Bottle gourd: a new host of Ralstonia solanacearum in China
G. Gao A B , L. P. Jin B , K. Y. Xie B , G. Q. Yan A and D. Y. Qu B CA College of Life Science, Shanxi Normal University, Linfen 041004, P. R. China.
B Institute of Vegetables and Flowers, Chinese Academy of Agricultural Sciences, Beijing 100081, P. R. China.
C Corresponding author. Email: dyqu@mail.caas.net.cn
Australasian Plant Disease Notes 2(1) 151-152 https://doi.org/10.1071/DN07059
Submitted: 15 October 2007 Accepted: 31 October 2007 Published: 15 November 2007
Abstract
The bottle gourd (Lagenaria siceraria) was confirmed as a new host of Ralstonia solanacearum. The strain isolated from the infected plants was identified as R. solanacearum race 1, biovar. 1.
Bottle gourd (Lagenaria siceraria) is one of the earliest cultivated plants in China. In autumn 2005, a new wilt disease was observed in bottle gourd seedlings growing in lowland fields in Shanxi Province. Disease incidence was ~2%, with symptoms appearing 1 week after transplanting. Initially, the upper leaves of affected plants became wilted, then 3 to 5 days later, almost all leaves of the diseased plants wilted. Symptoms were characterised by wilting, yellowing of leaves extending to the stem and then death 7–14 days after the first appearance of wilt. Five isolations were made from each of two plants, using an agar medium containing peptone (5 g/L), yeast extract (1 g/L), beef extract (3 g/L) and glucose (10 g/L). Bacteria of the 10 isolates showed similar colony morphology on solid media. A representative isolate was purified for identification and inoculation on gourd seedling plants.
Based on the morphological characteristics, physiological and molecular methods, the strain was identified as Ralstonia solanacearum. Two standard R. solanacearum strains GMI1000 and UW551 were used for control (Swanson et al. 2005). The strain produced fluidal colonies with a pink to red centre on TTC and a fluidal creamy colony on CPG medium, which is typical of the pathogen (Hayward 1964; He et al. 1983).
The strain could not oxidise mannitol, sorbitol, dulcitol, maltose, lactose or cellobiose, indicating that it belongs to the biovar. 1 subgroup (Hayward 1964; He et al. 1983). The strain designation was confirmed by comparing the DNA region coding for 16S rRNA with those of other standard R.solanacearum strains in GenBank. Two 16S rDNA fragments of 409 and 164 bp were amplified using two PCR primers OLI1/OLI2 (Caruso et al. 2003) and RSF/RSR (5′-GTAGCAGTGAAATGCGTAG-3′, 5′-GGAAATGAATCCCCAAC-3′, designed based on 16S rDNA gene sequences of strain GMI1000), and the nucleotide sequence of PCR products was submitted to GenBank as accession numbers EF587900 and EF587901, respectively.
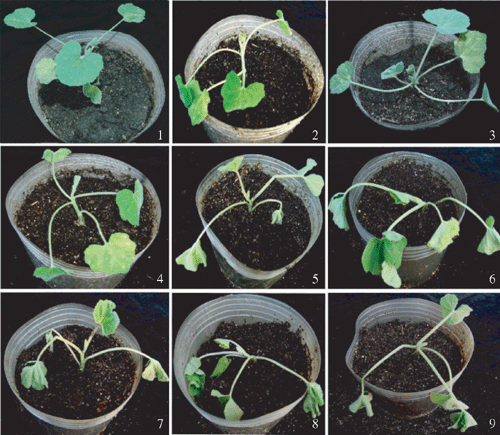
Pathogenicity tests were performed on gourd, tomato and potato seedlings as hosts, as previously described (Williamson et al. 2002). After a 24-h period without water, roots of each plant were cut 1 cm from the stem on one side, and ~10 mL of a bacterial suspension (~5 × 108 CFU/mL) was poured around the base of the stem. Negative control plants were inoculated with water. Plants were incubated at 28°C and observed daily. Approximately 1 week after inoculation, lower leaves of the gourd seedlings began to wilt, followed by the upper leaves of affected plants (Fig. 1). Whole plants wilted and died 7–14 days after the first appearance of the symptoms. However, R. solanacearum commonly developed latent infections on gourd and the proportion of gourd plants that developed active infections ranged from 3 to 10%. These results showed differences in symptom expression between field observations and their inoculations, suggesting that disease progress may be influenced by complicated environmental factors in the field. Inoculation tests indicated that the strain showed high virulence on tomato and potato (Fig. 2) and low virulence on gourd. To our knowledge, this is the first record of the gourd as a host of R. solanacearum in China.
|

|
Acknowledgements
This research was supported by National 863 Program (grant No. 2003AA207130) and Natural Science Foundation of Shanxi Province of China (grant No. 20051042).
Caruso P,
Bertolini E,
Cambra M, López MM
(2003) A new and sensitive co-operational polymerase chain reaction for rapid detection of Ralstonia solanacearum in water. Journal of Microbiological Methods 55, 257–272.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Hayward AC
(1964) Characteristics of Pseudomomas solanacearum. Journal of Applied Bacteriology 27, 265–277.

He LY,
Sequeira L, Kelman A
(1983) Characteristics of strains of Pseudomomas solanacearum from China. Plant Disease 67, 1357–1361.

Swanson JK,
Yao J,
Tans-Kersten J, Allen C
(2005) Behavior of Ralstonia solanacearum race 3 biovar 2 during latent and active infection of geranium. Phytopathology 95, 136–143.
| Crossref | GoogleScholarGoogle Scholar |

Williamson L,
Nakaho K,
Hudelson B, Allen C
(2002) Ralstonia solanacearum race 3, biovar 2 strains isolated from geranium are pathogenic on potato. Plant Disease 86, 987–991.
| Crossref | GoogleScholarGoogle Scholar |



