Toxicity of pure compounds isolated from Tagetes minuta oil to Meloidogyne incognita
O. K. Adekunle A C D , Ruchi Acharya B and Bikram Singh BA Floriculture Division, Institute of Himalayan Bioresource Technology, Palampur, HP 176 061, India.
B Natural Plant Products Division, Institute of Himalayan Bioresource Technology, Palampur, HP 176 061, India.
C Present address: Plant Nematology Laboratory, Department of Plant Science, Obafemi Awolowo University, Ile-Ife, Nigeria.
D Corresponding author. Email: kolaade2002@yahoo.co.uk
Australasian Plant Disease Notes 2(1) 101-104 https://doi.org/10.1071/DN07042
Submitted: 9 October 2006 Accepted: 21 June 2007 Published: 3 July 2007
Abstract
Essential oils are natural volatile substances found in a variety of plants. The toxicity of z-β-ocimene and dihydrotagetone (isolated from the oil of Tagetes minuta) to eggs and juveniles of Meloidogyne incognita was investigated in vitro. Tagetes minuta oil at 4%, 3%, 2% and 1% was strongly toxic to eggs and juveniles of M. incognita. Further studies revealed that dihydrotagetone and z-β-ocimene isolated from the oil showed strong nematicidal activity against M. incognita, with dihydrotagetone showing a higher level of toxicity than z-β-ocimene. The results of this study suggest that dihydrotagetone and z-β-ocimene isolated from T. minuta oil are potential sources of botanicals for control of the root-knot nematode M. incognita.
Additional keywords: root-knot nematode, characterisation.
Introduction
Plant-parasitic nematodes affect many crops worldwide, and the economic losses caused are estimated to be in the order of $77 billion each year (Sasser and Freckman 1987). Plant-parasitic nematodes are either ectoparasites (living outside the root) or endoparasites (living inside the root). The most economically important of the endoparasites are sedentary cyst nematodes of the genera Heterodera and Globodera, and the root-knot nematodes of the genus Meloidogyne. They invade the host root as juveniles and then induce the formation of highly specialised feeding structures within the vascular cylinder. These serve to satisfy the nutritional demand of the developing animal (Sijmons et al. 1994). Root-knot nematodes feed from multinucleate giant cells developed by the expansion of cambial cells within the differentiating vascular cylinder.
Essential oils are natural volatile substances found in a variety of plants. They are composed of isoprenoid compounds, mainly mono- and sesquiterpenes which are the carriers of the smell found in aromatic plants (Franzios et al. 1997). Particular emphasis has been placed on their antibacterial, antifungal, antimite, antitermite and insecticidal activities (Franzios et al. 1997; Chang et al. 2000a, 2000b, 2001a, 2001b, 2001c; Chang and Cheng 2002), but there has been little emphasis on their antinematicidal activities.
Volatile oils of Tagetes minuta have a biological activity against a wide range of microorganisms and insects (Mohamed et al. 2000). They are also used in perfumes and as flavour components in many food products. The plants have a suppressive effect on free-living nematodes and have been used as an intercrop in rotation to protect crops (Kimpinski and Arsenault 1994). However antinematicidal activities of pure compounds isolated from the oil against root-knot nematodes have not been reported. This study was, therefore, designed to investigate the antinematicidal activity of z-β-ocimene and dihydrotagetone on M. incognita in vitro.
Materials and methods
Cultures of Meloidogyne incognita and extraction of eggs
Pure cultures of M. incognita collected from Aligarh Muslim University, Aligarh, India were multiplied on tomato cv. Rutgers in the screenhouse. M. incognita eggs were extracted by shaking infected tomato root pieces with 0.5% sodium hypochlorite (Hussey and Barker 1973). Some of the extracted eggs were incubated using extraction trays to obtain second-stage juveniles (J2).
Extraction of essential oil from Tagetes minuta
In total, 2.5 kg fresh T. minuta (whole plant) was subjected to hydro-distillation for 4 h using Clevenger’s apparatus (Clevenger 1928) and the oil obtained (10 mL, 0.4%, v/v) was dried over anhydrous sodium sulfate. The oil was stored under refrigerated conditions before use.
Isolation and characterisation of pure compounds from Tagetes minuta oil
In total, 10 mL acetonitrile and 5 mL n-pentane were added to 10 mL T. minuta oil in a separating funnel. The two layers were gently shaken and acetonitrile and n-pentane fractions were collected in separate flasks after 30 min. To the acetonitrile fraction, 5 mL n-pentane was added and partitioned in a separating funnel. The resulting acetonitrile and n-pentane fractions were collected in separate flasks. This process was repeated seven times, by adding 5 mL n-pentane to acetonitrile fraction each time to completely remove n-pentane soluble substances from the acetonitrile fraction. Similarly, 5 mL acetonitrile was added to n-pentane fraction and mixed in a separating funnel. The two resulting fractions were collected in separate flasks and this process was repeated seven times as described above. The two composite n-pentane (ocimene-rich) and acetonitrile (dihydrotagetone-rich) fractions were separately distilled in rotavapour at 40°C under reduced pressure to completely remove n-pentane and acetonitrile. Further purification of ocimene and dihydrotagetone was achieved by repeated column chromatography over silica gel of 60–120 mesh with pure hexane and 1%, 2% and 5% ethyl acetate in hexane. The purity of components was monitored on a 14B Shimadzu gas chromatograph fitted with a dimethyl siloxane phase, SE-30 capillary column (30 m × 0.25 mm, with 0.25 mm film thickness) using nitrogen as a carrier gas with a flow rate of 1 mL/min on split mode. Temperature was programmed from 40 to 200°C at 10°C/min; the sample was held at 40°C for 2 min and at 200°C for 15 min. Injector and detector temperatures were at 250°C and 230°C, respectively. Samples were injected in 0.2 μL quantities. Gas chromatography was equipped with FID and C-R7A Chromatopac systems.
Gas chromatography–mass spectrometry analysis was conducted on a Perkin-Elmer, quadripole MS system (model Q-mass 910), equipped with an SE-30 capillary column, using a similar temperature programme to that described above. Injection temperature was programmed to be 250°C and transfer line temperature was maintained at 230°C. Helium was used as a carrier gas with flow rate of 6.4 mL/min. Ionisation energy in mass was 70 ev, mass range 40–300 amu and scan rate was 1.92. The constituents were identified by computer library search, visual interpretation of mass spectra (Jennings and Shibamoto 1988; Adams 1989; Stein 1990), comparison with available authentic samples and composition of mass spectra available in our own library.
Egg-hatch and juvenile mortality tests
In total, 3-mL aliquots of ~200 eggs of M. incognita were transferred separately into Petri dishes (60-mm diameter) containing 3 mL each of 8%, 6%, 4% and 2% T. minuta oil, thus reducing effective concentrations of the oil to 4%, 3%, 2% and 1% respectively. Petri dishes containing 3 mL of distilled water served as controls. Each treatment was replicated five times with separate controls. Petri dishes were incubated in the laboratory at ambient temperature. The numbers of hatched juveniles were counted every 24 h for 14 days. For the juvenile mortality test, 3 mL aliquots of ~200 J2 (freshly hatched) were transferred separately into 60-mm Petri dishes containing 3 mL of T. minuta oil or distilled water as described for the egg-hatch study. The numbers of immobilised nematodes were counted every 24 h for 14 days. Nematodes were confirmed dead when they remained immobile and failed to respond to touch by picking brush on transferring them into distilled water. In separate trials, z-β-ocimene and dihydrotagetone at 2% and 1% were tested on eggs and J2 of M. incognita as described earlier. The egg-hatch and juvenile mortality trials were performed twice.
Statistics
Egg-hatch data were subjected to statistical analysis using the SAS (1985) statistical package. Differences between means were tested using Duncan’s Multiple Range Test at P = 0.05
Results
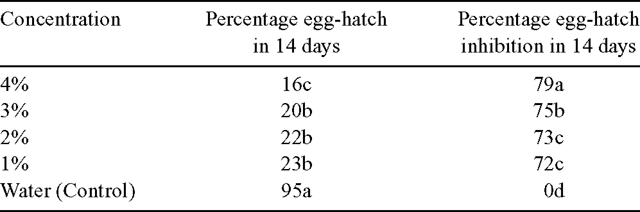
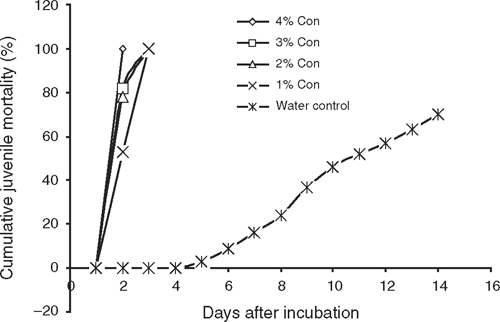
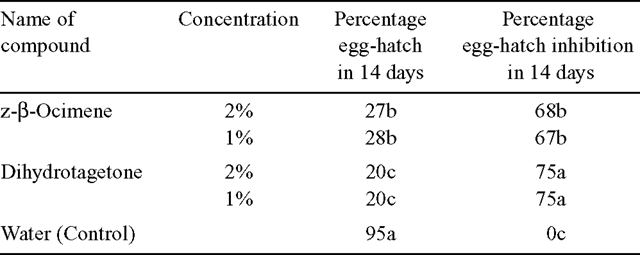
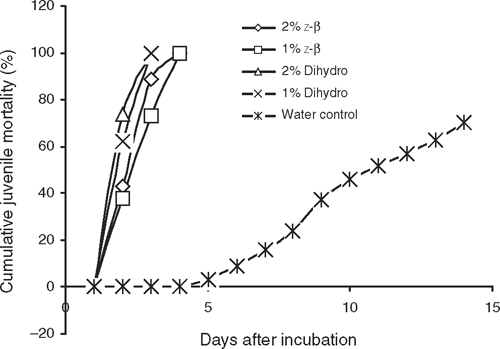
The results presented in Table 1 indicate that T. minuta oil was very toxic to eggs of M. incognita as shown by the 72–79% inhibition in egg-hatch in 14 days. Egg-hatch inhibition was found to be directly proportional to concentration of the oil. Similarly, M. incognita juveniles incubated in T. minuta oil were killed within 72 h (Fig. 1). Also, dihydrotagetone at 2% and 1% inhibited egg-hatch of M. incognita (75%) more than z-β-ocimene at 2% and 1% (67–68%). Dihydrotagetone was significantly more toxic to eggs of M. incognita than z-β-ocimene (Table 2). Similarly, juveniles of M. incognita incubated in dihydrotagetone were all killed within 72 h, while those incubated in z-β-ocimene were all killed in 96 h (Fig. 2).
|
|
|
|
Discussion
T. minuta oil, as well as dihydrotagetone and z-β-ocimene isolated from the oil, were strongly toxic to eggs and juveniles of M. incognita. The results of the present study also suggest that dihydrotagetone showed a stronger nematicidal activity against M. incognita than z-β-ocimene. It is well known that plant-derived natural products are extensively used as biologically active compounds. Among them, essential oils were the first preservatives used by man, originally in their natural state within plant tissues and then as oils obtained by water distillation. Essential oils and extracts obtained from trees have been studied for use as natural insecticides instead of organic phosphorus materials or other synthetic agents (Cheng et al. 2003). In a related study, bark essential oil of Calocedrus formosana and heartwood and bark essential oils of Cryptomeria japonica, among 14 essential oils, showed stronger toxicity against brine string (Cheng et al. 2003). The results of the present study are corroborated by the findings of Mohamed et al. (2000) who reported that aromatic Tagetes plants produce secondary products which have a biological activity against a wide range of microorganisms, insects and nematodes. Our results are also consistent with the reports that the bioassay of T. minuta and S. areira oils and their pure principal components revealed strong inhibitory activity of the root growth of Zea mays seedlings (Scrivanti et al. 2003). The use of T. minuta essential oil components in nematode control could be an alternative pest control method for minimising the side effects of some synthetic nematicides on the environment. The results of this study suggest that dihydrotagetone and z-β-ocimene isolated from T. minuta oil are potential sources of botanicals for control of the root-knot nematode, Meloidogyne incognita.
Acknowledgements
Dr O. K. Adekunle acknowledges award of a postdoctoral fellowship from Third World Academy of Sciences (TWAS), Trieste, Italy. Authors are thankful to the Director, Institute of Himalayan Bioresource Technology for encouragement and providing necessary research facilities. Professor Akhtar Hasseb of the Institute of Agriculture, Aligarh Muslim University, India is gratefully acknowledged for providing cultures of Meloidogyne incognita. Drs Sandeep Manuja and Raja Ram, IHBT, Palampur are thanked for running statistical analysis. IHBT Comm. No. is 0503.
Chang ST, Cheng SS
(2002) Antitermitic activity of leaf essential oils and components from Cinnamomum osmophleum. Journal of Agricultural and Food Chemistry 50, 1389–1392.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Chang ST,
Wang SY,
Wu CL,
Chen PF, Kuo YH
(2000a) Comparisons of the antifungal activities of cadinane skeletal sesquiterpenoids from Taiwania (Taiwania cryptomerioides Hayata) heart-wood. Holzforschung 54, 241–245.
| Crossref | GoogleScholarGoogle Scholar |

Chang ST,
Chen PF, Chang SC
(2000b) Antibacterial activity of essential oils and extracts from Taiwania (Taiwania cryptomerioides Hayata). Quarterly Journal of Chinese Forest 33, 119–125.

Chang ST,
Chen PF,
Wang SY, Wu HH
(2001a) Antimite activity of essential oils and their constituents from Taiwania cryptomerioides. Journal of Medical Entomology 38, 455–457.
| PubMed |

Chang ST,
Chen PF, Chang SC
(2001b) Antibacterial activity of leaf essential oils and their constituents from Cinnamomum osmophleum. Journal of Ethnopharmacology 77, 123–127.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Chang ST,
Cheng SS, Wang SY
(2001c) Antitermitic activity of essential oils and components from Taiwania (Taiwania cryptomerioides). Journal of Chemical Ecology 27, 717–724.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Cheng SS,
Chung HT,
Chang ST,
Tsai KH, Chen WJ
(2003) Bioactivity of selected plant essential oils against the yellow fever mosquito, Aedes aegypti larvae. Bioresource Technology 89, 99–102.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Clevenger JF
(1928) Apparatus for the determination of volatile oil. Journal of the American Pharmaceutical Association 17, 346.

Franzios G,
Morotson M,
Hatziapostolou E,
Kral J,
Scouras ZG, Mavragani TP
(1997) Insecticidal and genotoxic activities of mint essential oils. Journal of Agricultural and Food Chemistry 45, 2690–2694.
| Crossref | GoogleScholarGoogle Scholar |

Hussey RS, Barker KR
(1973) A comparison of methods of collecting inocula of Meloidogyne spp. including a new technique. Plant Disease Reporter 57, 1025–1028.

Kimpinski J, Arsenault WJ
(1994) Nematodes in annual ryegrass, marigold, mustard, red clover and soybean. Forage Notes 37, 52–53.

Mohamed MAH,
Harris PJC, Handerson J
(2000) In vitro selection and characterization of a drought tolerant clone of Tagetes minuta. Plant Science 159, 213–222.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Scrivanti LR,
Sunino MP, Zygadlo JO
(2003) Tagetes minuta and Schinus areira essential oils as allelopathic agents. Biochemical Systematics and Ecology 31, 563–572.
| Crossref | GoogleScholarGoogle Scholar |

Sijmons PC,
Atkinson HJ, Wyes U
(1994) Parasitic strategies of root nematodes and associated host cell responses. Annual Review of Phytopathology 32, 235–259.
| Crossref | GoogleScholarGoogle Scholar |



