First record of a Candidatus phytoplasma associated with little leaf disease of Portulaca grandiflora
P. V. Ajayakumar A , A. Samad A B , A. K. Shasany A , M. K. Gupta A , M. Alam A and S. Rastogi AA Central Institute of Medicinal and Aromatic Plants (CIMAP), PO CIMAP, Lucknow 226015, India.
B Corresponding author. Email: samad_cimap@yahoo.co.in
Australasian Plant Disease Notes 2(1) 67-69 https://doi.org/10.1071/DN07028
Submitted: 14 March 2007 Accepted: 16 April 2007 Published: 24 April 2007
Abstract
This paper reports Portulaca grandiflora as a new host of a Candidatus phytoplasma and demonstrates its association with the little leaf disease on P. grandiflora. Plants exhibited typical visual symptoms of a phytoplasma, with reduced leaf size. Pleomorphic bodies, observed by transmission electron microscopy, were restricted to the phloem of symptomatic tissue. However, no bacteria, fungi or viruses were observed under transmission electron microscopy. Products of the expected size were amplified by PCR using nested, phytoplasma-specific primer pairs. In addition, plants temporarily showed amelioration of phytoplasma symptoms following treatment with the antibiotic tetracycline.
Additional keywords: mycoplasma-like organism, electrophoresis, witches’ broom, proliferation, pathogen.
Portulaca grandiflora (family Portulacaceae), commonly known as moss-rose purslane, is widely grown in temperate climates and is popular as an ornamental as it blooms all summer long. Portulaca is also used for medicinal purposes such as for relieving teeth grinding and muscle spasms, and for soothing gunpowder burns. As a food, Portulaca is used raw in salads or cooked like spinach. Portulaca is very rich in vitamins A, B1 and C and has antimicrobial and cytotoxic activity (Abou El Seoud et al. 2003).
Phytoplasmas (earlier mycoplasma-like organisms) are emerging as devastating plant pathogens and are associated typically with little leaf diseases of several hundred plant species. Phytoplasmas cause loss in biomass and quality of plant products including flowers. Many plant species have been recorded as phytoplasma hosts without further characterisation of the causal organism. This is largely due to the inability of the pathogen to grow in axenic cultures, little information about pathogenicity, presence only in the phloem region of infected tissues, variable titre and uneven distribution. Phytoplasmas are known to be transmitted by vegetative propagation and leafhoppers (Bhat et al. 2006). Since P. grandiflora is propagated through cuttings, it is highly desirable to identify the causal agent of the little leaf disease and to develop a sensitive detection system.
Since March 2005, 30–50% of P. grandiflora plants in the ornamental gardens as well as in pots at Central Institute of Medicinal and Aromatic Plants (CIMAP), Lucknow, India have displayed symptoms resembling phytoplasma infection. The disease symptoms start as a typical bud proliferation, downward curling and diminishing size of leaves, followed by overall stunted growth and yellowing of the whole plant (Fig. 1). Some plants also formed rosettes and had a typical proliferation of axillary shoots resulting in a witches’ broom appearance (Fig. 1c). In advanced stages of disease development, the infected plants would either fail to produce flowers or to set seeds. Symptomatic plants were generally half the size (height of plants, number of branches/plant, and size of leaves) of healthy plants. Such symptoms are generally not incited by fungi, bacteria or viruses. Occasionally, symptomatic plants shed all their leaves and died prematurely.

|
Under glasshouse conditions, sap transmission of the infectious agent could not be achieved. Tissue samples from 19 infected and 26 uninfected plants were collected and processed for ultrastructural examinations using a transmission electron microscope (TEM). Typical pleomorphic bodies, mostly spherical to oval, of sizes ranging from ~340–1100 nm were observed. These were found by TEM only in the sieve elements of infected samples (Fig. 2). The bodies had opaque, low electron density cytoplasms that contained ribosome-like granules, DNA-strand like structures and that lacked nuclear membranes. These appeared to be similar to previously reported phytoplasmas (Credi 1994; Samad et al. 2002). Such bodies were absent from healthy samples and from the xylem and companion cells of infected tissue. Necrosis of phloem tissue was also often observed. In ultra thin sections from infected leaves, the chloroplasts showed the signs of degeneration that might have contributed to growth retardation and production of yellows and little leaf symptoms (Ghosh et al. 1988). The intra- or intercellular presence of any viruses, bacteria or fungus pathogens was not detected in naturally infected tissues. Samples from antibiotic treated infected plants (see below) showed degraded pleomorphic bodies under TEM.
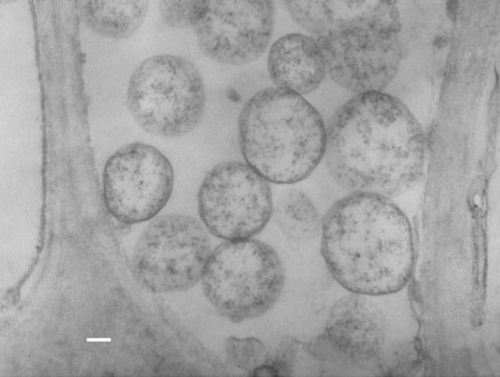
|
Total genomic DNA was isolated by a modified CTAB procedure (Khanuja et al. 1999) from leaves of 35 infected and 26 healthy plants. Approximately 2 g of leaves from each sample was ground in liquid nitrogen then mixed immediately in extraction buffer (2.5% CTAB, 100 mM Tris-HCl pH 8.0, 20 mM EDTA pH 8.0, 1.4 M NaCl, 1% PVP, and 0.3% β-mercaptoethanol). Following 2 h at 65°C the mixture was extracted with phenol : chloroform : isoamyl alcohol (25 : 24 : 1) and then chloroform : isoamyl alcohol (24 : 1). DNA from the resulting supernatant was precipitated with absolute ethanol overnight at –20°C. The DNA was pelleted by centrifugation then dissolved in 100 µL sterile distilled water. The quantity and quality of the isolated DNA was estimated by ethidium bromide staining of an 0.8% agarose gel.
For PCR-based detection, a universal primer pair P1/P6 (P1, 5′-AAGAGTTTGATCCTGGCTCAGGATT3′, P6, 5′CGGTAGGGATACCTTGTTACGA CTTA-3′) was synthesised on the basis of the 16S rDNA sequence of mollicutes (Deng and Hiruki 1991). For nested PCR another specific primer pair R16F2n/R16R2 (R16F2n, 5′-GAAACGACTGCTAAGACTGG-3′, R16R2, 5′TGACGGGCGGTGTGTACAAACCCCG-3′) was used (Lee et al. 1993). The PCR amplification reaction was set up in 25 µL containing 25 ng of template DNA, 1.5 units of Taq DNA polymerase, 2.5 µL of 10× buffer containing 15 mM MgCl2, 1 µL of 10 mM dNTP mix, and 25 ng of each forward and reverse primer (P1/P6). The reactions were carried out in a thermal cycler (PTC-200 DNA Engine, MJ Research, USA) with an initial denaturation at 94°C for 5 min, followed by 35 cycles (94°C for 1 min, 55°C for 60 s, and 72°C for 90 s) and a final extension at 72°C for 5 min.
The amplified products obtained with the primer pair P1/P6 were further diluted (1 : 200) for nested PCR with the primer pair R16F2n/R2. The PCR was repeated as above except that 2 µL of the 200-fold diluted amplified product from the initial reaction was used as DNA template and the annealing temperature was changed from 55°C to 50°C. The amplicons obtained were resolved by electrophoresis through a 1.2% agarose gel, stained with ethidium bromide and photographed using an Image Master VDS (Pharmacia).
The primer pairs P1/P6 amplified a product of the expected 1.5 kb size from DNA from infected plants (Fig. 3, lane 2). The same primer pairs failed to generate amplicons in a PCR mixture containing DNA from the healthy plants (Fig. 3, lane 3). The amplified product of P1/P6 primers was reamplified by nested PCR. The resultant amplified products of ~1.2 kb were consistently obtained (Fig. 3, lanes 4 to7). No such DNA band was observed with the R16F2n/R16R2 primers on DNA from healthy plants (Fig. 3, lane 8).
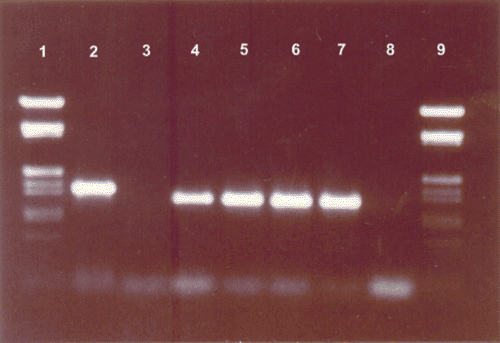
|
A set of infected plants displaying symptoms were treated with one of two antibiotics. Each set of 20 plants was treated for 6 weeks by foliar spray with tetracycline hydrochloride or penicillin at 250, 500 and 1000 μg/mL concentration at weekly intervals. Plants sprayed with distilled water served as controls. The antibiotic was dissolved in sterile water and applied on the infected plants by hand sprayer in the evening under glasshouse conditions.
All infected plants showed signs of recovery from the disease symptoms 4 weeks after treatment with tetracycline hydrochloride and appeared healthy after 6 weeks. However, by 6 weeks after ceasing the antibiotic treatment, the characteristic symptoms of the disease reappeared on newly emerged leaves and branches. On the contrary, symptoms of the disease became more severe following penicillin or distilled water treatment.
On the basis of these studies it is concluded that the little leaf disease on P. grandiflora is due to the association of phytoplasma. Evidence for the presence of a phytoplasma includes: transient recovery from the disease symptoms following tetracycline treatment; presence of pleomorphic bodies and the absence of bacteria, fungi or viruses under TEM; and the inability to transmit the disease through seeds or by mechanical means (Credi 1994). Furthermore, products of the expected size were amplified using phytoplasma 16S rDNA-specific primers in PCR or nested PCR (Samad et al. 2006).
A review of the literature revealed that P. oleracea can be infected by several pathogens such as Cucumber mosaic virus (Navas et al. 1998), nematodes (Walker et al. 2002), fungus (Hennessy et al. 2005), bacteria (Sato et al. 2001) and phytoplasma (Schneider et al. 1997b). However, a potexvirus is reported to infect P. grandiflora (Ciuffo and Turina 2004). To the best of our knowledge the little leaf disease of P. grandiflora caused by phytoplasma is a new record.
Acknowledgement
The authors are grateful to Dr S. P. S. Khanuja (Director, CIMAP) for encouragement and for providing necessary facilities during the course of this investigation.
Abou El Seoud KAEH,
Bibby MC,
Nagwa S, Wright CW
(2003) Evaluation of some Egyptian plant species for in vitro antimycobacterial and cytotoxic activities. Pharmaceutical Biology 41, 463–465.

Bhat AI,
Madhubala R,
Hareesh PS, Anadaraj M
(2006) Detection and characterization of the phytoplasma associated with a phyllody disease of black pepper (Piper nigrum) in India. Scientia Horticulturae 107, 200–204.
| Crossref | GoogleScholarGoogle Scholar |

Ciuffo M, Turina M
(2004) A Potexvirus related to papaya mosaic virus isolated from moss rose (Portulaca grandiflora) in Italy. Plant Pathology 53, 515.
| Crossref | GoogleScholarGoogle Scholar |

Credi R
(1994) Occurrence of anomalous mycoplasma like organisms in grapevine yellows diseased phloem. Journal of Phytopathology 142, 310–316.

Deng S, Hiruki C
(1991) Amplification of 16S rRNA genes from culturable and non-culturable mollicutes. Journal of Microbiological Methods 14, 53–61.
| Crossref | GoogleScholarGoogle Scholar |

Hennessy C,
Walduck G,
Daly D, Padovan A
(2005) Weed hosts of Fusarium oxysporum f. sp. cubence tropical race 4 in northern Australia. Australasian Plant Pathology 34, 115–117.
| Crossref | GoogleScholarGoogle Scholar |

Khanuja SPS,
Shasany AK,
Darokar MP, Kumar S
(1999) Rapid isolation of DNA from dry and fresh samples of plants producing large amounts of secondary metabolites and essential oils. Plant Molecular Biology Reporter 17, 74.
| Crossref | GoogleScholarGoogle Scholar |

Lee IM,
Hammond RW,
Davis RE, Gundersen DE
(1993) Universal amplification and analysis of pathogen 16S rDNA for classification and identification of mycoplasma like organisms. Phytopathology 83, 834–842.
| Crossref | GoogleScholarGoogle Scholar |

Navas M-L,
Friess N, Maillet J
(1998) Influence of cucumber mosaic virus infection on the growth response of Portulaca oleracea (purslane) and Stellaria media (chickweed) to nitrogen availability. The New Phytologist 139, 301–309.
| Crossref | GoogleScholarGoogle Scholar |

Samad A,
Ajayakumar PV,
Zaim M,
Lal RK, Khaliq A
(2002) Phytoplasma associated with little leaf disease of psyllium (Plantago ovata) in India. Journal of Herb Spices and Medicinal Plants 9, 55–61.
| Crossref | GoogleScholarGoogle Scholar |

Samad A,
Shasany AK,
Gupta S,
Ajayakumar PV,
Darokar MP, Khanuja SPS
(2006) First report of a 16SrVI group Phytoplasma associated with witches broom disease on Withania somnifera. Plant Disease 90, 248.

Sato M,
Watanabe K, Yoko S
(2001) Pseudomonas syringae pv. Solidagao pv. Nov., the causal agent of bacterial leaf spot of Tall Goldenrod Solidago altissima L. Journal of General Plant Pathology 67, 303–308.
| Crossref | GoogleScholarGoogle Scholar |

Schneider B,
Marcone C,
Kampmann M,
Ragozzino A,
Lederer W,
Cousin MT, Seemuller E
(1997b) Characterisation and classification of phytoplasmas from wild and cultivated plants by RFLP and Sequence analysis of ribosomal DNA. European Journal of Plant Pathology 103, 675–686.
| Crossref | GoogleScholarGoogle Scholar |

Walker GE,
Gobonand J, Nobbs J
(2002) New Australian record of Meloidogyne javanica on Portulaca oleracea (pigweed). Australasian Plant Pathology 31, 301.
| Crossref | GoogleScholarGoogle Scholar |



