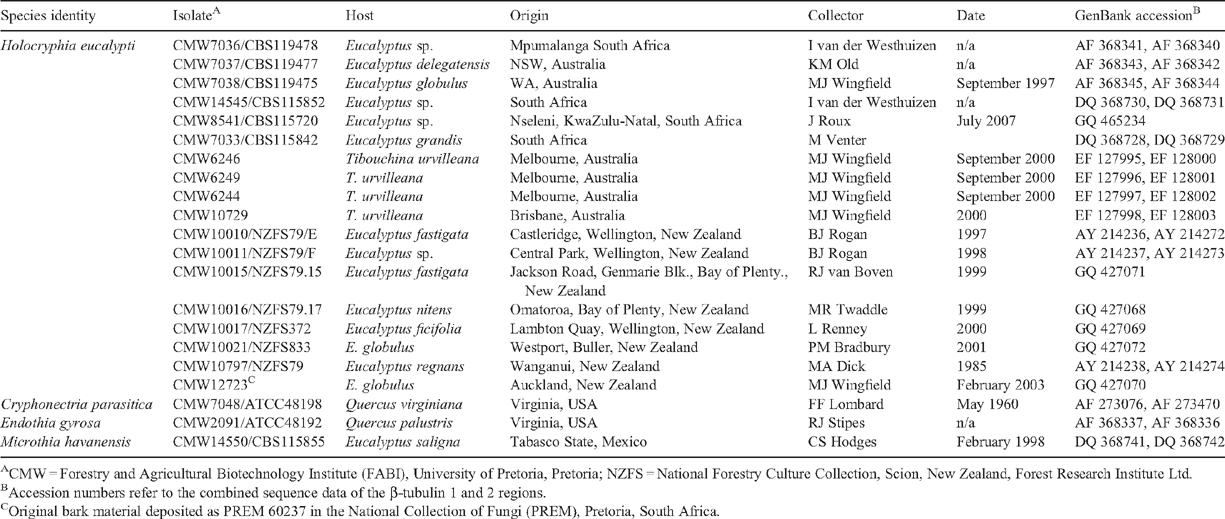
During the course of the past 24 years, isolates of a fungus in the Cryphonectriaceae have been collected from various Eucalyptus species in New Zealand. Most locations were in the southern half of the North Island with one from the north of the South Island (Table 1). Based on cultural characteristics and the morphology of conidia produced in culture, the isolates were identified as Endothiella-like, which represent the anamorph state of some species in the Cryphonectriaceae (Gryzenhout et al. 2009). However, in the absence of sexual fruiting bodies or well-defined fruiting bodies on host tissue and in culture, a definitive identification could not be made. The aim of this study was thus to characterise the isolates from Eucalyptus stems in New Zealand based on DNA sequence comparisons for the β-Tubulin gene region.
|
Original isolates curated in NZFS (National Forestry Culture Collection) were obtained from discoloured yellowish wood, from branch cankers and die-back. These isolates are maintained at 4°C in the culture collection (NZFS) of Scion, New Zealand Forest Research Institute, Rotorua, New Zealand (Table 1). Duplicates of the cultures, as well as an isolate (CMW12723) obtained from asexual fruiting bodies from a later collection, are housed in the culture collection (CMW) of the Forestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, Pretoria, South Africa (Table 1). The representative specimen of the original bark material from which the culture CMW12723 was made has been deposited in the National Collection of Fungi (PREM), Pretoria, South Africa (Table 1).
For DNA sequence comparisons, isolates were grown in 2% Malt Extract broth (100 μL in 1.5 μL Eppendorf tubes), the mycelium harvested and the DNA extracted following Gryzenhout et al. (2006). The DNA was used in polymerase chain reactions (PCR) of the β-Tubulin gene regions 1 and 2 and sequenced using the same protocols as those described by Gryzenhout et al. (2006). The sequences were included in a data matrix containing sequences of H. eucalypti isolates previously published (Gryzenhout et al. 2006; Heath et al. 2007). Sequences of isolates CMW10010 and CMW10011 were obtained from the study of Myburg (2003). Species of other genera in the Cryphonectriaceae (Endothia gyrosa, Cryphonectria parasitica, Microthia havanensis) were defined as outgroups. The dataset consisted of 20 taxa and the sequences were aligned with the CLUSTAL function of the program MEGA ver. 4 (Tamura et al. 2007) and verified manually.
Phylogenetic analyses were run with PAUP (Phylogenetic Analysis Using Parsimony) version 4.0b10 (Swofford 2002). Phylogenetic trees were obtained with maximum parsimony (uninformative sites excluded, heuristic search with 100 random sequence additions and tree-bisection-reconnection branch swapping, MULTREES off, base pairs re-weighted according to the consistency index). The strength of branches was tested with a 70% bootstrap analysis (1000 replicates).
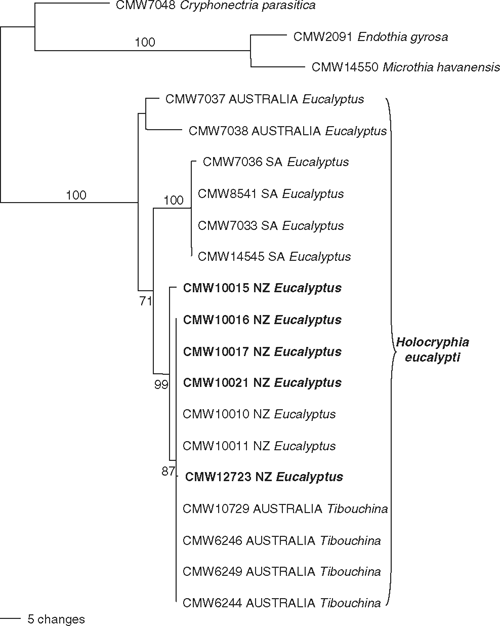
Of the 928 characters in the DNA matrix, 165 were uninformative, 645 were constant and 118 were informative. Two trees that were identical topologically but differed slightly based on length of some branches (Tree Length = 170.3, Consistency Index = 0.882, Retention Index = 0.895, g1 value = –2.62), were obtained in the analysis. The phylogenetic trees (Fig. 1) clearly showed that the isolates from New Zealand grouped with those of H. eucalypti from South Africa and Australia (bootstrap support 100%). The isolates from New Zealand formed a group with those from T. urvilleana in Australia (bootstrap support 87%). Two other groups, including South African and Australian isolates, were also evident (bootstrap support 100% and below 50%, respectively).
|
This study reports the presence of the Eucalyptus pathogen H. eucalypti in New Zealand for the first time. This report further contributes to the databases and checklists of pathogens and fungi in New Zealand (http://nzfungi.landcareresearch.co.nz/html/mycology.asp database; Dingley 1969; Pennycook 1989; McKenzie et al. 2000; Pennycook and Galloway 2004). Due to the opportunistic nature of H. eucalypti on Eucalyptus and Tibouchina, the occurrence of the fungus in New Zealand is most likely not of economic significance, although its presence deserves to be monitored.
Relatively little is known regarding the Cryphonectriaceae in New Zealand. Although there are reports of Endothia gyrosa, a previous name used for H. eucalypti (Gryzenhout et al. 2006), in the country (Gryzenhout et al. 2009), these reports are from Nothofagus (McKenzie et al. 2000; Pennycook and Galloway 2004), Myrsine salicina and dead wood of an unknown host (http://nzfungi.landcareresearch.co.nz/html/mycology.asp database) and need to be verified based on recent taxonomic changes to the group (Gryzenhout et al. 2009). Reports of other Cryphonectriaceae that could represent incorrectly identified H. eucalypti specimens in New Zealand, include those of Rostraureum longirostris on Nothofagus (Nothofagaceae, Fagales), Amphilogia gyrosa and Amphilogia major on Elaeocarpus spp. (Elaeocarpaceae, Oxalidales), an Endothiella sp. and Cryphonectria radicalis (http://nzfungi.landcareresearch.co.nz/html/mycology.asp database; Dingley 1969; Pennycook 1989; McKenzie et al. 2000; Pennycook and Galloway 2004; Gryzenhout et al. 2005). In the NZFS collection, cultures of an Endothiella sp. identified only on the basis of culture characteristics, originate from Myrsine chathamica and Leptospermum scoparium as well as various species of eucalypts. A member of the Cryphonectriaceae has also been found on a Coriaria sp. (Coriariaceae, Cucurbitales) in New Zealand (PDD28477, Waiomu, Thames, Auckland, J.M. Dingley, August 1958) but the identity of this collection relies only on a herbarium specimen and cannot be confirmed (M. Gryzenhout, unpubl. data). Thus, only the identities of A. gyrosa and A. major have been verified based on recent phylogenies (Gryzenhout et al. 2005).
The relative proximity of New Zealand to Australia, as well as the common occurrence of H. eucalypti in the eastern part of Australia indicates a possibility that the fungus originated in the latter country. However, it is also possible that H. eucalypti occurs on native Myrtaceae in New Zealand. It may also occur on other non-native shrubs such as Tibouchina spp. (Melastomataceae, Myrtales), which are hosts of this fungus (Heath et al. 2007) and many other members of the Cryphonectriaceae (Gryzenhout et al. 2009). Answers to these intriguing questions will require further surveys and population genetic studies.
Acknowledgements
Funding from the National Research Foundation (NRF), members of the Tree Protection Co-operative Program (TPCP), the THRIP support program of the Department of Trade and Industry, and the Department of Science and Technology/National Research Foundation Centre of Excellence in Tree Health Biotechnology, South Africa made this study possible.
Carnegie AJ
(2007) Forest health condition in New South Wales, Australia, 1996–2005. II. Fungal damage recorded in eucalypt plantations during forest health surveys and their management. Australasian Plant Pathology 36, 225–239.
| Crossref | GoogleScholarGoogle Scholar |

Davison EM, Coates DJ
(1991) Identification of Cryphonectria cubensis and Endothia gyrosa from eucalypts in Western Australia using isozyme analysis. Australasian Plant Pathology 20, 157–160.
| Crossref | GoogleScholarGoogle Scholar |

Dingley JM
(1969) Records of plant diseases in New Zealand. New Zealand Department of Scientific and Industrial Research Bulletin 192, 120.

Gryzenhout M,
Eisenberg BE,
Coutinho TA,
Wingfield BD, Wingfield MJ
(2003) Pathogenicity of Cryphonectria eucalypti to Eucalyptus clones in South Africa. Forest Ecology and Management 176, 427–437.
| Crossref | GoogleScholarGoogle Scholar |

Gryzenhout M,
Glen HF,
Wingfield BD, Wingfield MJ
(2005) Amphilogia gen. nov. for Cryphonectria-like fungi from Elaeocarpus spp. in New Zealand and Sri Lanka. Taxon 54, 1009–1021.

Gryzenhout M,
Myburg H,
Hodges CS,
Wingfield BD, Wingfield MJ
(2006) Microthia, Holocryphia and Ursicollum, three new genera on Eucalyptus and Coccoloba for fungi previously known as Cryphonectria. Studies in Mycology 55, 35–52.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Heath RN,
Roux J,
Gryzenhout M,
Carnegie AJ,
Smith IW, Wingfield MJ
(2007) Holocryphia eucalypti on Tibouchina urvilleana in Australia. Australasian Plant Pathology 36, 560–564.
| Crossref | GoogleScholarGoogle Scholar |

McKenzie EHC,
Buchanan PK, Johnston PR
(2000) Checklist of fungi on Nothofagus species in New Zealand. New Zealand Journal of Botany 38, 635–720.

Nakabonge G,
Burgess T,
Gryzenhout M,
Roux J,
Wingfield BD, Wingfield MJ
(2008) Population structure of the fungal pathogen Holocryphia eucalypti in Australia and South Africa. Australasian Plant Pathology 37, 154–161.
| Crossref | GoogleScholarGoogle Scholar |

Old KM,
Murray DIL,
Kile GA,
Simpson J, Malafant KWJ
(1986) The pathology of fungi isolated from eucalypt cankers in south-eastern Australia. Australian Forest Research 16, 21–36.

Tamura K,
Dudley J,
Nei M, Kumar S
(2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology and Evolution 24, 1596–1599.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Van der Westhuizen IP,
Wingfield MJ,
Kemp GHJ, Swart WJ
(1993) First report of the canker pathogen Endothia gyrosa on Eucalyptus in South Africa. Plant Pathology 42, 661–663.
| Crossref | GoogleScholarGoogle Scholar |

Walker J,
Old KM, Murray DIL
(1985) Endothia gyrosa on Eucalyptus in Australia with notes on some other species of Endothia and Cryphonectria. Mycotaxon 23, 353–370.

Wardlaw TJ
(1999) Endothia gyrosa associated with severe stem cankers on plantation grown Eucalyptus nitens in Tasmania, Australia. European Journal of Forest Pathology 29, 199–208.
| Crossref | GoogleScholarGoogle Scholar |

Yuan ZQ, Mohammed C
(2000) The pathogenicity of isolates of Endothia gyrosa to Eucalyptus nitens and E. globulus. Australasian Plant Pathology 29, 29–35.
| Crossref | GoogleScholarGoogle Scholar |



