Molecular evidence for the presence of huanglongbing in Pakistan
Shahid Nadeem Chohan A B , Raheel Qamar A , Irfan Sadiq A , Maleeha Azam A , Paul Holford B C and Andrew Beattie BA Department of Biosciences, COMSATS Institute of Information Technology, Islamabad, Pakistan.
B Centre for Plant and Food Science, University of Western Sydney, Locked Bag 1797, Penrith South DC, NSW 1797, Australia.
C Corresponding author. Email: p.holford@uws.edu.au
Australasian Plant Disease Notes 2(1) 37-38 https://doi.org/10.1071/DN07019
Submitted: 15 January 2007 Accepted: 19 March 2007 Published: 29 March 2007
Abstract
The presence of Candidatus Liberibacter asiaticus in citrus orchards in the North-West Frontier Province of Pakistan has been confirmed. Samples of DNA extracted from leaves of putatively infected citrus plants and from the vector of the disease, Diaphorina citri, were subjected to analysis using PCR and produced DNA amplicons characteristic of this bacterium. To confirm the exact nature of the pathogen, amplicons were sequenced and the resulting data showed 100% identity with published sequences of the Ca. L. asiaticus rplKAJL-rpoBC operon.
Citrus decline is widespread throughout Pakistan and is variously attributed to orchard neglect, poor plant nutrition, water logging, fungal diseases or nematodes (Singh 1998). While these causes of decline can be expected to affect citrus orchards in Pakistan, it is more likely that the main cause of decline is due to a bacterial disease called huanglongbing (HLB: also known as citrus greening) caused by the bacterium Candidatus Liberibacter asiaticus, a bacterium that is transmitted by the Asiatic citrus psyllid, Diaphorina citri. It is likely that HLB is widespread throughout Pakistan as, first, the disease is present in all of the countries neighbouring Pakistan, and second, in India, Ahlawat and Raychaudhuri (1988) have reported HLB to be present in all citrus-growing regions. In particular, HLB occurs in the provinces of Jammu, Kashmir, Punjab and Rajasthan, which border Pakistan. Third, a survey by Akhtar and Ahmad (1999), who used symptomatology as a diagnostic tool, suggested that the disease is present in Punjab and Peshawar. However, their study was not conclusive since the evidence presented, which was based upon chemical staining and microscopy, is not specific for the identification of HLB. Lastly, the first detailed description of visual symptoms of the disease appears to have been based on observations of severely affected trees at Sargodha, Lyallpur (now Faisalabad) and Gujranwala in the Punjab (Husain and Nath 1927). The symptoms of HLB are similar to those caused by iron and zinc deficiency, such that trees suffering from the disease can be easily misdiagnosed and the presence of Ca. L. asiaticus needs to be confirmed through molecular analysis. Both the occurrence of HLB and the extent of the disease are questioned within Pakistan. Thus, it is imperative that the true extent of the disease is recognised so that management strategies can be put into place. Therefore, as a first step towards the overall management of the disease, we report molecular evidence confirming the presence of the disease in Pakistan.
Putatively infected trees were identified based upon visual symptoms (Fig. 1). The midribs from three to five symptomatic leaves were excised and washed in distilled water before grinding in liquid nitrogen. Total genomic DNA was prepared from the pulverised midribs by extraction with 1 mL of extraction buffer [2% (w/v) CTAB, 1% (v/v) β-mercaptoethanol, 100 mM EDTA, 50 mM Tris, 1.4 M NaCl, 1% (w/v) PVP, pH 8.0]. The suspension was incubated at 65°C for 20 min following which it was centrifuged at 6000 g for 10 min. The supernatant was then extracted with an equal volume of chloroform : isoamyl alcohol (24 : 1). The aqueous phase was centrifuged for 10 min at 13000 g and the DNA precipitated at –20°C for 2 h by the addition of two volumes of cold ethanol. The DNA was pelleted by centrifuging at 13000 g for 15 min after which the pellet was washed in 70% ice-cold ethanol, centrifuged again at 13000 g for 5 min and the supernatant discarded. The DNA pellet was air-dried and dissolved in 100 μL TE buffer. Psyllids were also collected from the vicinity of three putatively infected trees and total genomic DNA prepared from single insects. Each psyllid was crushed in an Eppendorf tube in 50 μL of extraction buffer. After crushing, 200 μL of extraction buffer was added and the suspension was incubated at 65°C for 1 h. The remainder of the extraction procedure is as reported above.

|
The presence of DNA from Ca. L. asiaticus was determined by touchdown PCR using 1 µL of leaf or psyllid DNA extract per amplification. Primers A2 and J5 (Hocquellet et al. 1999) were used to amplify sequences from the pathogen and whilst primers fD1 and rP2 that amplify 16S rDNA (Weisburg et al. 1991) provided an internal control for the amplification reaction for extracts from both plants and psyllids. The PCR reaction mixture consisted of: 0.12 µM dNTPs, 1 × manufacturer’s buffer (Biotools, Spain), 2 mM MgCl2, 0.75 µM primers A2 and J5, 0.1 µM primers fD1 and rP2, 2.5 U Taq polymerase (Biotools, Spain). The cycling conditions were: 92°C for 205 s, 1 cycle; 92°C for 45 s, 65–60°C for 45 s (the annealing temperature reducing 1°C for each of the 5 touchdown cycles), 72°C for 1 min, 5 cycles; 92°C for 45 s, 60°C for 45 s, 72°C for 1 min, 30 cycles; 72°C for 10 min.
The leaf samples were collected from suspect trees in orchards at Rabaat, Temurgrah and Peshawar in the North-West Frontier Province. From the samples collected, 6 of 6, 8 of 13 and 1 of 5 at the three sites, respectively, were positive for HLB. The primer pair of Hocquellet et al. (1999) produces an amplicon from the DNA of both Ca. L. asiaticus and Ca. L. africanus. However, the amplicon size is different for the two species and all samples in this study produced the 700-bp amplicon that is typical of infection with Ca. L. asiaticus (Fig. 2). Psyllids were collected from Rabaat from trees surrounding the trees from which HLB-positive leaf samples were collected. Six insects were tested separately by PCR from which only two were HLB-positive (Fig. 2). All HLB-negative samples from plants and psyllids produced a band that is typical of the bacterial 16 S rDNA (1500 bp) indicating the success of the amplification process. The plant samples also produced a second band (2000 bp) amplified from the chloroplast DNA by the 16S primers.
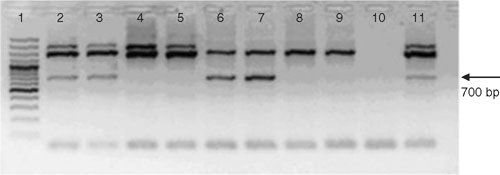
|
To confirm Ca. L. asiaticus as the causal organism of disease, amplicons from leaves and psyllids were sequenced and one sequence lodged with GenBank (EF493182). All sequences were identical with each other as well as with the previously published sequence of the area around the rplJ gene of the Ca. L. asiaticus rplKAJL-rpoBC operon (AY342001). This study is the first to provide molecular evidence for the presence of Ca. L. asiaticus in Pakistan and future work will determine the extent and occurrence of the disease.
Acknowledgements
The authors would like to thank the Higher Education Commission of Pakistan for providing funding for this research through a HEC Foreign Faculty Start-up research grant.
Akhtar MA, Ahmad I
(1999) Incidence of citrus greening in Pakistan. Pakistan Journal of Phytopathology 11, 1–5.

Hocquellet A,
Toorawa P,
Bové JM, Garnier M
(1999) Detection and identification of the two Candidatus Liberobacter species associated with citrus huanglongbing by PCR amplification of ribosomal protein genes of the β operon. Molecular and Cellular Probes 13, 373–379.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Husain MA, Nath D
(1927) The citrus psylla (Diaphorina citri Kuw.) [Psyllidae: Homoptera]. Memoirs of the Department of Agriculture in India, Entomological Series 10, 5–27.

Singh S
(1998) Status of citrus decline in India: a review. Agricultural Reviews 19, 227–238.

Weisburg S,
Barnes SM,
Pelletier DA, Lane DJ
(1991) 16S ribosomal DNA amplification for phylogenetic study. Journal of Bacteriology 173, 697–703.
| PubMed |



