Detection of Corynespora cassiicola in Hevea rubber tree from China
Y. X. Qi A B D , X. Zhang A , J. J. Pu A , Y. X. Xie A D , H. Q. Zhang A , S. L. Huang B C and H. Zhang AA Hainan Key Laboratory of Monitoring and Management of Pest of Tropical Agriculture, Environment and Plant Protection Institute, Chinese Academy of Tropical Agricultural Sciences, Danzhou, Hainan 571737, P. R. China.
B College of Life Science and Technology, Guangxi University, Nanning, Guangxi 530004, P. R. China.
C Nanyang Normal University, Nanyang, Henan 473061, P. R. China.
D Corresponding author. Email: qiyanxiang@126.com; yixian81@126.com
Australasian Plant Disease Notes 2(1) 153-155 https://doi.org/10.1071/DN07060
Submitted: 8 October 2007 Accepted: 21 November 2007 Published: 5 December 2007
Abstract
Corynespora cassiicola, which causes Corynespora leaf fall disease, is one of the most destructive and economically important fungal pathogens of Hevea rubber trees in Asian and African countries. This fungus is present in some Hevea rubber plantations and nurseries in Hainan and Yunnan provinces of South China. We describe the development of a PCR assay for C. cassiicola. With this test, of the 32 leaf samples obtained from 28 Hevea rubber trees in 15 rubber plantations and nurseries of Hainan and Yunnan provinces, 21 tested positive for the fungus. This assay is useful for the screening and certification of young C. cassiicola-free Hevea rubber plants (for distribution to commercial growers) due to its high sensitivity, reliability and efficacy.
Corynespora cassiicola, the causal agent of Corynespora leaf fall disease, is one of the most economically important fungi of Hevea rubber trees in Asian and African countries (Ismail and Jeyanayagi 2003). It was first isolated from Hevea rubber in Malaysia (Newsam 1960) and afterwards in India (Ramakirishnan and Pillai 1961). It can cause circular or irregular amphigenous spots on both young and old leaves and ‘fishbone’ or ‘railway-track’ appearances on the main vein or small veinlets of the leaves, which results in leaves falling all year round, yield reduction of mature rubber trees and even plant death on susceptible clones. Therefore, the International Rubber Research and Development Board (IRRDB) has repeatedly warned rubber producers of this disease and of the risk of outbreaks (IRRDB 2000). Corynespora cassiicola was isolated from Hevea rubber trees in rubber nurseries and plantations of Hainan and Yunnan provinces, China (Pu et al. 2007). Since the rubber tree is vegetatively propagated, the use of the C. cassiicola-free planting materials is the primary method of disease control. To ensure the production of C. cassiicola-free young rubber plants, it is necessary to have a sensitive disease detection method. In this communication, we describe a PCR detection method for C. cassiicola, which we have used to detect diseased leaves of Hevea rubber trees from rubber plantations and nurseries in Hainan and Yunnan provinces of China.
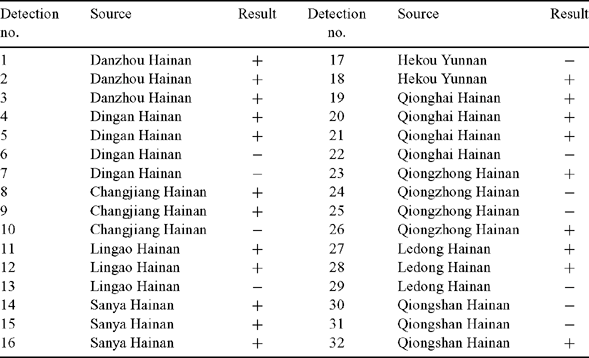
Thirty-two Hevea rubber leaf samples obtained from 28 Hevea rubber trees in 15 rubber plantations and nurseries of Yunnan and Hainnan Provinces, China (Table 1) were screened for C. cassiicola by PCR detection. Primers CCF and CCR (CCF: 5′-CCC TTC GAG ATA GCA CCC-3′; CCR: 5′-ATG CCC TAA GGA ATA CCA AA-3′) were designed by the alignment software Omega 2.0, Primer Express and Primer 5.0, on the basis of the ITS sequence of the rRNA gene of C. cassiicola [GenBank accessions EF490450, EF471932, EF198115, EF198116, EF198117, EF545008, DQ780453, DQ780421, AY238606, AY237605, AF163087 and U95173]. Primers were designed to amplify a fragment of 272 bp. The specificities of the primers were computer-tested, as was the theoretical PCR product. No significant alignments were found with sequences deposited in the public database of the US National Center for Biotechnology Information (NCBI).
|
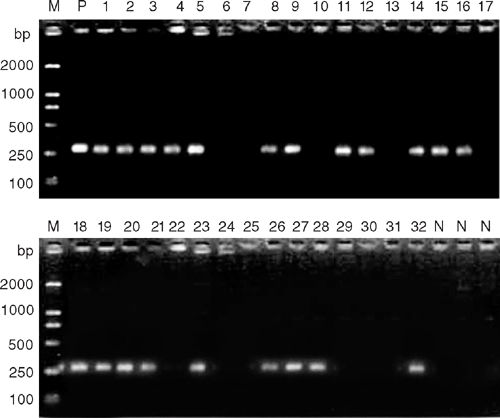
DNA was extracted from Hevea rubber tree leaves according to the method described by Ausubel et al. (1998). Negative controls (no template and healthy rubber leaves) and a positive control (a recombinant plasmid CATAS001) were used. PCR reactions were performed in a total volume of 20 μL with 10× concentrated PCR reaction buffer containing 1.5 mM MgCl2 and a final concentration of 200 μM of each dNTP, 1 μM of each primer and 2.5 units of Taq DNA polymerase per reaction. Amplification was performed in thin-walled PCR tubes in a PTC200 DNA thermal cycler (TM Research) with heated lid, which was programmed as follows: 1 cycle of 2 min at 94°C, followed by 30 cycles of 1 min at 94°C, 1 min at 62°C. One cycle of 5 min at 72°C was conducted after the 30 cycles. After amplification, 8 μL of the reaction mixture was loaded onto a 0.8% agarose gel in 1 × TAE buffer, separated by electrophoresis, stained with ethidium bromide and viewed and photographed under UV light. A 2000-bp DNA ladder standard (TaKaRa Biotech) was used as the marker.
Of the 32 samples tested for C. cassiicola by PCR detection, 21 were found to be infected (Fig. 1). Negative controls (no DNA target and healthy rubber leaves) were included in every experiment to test for contamination, as well as a positive control (a recombinant plasmid CATAS001). The positive control, but not the negative controls, was to yield an amplicon of 272 bp with the primer pair CCF–CCR. Twenty-one samples yielding amplicons were identical to the positive control in size and shared 99–100% nucleotide sequence homology with the positive control by cloning and sequence analysis.
|
Using this PCR technique, it is feasible to process a large number of Hevea rubber leaf samples at a single time. This will be useful for plant diagnostic clinics, for C. cassiicola-free certification of tissue culture plants, quarantine verification, germplasm screening and selection of disease-resistant plants. The symptomatic expression of C. cassiicola is variable and cannot be relied on as a means of diagnosis and indeed most of samples tested were symptomless. Infected plants may, therefore, be inadvertently propagated if diagnosis is only by visual screening.
Acknowledgements
This work was supported by the grants 2004DIA4J012 and 2006NKJ-5 from the Ministry of Agriculture, and Ministry of Science and Technology, the People’s Republic of China, respectively.
Pu JJ,
Zhang X,
Qi YX,
Xie YX,
Zhang HQ, Zhang H
(2007) First record of Corynespora leaf fall disease of Hevea rubber tree in China. Australasian Plant Disease Notes 2, 35–36.
| Crossref | GoogleScholarGoogle Scholar |

Ramakirishnan TS, Pillai PNR
(1961) Leaf spots of rubber caused by Corynespora cassiicola. Rubber Board Bulletin 5, 52–53.



