First report of Colombian datura virus from Australia
V. Steele A and J. E. Thomas A BA Primary Industries and Fisheries, Department of Employment, Economic Development and Innovation, Plant Pathology Building, 80 Meiers Road, Indooroopilly, Qld 4068, Australia.
B Corresponding author. Email: john.thomas@dpi.qld.gov.au
Australasian Plant Disease Notes 4(1) 108-109 https://doi.org/10.1071/DN09045
Submitted: 12 May 2009 Accepted: 12 October 2009 Published: 4 November 2009
Abstract
Colombian datura virus was identified from the ornamental plant Brugmansia sp., showing leaf mosaic symptoms. The nucleotide sequence of the 3′ untranslated region and the amino acid sequence of the 3′ portion of the coat protein were 100% identical to those from a Hungarian isolate of the virus. This represents the first record of this virus in Australia.
Brugmansia sp. (Angel’s trumpet; Family: Solanaceae) is a shrub or small tree with flowers that are single, pendent, trumpet-like or funnel-shaped to 30 cm long. Originally from South America, it is used mainly as a houseplant or for landscaping (Russell et al. 1997). In 1968, a virus named Colombian datura virus (CDV, genus Potyvirus) was reported from plants of B. (syn. Datura) candida and B. (syn. Datura) sanguinea originating from Colombia (Kahn and Bartels 1968). The virus has subsequently been reported from Europe, Japan, Canada and the USA in a range of hosts including tomato, pepino, tobacco, cape gooseberry, petunia, the terrestrial orchid Spiranthes ceruna and Brugmansia spp. (Fry et al. 2004; Schubert et al. 2006; Rott et al. 2009).
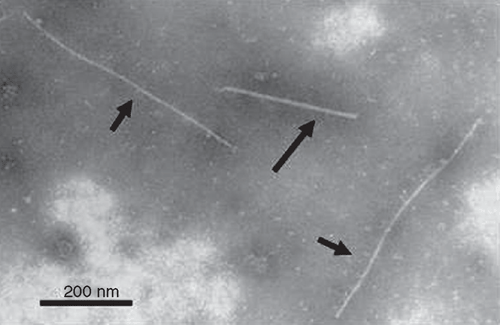
In 2007, a sample of Brugmansia sp. from Bomaderry, New South Wales, Australia was submitted for disease diagnosis. The sample displayed a chlorotic mosaic pattern (Fig. 1) and flexuous filamentous virus-like particles were observed by electron microscopy of a sap preparation (Fig. 2). The particles were estimated to have a mean length of 836 ± 23 nm (42 particles measured), using Tomato mosaic virus as an internal size standard (300 nm × 18 nm; Thomas 1985).

|
|
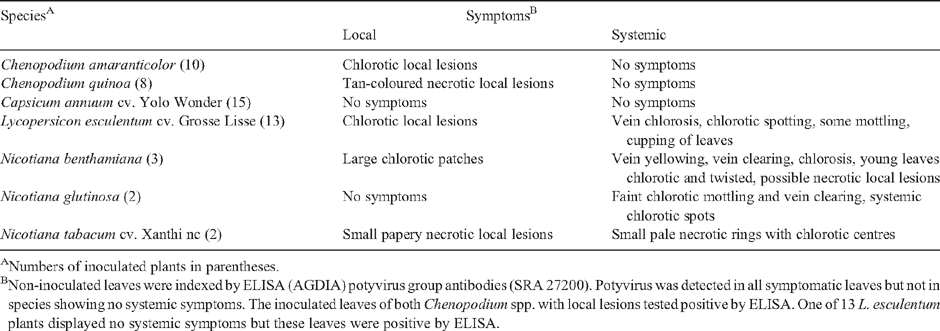
Symptomatic leaves of the Brugmansia sp. sample were used for the mechanical inoculation of a range of test plants and symptoms were recorded after 15 days (Table 1). All inoculated species showed local and/or systemic symptoms, except for Capsicum annuum cv. Yolo Wonder, and were generally consistent with those previously reported for CDV (Kahn and Bartels 1968; Verhoeven et al. 1996; Fry et al. 2004). A random sample of all symptomatic species and the 15 symptomless Yolo Wonder plants were indexed by antigen-coated plate enzyme-linked immunosorbent assay. This was done by using a potyvirus group antibody kit (SRA 27200, Agdia Inc., Elkhart, IN, USA) essentially following the manufacturer’s instructions. All symptomatic plants were positive for potyvirus. All the Yolo wonder plants were negative, except two which gave borderline results. These latter two plants were shown to be uninfected by back-inoculation onto Nicotiana tabacum cv. Xanthi nc.
|
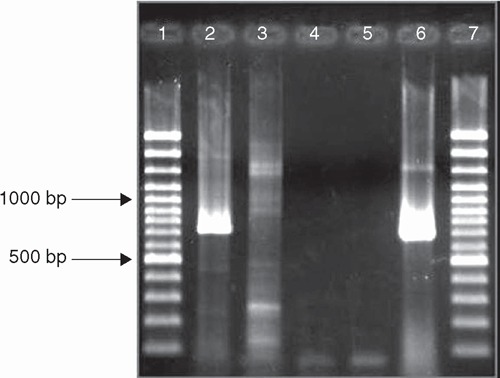
Total RNA was extracted from the Brugmansia leaves using an RNeasy Plant Mini Kit (QIAGEN, Hilden, Germany) following the manufacturer’s instructions. The RNA was eluted from the spin column using 50 μL of RNase-free water. A reverse transcription polymerase chain reaction (RT–PCR) was done using degenerate potyvirus primers U341 (Langeveld et al. 1991) and poty 1 (Gibbs and Mackenzie 1997). The ≈720 bp DNA fragment (Fig. 3) was excised from the agarose gel, cloned and sequenced as described by Schwinghamer et al. (2007). The consensus sequence of 684 nt from three independent clones was lodged on GenBank (FJ821796) and covered the 3′ portion of the coat protein coding region and the 3′ untranslated region (UTR). It was 99.4–99.6% identical to CDV (GenBank accessions AM113754, AM113757 and AY621656). When translated, the sequence of the 3′ 147 amino acids of the coat protein was found to be 100% identical, and the nt sequence of the 3′UTR 99.6–100% identical to these same CDV isolates.
|
This report represents the first record of CDV from Australia. As CDV can also infect tomato and potato, it may constitute a threat to these crops. The Australian Brugmansia isolate is stored in the QPIF Indooroopilly Plant Virus Collection as virus isolate number 2079.
Fry CR,
Zimmerman MT, Scott SW
(2004) Occurrence of Colombian datura virus in the Terrestrial Orchid, Spiranthes cernua. Journal of Phytopathology 152, 200–203.
| Crossref | GoogleScholarGoogle Scholar |

Gibbs A, Mackenzie A
(1997) A primer for amplifying part of the genome of all potyvirids by RT-PCR. Journal of Virological Methods 63, 9–16.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Kahn RP, Bartels R
(1968) The Colombian datura virus – a new virus in the potato virus Y group. Phytopathology 58, 587–592.

Langeveld SA,
Dore J-M,
Memelink J,
Derks AFLM,
van der Vlugt CIM,
Asjes CJ, Bol JF
(1991) Identification of potyviruses using the polymerase chain reaction with degenerate primers. The Journal of General Virology 72, 1531–1541.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Rott M,
Schmidt AM,
Joshi V,
Masters C,
Godkin S, Johnson R
(2009) First Report of Colombian datura virus in Brugmansia in Canada. Plant Disease 93, 196.
| Crossref | GoogleScholarGoogle Scholar |

Schubert J,
Doroszewska T,
Chrzanowska M, Sztangret-Wisniewska J
(2006) Natural infection of tobacco by Columbian datura virus in Poland, Germany and Hungary. Journal of Phytopathology 154, 343–348.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Schwinghamer MW,
Thomas JE,
Parry JN,
Schilg MA, Dann EK
(2007) First record of natural infection of chickpea by Turnip mosaic virus. Australasian Plant Disease Notes 2, 41–43.
| Crossref | GoogleScholarGoogle Scholar |

Thomas JE
(1985) Purification and properties of ginger chlorotic fleck virus. The Annals of Applied Biology 108, 43–50.
| Crossref | GoogleScholarGoogle Scholar |

Verhoeven JTJ,
Lesemann D-E, Roenhorst JW
(1996) First report of Colombian datura potyvirus in tomato. European Journal of Plant Pathology 102, 895–898.
| Crossref | GoogleScholarGoogle Scholar |



