First record of Cryptosporiopsis actinidiae in Australia
E. M. Davison A B D , J. H. Cunnington C and F. C. S. Tay A BA Department of Conservation and Land Management, Kensington, WA 6151, Australia.
B Present address: Department of Environmental and Aquatic Sciences, Curtin University, GPO Box U1987, Perth, WA 6845, Australia.
C Primary Industries Research Victoria, Department of Primary Industries, Knoxfield Centre, Private Bag 15, Ferntree Gully Delivery Centre, Vic. 3156, Australia.
D Corresponding author. Email: e.davison@curtin.edu.au
Australasian Plant Disease Notes 4(1) 66-67 https://doi.org/10.1071/DN09028
Submitted: 20 April 2009 Accepted: 7 June 2009 Published: 17 June 2009
Abstract
Cryptosporiopsis actinidiae, the cause of post-harvest rot in yellow-fleshed kiwifruit in New Zealand, was isolated from freshly sawn Eucalyptus diversicolor scantling in the south-west of Western Australia in 1986. This isolate was identified by rDNA internal transcribed spacer sequencing in 2006. It is the first record of C. actinidiae in Australia.
In 1986 a large number of fungal isolations were made from rot and discolouration in karri trees (Eucalyptus diversicolor) from regrowth stands in the south-west of Western Australia (Davison and Tay 2008). This was part of a study to determine which fungi caused these symptoms and whether all symptoms of discolouration would ultimately develop into rot. Many different symptoms were sampled including a dark discolouration associated with galleries of larvae of the common wood boring beetle Phoracantha acanthocera. One mitosporic fungus (DCE 312) obtained from this symptom in freshly sawn scantling was used in subsequent experiments. It was sent to the Commonwealth Mycological Institute and was identified as Colletotrichum acutatum. This isolate caused a brown discolouration when grown on tannic acid agar, indicating that it produced extracellular oxidases (Nobles 1965). When inoculated into the sapwood and heartwood of regrowth karri trees it persisted in some inoculations for up to 4 years, and caused a dark discolouration in the sapwood.
The identity of DCE 312 was checked in 2006 as part of an attempt to identify some of the cultures isolated from rot and discoloured wood. The rDNA (rDNA) internal transcribed spacer (ITS) region was amplified and sequenced using primers ITS5 and ITS4 (White et al. 1990). BLAST searches revealed this sequence to be identical or up to four bases different to the Cryptosporiopsis actinidiae ITS sequences on GenBank.
Cryptosporiopsis actinidiae was described from New Zealand by Johnston et al. (2004). It causes an economically important post-harvest rot of yellow-fleshed kiwifruit (Actinidia chinensis var. chinensis ‘Hort16A’) (Fullerton et al. 2007; Wurms et al. 2007) but has a wider host range including other species of kiwifruit (A. arguta, A. deliciosa and A. indochinensis), apple (Malus × domesticus) and persimmon (Diospyros virginiana). It has also been isolated at low levels from native forest in New Zealand (Johnson et al. 2005). Genetic analysis of isolates from several different locations and hosts has shown that it is outbreeding with five vegetative compatibility genes; the teleomorph is likely to be a Neofabraea sp., but has not been identified (Beever and Parkes 2007).
The morphology and cultural characteristics of isolate DCE 312 agree with the original description of C. actinidiae (Johnston et al. 2004). In particular, this isolate was a characteristic intense red on the reverse when grown on potato dextrose agar. The conidia were hyaline, smooth, cylindrical, rounded at the apex, tapered at the base with a truncate scar, [20/1] (5−) 7.5–13.5 (−15) × 1.5–3 µm (Fig. 1). The only discrepancy was that the conidia in the original description were wider, ranging from 3–5.5 µm. To our knowledge this is the first report of C. actinidiae in Australia.
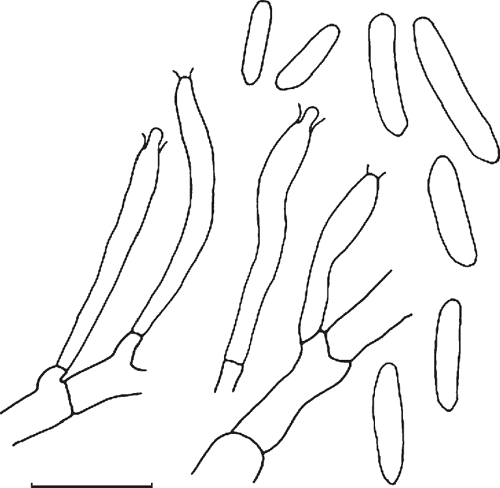
|
Cultures of C. actinidiae (DCE 312) have been deposited at the Department of Agriculture and Food Western Australia as WAC 13024, and at the International Mycological Institute, UK, as IMI 316717. The rDNA ITS sequence has been deposited on GenBank as accession FJ974105.
Beever RE, Parkes SL
(2007) Vegetative compatibility groups in the fungus Cryptosporiopsis actinidiae. New Zealand Journal of Crop and Horticultural Science 35, 67–72.

Davison EM, Tay FCS
(2008) Causes of incipient rot and rot in regrowth Eucalyptus diversicolor (karri) trees. Plant Pathology 57, 1097–1102.
| Crossref | GoogleScholarGoogle Scholar |

Fullerton RA,
Rheinländer PA,
Manning MA, Casonato SG
(2007) Infection and rot expression by Cryptosporiopsis actinidiae and Phomopsis sp. in kiwifruit, Actinidia chinensis ‘Hort16A’. Acta Horticulturae 753, 653–660.

Johnston PR,
Manning MA,
Meier X,
Park D, Fullerton RA
(2004)
Cryptosporiopsis actinidiae sp. nov. Mycotaxon 89, 131–136.

Johnson PR,
Pennycook SR, Manning MA
(2005) Taxonomy of fruit-rotting fungal pathogens: what’s really out there? New Zealand Plant Protection 58, 42–46.

Nobles MK
(1965) Identification of cultures of wood-inhabiting Hymenomycetes. Canadian Journal of Botany 43, 1097–1139.
| Crossref | GoogleScholarGoogle Scholar |

Wurms KV,
Reglinski T,
Friel EN,
Wang M,
Chynoweth R,
Taylor JT, Ah Chee A
(2007) Postharvest biochemical changes in ‘Hort16A’ kiwifruit: effects of fungal inoculation and storage environment. Acta Horticulturae 753, 677–684.



