First report of Pseudomonas marginalis pv. marginalis as a cause of soft rot of potato in China
Jinhua Li A B , Zhaoxiang Chai A , Hetong Yang C , Guoquan Li D and Di Wang B EA Department of Plant Pathology, College of Grassland Science, Gansu Agricultural University, Lanzhou 730070, China.
B Gansu Key Laboratory of Crop Genetic and Germplasm Enhancement, Gansu Agricultural University, Lanzhou 730070, China.
C Biology Institute of Shandong Academy of Sciences, Jinan 250014, China.
D Zhangye Trial Farm of Gansu Agricultural Science Academy, Zhangye 734000, China.
E Corresponding author. Email: jiangze@public.lz.gs.cn
Australasian Plant Disease Notes 2(1) 71-73 https://doi.org/10.1071/DN07029
Submitted: 5 March 2007 Accepted: 22 April 2007 Published: 4 May 2007
Abstract
Pseudomonas marginalis pv. marginalis was identified by physiological and molecular methods (16 S rDNA sequence), as a cause of bacterial soft rot of potato (Solanum tuberosum) in Zhangye District of central Gansu, China. Pathogenicity was confirmed in whole potato microtubers, seedlings and tuber pieces and the pathogen reisolated from the inoculated plants. This is the first report of bacterial soft rot of potato caused by P. marginalis pv. marginalis in China.
Potato, after wheat and maize, is the third most important crop in Gansu, China, with over 600 000 ha planted annually (Wang 2004; Qu et al. 2005), a third of China’s total potato production area. Growing areas are characterised by poor soils and frequent growing season droughts. However, the rainfall increases from August to October, about the time of the potato harvest, increasing the risk of pre- and postharvest disease development, especially bacterial soft rots. This, and the problem with storage conditions, results in the ratio of merchandisable tubers to gross yield being well below production systems in developed countries. Also, bacterial colonisation of tubers can further limit their use for starch production or for stockfeed though ptomaine poisoning.
Despite the national and regional significance of potato production in Gansu, there has been little research on disease, other than some work on integrated management of storage rots (Wang 2004). A study was thus undertaken to isolate and identify bacteria responsible for soft rot of potato in Zhangye District (38.55°N, 100.28°E) of central Gansu.
Rotten potato cv. Atlantic tubers were collected in April 2004 from the field at the Zhangye Experimental Farm of Gansu Agricultural Academy College. The tubers that had brown rot and ooze in microtubule fasciculus were rinsed, surface sterilised (70% ethanol) for 3 min, dried and broken open just past the margin of the rot. Sections (5–10 mm square) were aseptically removed and suspended in sterile water, immediately streaked on peptone dextrose yeast medium (PDYM) (Fang 2003) and incubated at 28°C for 24 to 48 h. Bacteria were readily isolated from rotten tubers, but not from healthy controls, and were purified from single colonies. Three strains were selected for pathogenicity testing.
To test pathogenicity, three methods were applied to three potato cultivars, Gannong 1, Shepody and Atlantic, cultured from virus-free microtubers grown in sterile vermiculite and nutrient solution for 60 days. In the first method (Method 1, after Wang et al. 1986) 10 rinsed and surface sterilised tubers of each cultivar were inoculated at five locations on the tuber with 200 μL bacterial suspension (108 cells/mL) produced in shaken liquid PDYM (140 rpm, 28°C, 24 h) by syringe 10 mm below the surface. Five control tubers were treated likewise with sterile water. Treated tubers were incubated at 28°C for 3 days before dissecting and measurement of the diameter of any rot. In the second method (Method 2, after Fang 2003) 15 plants (10 and 30 days old) of each cultivar, grown five per pot, were inoculated with a bacterial suspension (as above) by dribbling 50 and 80 mL, respectively, down the stem and into the soil. Sterile water was applied likewise to 15 control plants of each cultivar. Daily observations were made for signs of pathological effects. For both methods, if symptoms were observed, reisolation was attempted. Cultures whose properties agreed with the inoculated bacteria were recovered.
Both methods provided evidence of pathogenicity of the three strains. Rot diameters of 1.3, 1.5 and 2.2 mm were observed in inoculated tubers (Method 1) of cvv. Gannong 1, Atlantic and Shepody, respectively. Reisolation was attempted and cultures of the same properties obtained. Inoculation of plants (Method 2) resulted in wilt, damping off and death. Gannong 1 wilted in 48 h, and Atlantic and Shepody in 72 h. Damping off in Gannong occurred by 72 h and Shepody by 11 days. Most Atlantic plants had died by 5 days.
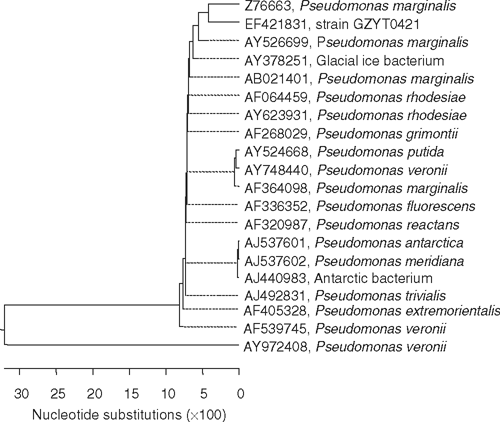
For one strain (GZYT0421, lodged in the Culture Collection of Plant Pathological Laboratory in Gansu Agricultural University and as CGMCC 070212 in China General Microbiological Culture Collection Centre), genomic DNA was extracted and purified (Sambrook and Russell 2001). The purification was assessed from the A260/A280 and A260/A230 extinction ratios. A partial segment of 16 S rDNA (~500 bp) was amplified by PCR using the universal forward primer P1 and reverse primer P6 (Suzuki and Takikawa 2004), purified on a 0.8% low-melting point agarose gel, commercially sequenced (Sangong Co., Shanghai) and lodged as GenBank Accession EF421831. Sequences were analysed and aligned with Blast in GenBank (www.ncbi.nlm.nih.gov), evolutionary distances calculated and a phylogenetic tree generated by the neighbour-joining method with DNAStar (www.biox.cn). Strain GZYT0421 had 97% sequence homology with Pseudomonas marginalis (Fig. 1).
|
Pure cultures of the pathogenic strain GZYT0421 were characterised by standard morphological, biochemical and physiological tests: LOPAT reaction (Lelliot and Stead 1987), colony and cell morphology, flagella, Gram reaction, fluorescent pigment, gelatin liquefaction, denitrification, lecithinase, lipase, pyocyanin, growth at 4 and 41°C, G + C nucleotide content of the DNA, optimum, minimum and maximum growth temperature, nutritional properties (utilisation of glucose, galactose, fructose, mannose, arabinose, xylose, rhamnose, mannitol, formate, lactate, succinate and tartrate) and starch hydrolysis (Lelliot and Stead 1987; Ren 1999; Dong and Cai 2001; Fang 2003).
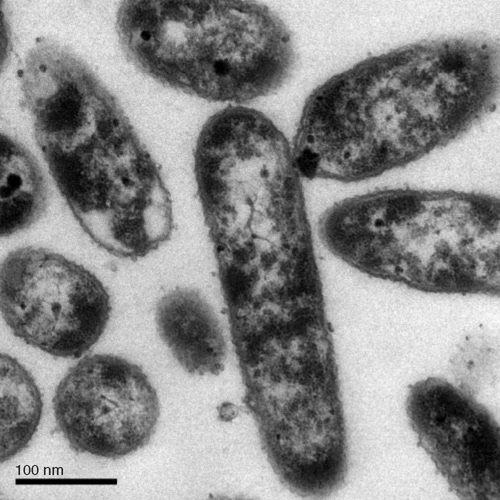
Colonies of GZYT0421 were white to yellowish and transparent with a white spot in the centre on PDYM. Cells were rods (Fig. 2) with one to three polar flagella. Biochemical and physiological characteristics were: fluorescent pigment produced on KBA; gelatine liquefied; LOPAT reaction +/+/+/+/–; denitrification positive; levan positive; pyocyanin negative; lipase negative; weak positive hydrolysis of starch; acid produced but no gas from glucose, galactose, fructose, mannose, arabinose, xylose, rhamnose, mannitol and glycerol; alkali produced from acetate, citrate, malate, formate, lactate, succinate and tartrate; sucrose, maltose, raffinose and salicin not degraded; optimum temperature 25–26°C, min. 0°C, max. 38°C; growth at 4°C but not 41°C.
|
The results indicated that strain GZYT0421 is a fluorescent pseudomonad fully consistent with Pseudomonas marginalis pv. marginalis (Ren 1999; Dong and Cai 2001; Brenner et al. 2005) and distinguishable from the other pathovars by its distinct LOPAT reaction. Pseudomonas marginalis pv. marginalis is known to cause rot in faba bean (Vassilev 1998) and cauliflower (Yang and Ren 1994; Obradovic et al. 2002), leaf spot of philodendron (Schollenberger 2005) and cucurbits (Ghobakhloo et al. 2002), but there has been no previous report of this bacterium in potato in China. Clearly, the implication of this finding is the need to determine the geographic extent of the pathogen in potato crops in Gansu, elsewhere in China and other countries, to determine the relative susceptibility of available cultivars and to develop approaches and recommendations for control.
Acknowledgements
The study received financial support from Agricultural Council of Gansu Province (project 2GS054-A41-005-01) and the Scientific and Technological Council of Gansu Province (project GNSW-2006-01). The authors thanks Prof. Wang Shengrong (Gansu Agricultural University) and Dr Ian Riley for their encouragement and advice.
Ghobakhloo A,
Shahriari D, Rahimian H
(2002) Occurrence of bacterial leaf spot and blight of cucurbits in Varamin and evaluation of the resistance of some cultivars and lines of cucumber to the disease. Iranian Journal of Plant Pathology 38, 59–60.

Obradovic A,
Mijatovic M,
Ivanovic M, Arsenijevic M
(2002) Population of bacteria infecting cauliflower in Yugoslavia. Acta Horticulturae 579, 497–500.

Qu DY,
Xie KY,
Jin L,
Pang WF,
Bian CS, Duan SG
(2005) Potato development and food safety in China. Agricultural Sciences in China 38, 358–362.

Schollenberger M
(2005) Bacterial leaf spot of philodendron. Phytopathologia Polonica 35, 103–108.

Suzuki A, Takikawa Y
(2004) Cloning of a specific DNA region of white top pathogen of pea and its detection by polymerase chain reaction. Journal of General Plant Pathology 70, 174–180.
| Crossref | GoogleScholarGoogle Scholar |

Vassilev VI
(1998) Pseudomonas marginalis pv. marginalis and some other bacteria on faba bean. Fabis Newsletter 41, 21–24.

Wang JS,
Zhang X, Fang ZD
(1986) Methods for assaying the resistance of potato tubers to the soft rot (Erwinia carotovora pv. carotovora Dye) and the reactions of potato varieties from some potato growing regions of China. Agricultural Sciences in China 4, 5–50.

Wang YH
(2004) Superiority on industrial development of potato and several problems needing to be resolved in Gansu province. Gansu Agricultural Science and Technology 2004, 3–6.

Yang HT, Ren ZX
(1994) Bacterial flower rot of Chinese cabbage caused by Pseudomonas marginalis pv. marginalis. Journal of Nanjing Agricultural University Supplement 17, 46–48.



