Yellow vein netting of Bimili jute (Hibiscus cannabinus L.) in India caused by a strain of Tomato leaf curl New Delhi virus containing DNA β
S. K. Raj A C , M. S. Khan A , S. K. Snehi A and R. K. Roy BA Molecular Virology, National Botanical Research Institute, Lucknow 226 001, India.
B Botanic Garden, National Botanical Research Institute, Lucknow 226 001, India.
C Corresponding author. Email: skraj2@rediffmail.com
Australasian Plant Disease Notes 2(1) 45-47 https://doi.org/10.1071/DN07021
Submitted: 20 February 2007 Accepted: 20 March 2007 Published: 2 April 2007
Abstract
A strain of DNA β-containing Tomato leaf curl New Delhi virus, causing yellow vein net disease of Bimili jute (Hibiscus cannabinus L.) in India, was identified by dot blot hybridisation, PCR and sequence analysis.
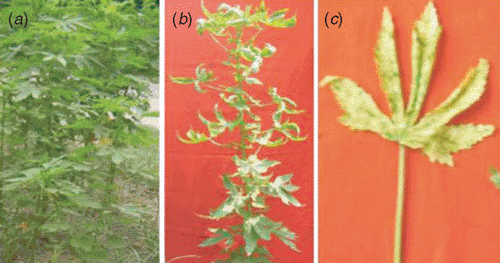
Bimili jute (Hibiscus cannabinus L.), a member of the family Malvaceae, is one of the important fibre yielding plants. The plant has been under cultivation for many years and is widely distributed throughout India. The leaves and tender twigs of the plant are used for cattle fodder, the dried stem stalks as firewood and fibre for sacking and cordage ropes. Recently, a cultivar of H. cannabinus was introduced as an ornamental plant at the NBRI garden because of its beautiful blooms (Sharga 2004). A yellow vein net disease on a H. cannabinus cultivar with >20% incidence in the garden has been observed over four successive years (2003–06). Naturally infected plants showed yellow leaf vein netting symptoms accompanied by excessive yellowing and curling of leaves and stunting of the whole plant (Fig. 1). A population of whiteflies (Bemisia tabaci) was also observed in the growing area, therefore, an association of begomovirus with the disease was suspected. To determine the cause of this yellow vein netting symptom, total DNA was isolated from symptomatic and asymptomatic H. cannabinus leaf samples, dot blotted on nylon membranes and allowed to hybridise with the radiolabelled probes prepared from cloned DNA A of a well-characterised begomovirus isolate of Indian tomato leaf curl virus (Srivastava et al. 1995). The total DNA of infected samples hybridised well with the probe and developed strong signals but not with DNA of healthy plants, indicating the presence of a begomovirus.
|
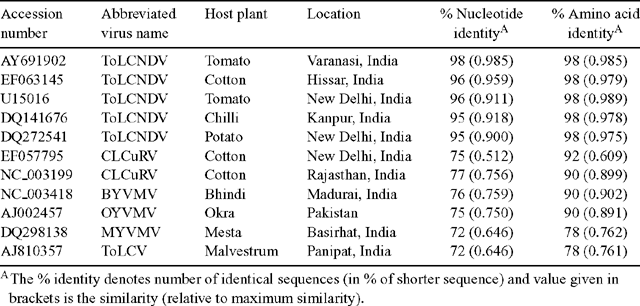
To identify the begomovirus associated with the disease, PCR was performed using the total DNA of infected and healthy samples with a pair of primers specific to the coat protein (CP) gene of the genus Begomovirus (Singh 2005). The electrophoresis of PCR products on 1% agarose gel showed the expected size (~800 bp) amplicons in infected samples but no such amplicons were obtained in healthy samples. The amplicon was cloned and sequenced and the data obtained from three clones was submitted to GenBank (Accession number EF123060). BLAST search analysis of nucleotide sequence EF123060 revealed 96–98% sequence identity with various strains of Tomato leaf curl New Delhi virus (ToLCNDV) isolated from cotton (EF063145), Luffa (AY939926), tomato (U15016, AY691902), potato (DQ272541), bottle guard (DQ272540), Solanum (DQ116885) and chilli (DQ141676, DQ116880).
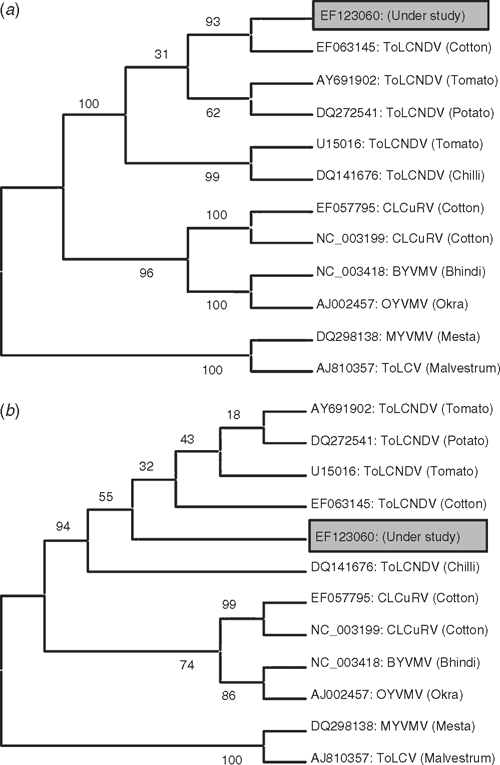
Pair-wise alignment and phylogenetic analyses of nucleotide (nt) and amino acid (aa) sequences of the CP gene of the virus isolate EF123060 was also completed to compare it with CP gene sequences of selected ToLCNDV isolates originating from diverse plant species, and other begomoviruses reported from malvacious host species. Pair-wise alignment of the virus isolate EF123060 revealed the 96–98% similarity at the nt level and 98% similarity at the aa level with various isolates of ToLCNDV (Table 1). The virus isolate did not reveal a close identity to Cotton leaf curl Rajasthana virus (NC_003199) used earlier for cross hybridisation (Chatterjee et al. 2005), nor with Mesta yellow vein mosaic virus (DQ298138) (Paul et al. 2006). Moreover, the phylogenetic analyses of both nt and aa sequences of the virus isolate showed a close relationship with strains of ToLCNDV (Fig. 2). Therefore, we identified the causal agent of yellow vein net symptoms of H. cannabinus as a strain of Tomato leaf curl New Delhi virus.
|
|
Recently, DNA β has been detected as being associated with ToLCNDV strains (Usharani et al. 2004; Reddy et al. 2005; Tahir and Haider 2005). A set of primers specific to the DNA β (Jose and Usha 2003) was therefore employed to test for its presence. Amplicons of the expected size, ~1350 bp, were successfully amplified from all of the infected samples. Furthermore, the amplicons cross-hybridised with the DNA β probe (DQ343289) under high stringency wash conditions. These findings confirm the association of the DNA β molecule with the ToLCNDV strain causing yellow vein net disease in H. cannabinus.
A literature survey revealed that Hibiscus chlorotic ringspot carmovirus, Hibiscus yellow mosaic tobamovirus and Hibiscus latent ringspot nepovirus have been reported on Hibiscus spp. (Brunt et al. 1996). Occurrence of a DNA β-containing begomovirus on H. cannabinus and H. sabdariffa has been recorded earlier (Chatterjee et al. 2005; Paul et al. 2006) based on PCR amplification and hybridisation with a Cotton leaf curl Rajasthan virus probe but without sequence analysis. Hence, molecular identification of a strain of Tomato leaf curl New Delhi virus containing DNA β as the cause of yellow vein net disease of H. cannabinus is a new report from India.
Acknowledgements
The authors are thankful to the Director, NBRI, Lucknow for facilities and Department of Science and Technology (DST), New Delhi for financial support.
Chatterjee A,
Roy A,
Padmalatha KV,
Malathi VG, Ghosh SK
(2005) Occurrence of a begomovirus with yellow vein mosaic disease of mesta (Hibiscus cannabinus and Hibiscus sabdariffa). Australasian Plant Pathology 34, 609–610.
| Crossref | GoogleScholarGoogle Scholar |

Jose J, Usha R
(2003) Bhendi yellow vein mosaic disease in India is caused by association of a β DNA satellite with a begomovirus. Virology 305, 310–317.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Paul S,
Ghosh R,
Roy A,
Mir JI, Ghosh SK
(2006) Occurrence of a DNA β-containing begomovirus associated with leaf curl disease of kenaf (Hibiscus cannabinus L.) in India. Australasian Plant Disease Notes 1, 29–30.
| Crossref | GoogleScholarGoogle Scholar |

Reddy CRV,
Colvin J,
Muniyappa V, Seal S
(2005) Diversity and distribution of begomoviruses infecting tomato in India. Archives of Virology 150, 845–867.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Srivastava KM,
Hallan V,
Raizada RK,
Chandra G,
Singh BP, Sane PV
(1995) Molecular cloning of Indian tomato leaf curl virus genome following a simple method of concentrating the supercoiled replicative form of viral DNA. Journal of Virological Methods 51, 297–304.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Tahir M, Haider MS
(2005) First report of Tomato leaf curl New Delhi virus infecting bitter gourd in Pakistan. Plant Pathology 54, 807.
| Crossref | GoogleScholarGoogle Scholar |

Usharani KS,
Surendranath B,
Paul-Khurana SM,
Garg ID, Malathi VG
(2004) Potato leaf curl – a new disease of potato in northern India caused by a strain of Tomato leaf curl New Delhi virus. Plant Pathology 53, 235.
| Crossref | GoogleScholarGoogle Scholar |



