First record of a begomovirus associated with yellow vein mosaic disease of Urena lobata in India
A. Chatterjee A , A. Roy A and S. K. Ghosh A BA Plant Virus Laboratory and Biotechnology Unit, Division of Crop Protection, Central Research Institute for Jute and Allied Fibres, Barrackpore, Kolkata 700 120, India.
B Corresponding author. Email: subratak_ghosh@yahoo.co.uk
Australasian Plant Disease Notes 2(1) 27-28 https://doi.org/10.1071/DN07012
Submitted: 4 October 2006 Accepted: 20 February 2007 Published: 2 March 2007
Abstract
A begomovirus with DNA A and a satellite DNA β molecule has been detected for the first time in the eastern part of India in Urena lobata showing yellow vein mosaic disease symptoms.
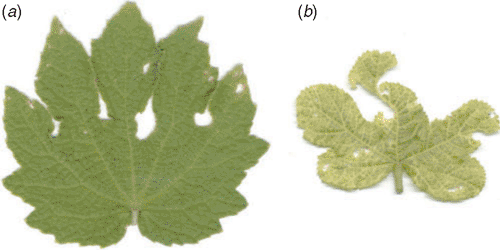
Urena lobata L. is popularly known as Aramina, Bun Ochra, Caesar Weed, or Congo Jute and is of the family Malvaceae; it is probably of Old World origin. It grows best in hot and humid climates in tropical and subtropical areas, where begomoviruses (family Geminiviridae) are a serious threat to dicotyledonous plants. This plant suffers a great deal due to attack by several diseases. Recently, yellow vein mosaic disease of U. lobata has been found in endemic form in different parts of eastern India. The association of begomoviruses with yellow vein mosaic disease in several crops and weeds, such as Ageratum spp., bhendi (Abelmoschus esculentus) and mesta (Hibiscus cannabinus and Hibiscus sabdariffa), grown in the eastern part of India has been noted (Saunders et al. 2000; Usha and Jose 2000; Chatterjee et al. 2005). Yellow vein mosaic disease, which has not been seen previously, has been observed in U. lobata. Symptomatologically the disease is characterised by yellowing of veins and veinlets followed by complete chlorosis of the leaves of affected plants at an advanced stage of infection (Fig. 1). The disease develops from one or both halves of the leaves or with the appearance of erratic chlorotic flakes in the veins and veinlets. In severe cases, these flakes gradually increase in size and form yellow netting. This disease does not exhibit vein clearing and vein thickening types of symptoms as have been noticed in other yellow vein mosaic diseases of bhendi, Croton spp. and Ageratum spp. Along with the vein yellowing, the leaf lamina started showing discolouration. This discolouration was greenish yellow at first, then developed to form yellow mosaic, chlorotic yellowing and complete chlorosis of foliage in a sequential chronology. The infected plants, in general, showed stunted growth with reduced leaf size.
|
In order to study the disease incidence pattern, an initial survey was carried out at different experimental fields of Central Research Institute for Jute and Allied Fibres (CRIJAF), Barrackpore and at experimental farm of BudBud (Burdwan), West Bengal. The results revealed that the disease intensity was slightly higher at CRIJAF (22.6%) than at the experimental farm of BudBud (11.2%). The study was also continued and validated in 20 farmers’ field in three districts of West Bengal and a low level of disease incidence (16.1%) was recorded in those fields. Transmission electron microscopy of typical symptomatic leaves of U. lobata using a 2% uranyl acetate stain revealed that the disease was associated with a geminivirus. The size of the geminate particle was 20 nm × 30 nm.
The virus was effectively transmitted in nature by whiteflies (Bemisia tabaci). Under glasshouse conditions with whitefly acquisition and inoculation access periods of 12 h each, a similar pattern of symptom appearance to that observed in nature was recorded. The whitefly transmission experiments were conducted with 25 plants in three replications. The same numbers of healthy plants were inoculated with non-viruliferous whitefly as a control in each experiment and no symptom expression was observed. Back-inoculation to a new set of healthy plants, 20 in each case, produced similar yellow vein mosaic symptoms and thus confirmed the whitefly transmissibility of the disease.
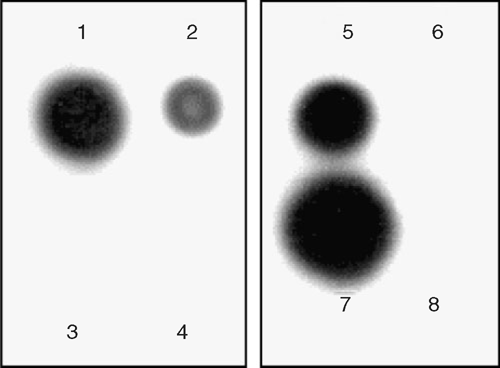
To confirm the presence of a geminivirus in the Urena plants showing yellow vein mosaic disease symptoms, nucleic acid spot hybridisation (NASH) tests were performed using α-32P radiolabelled probes (prepared using a High Prime DNA Labelling Kit, Roche Applied Science, USA) complementary to the clone of a complete DNA β molecule (GenBank accession number DQ298137) and a DNA A coat protein gene (accession number DQ298138) isolated from a begomovirus associated with yellow vein mosaic disease of mesta. Both the NASH tests gave a positive hybridisation signal and thus indicated the involvement of a begomovirus, having both DNA A and DNA β molecules, with the disease (Fig. 2).
|
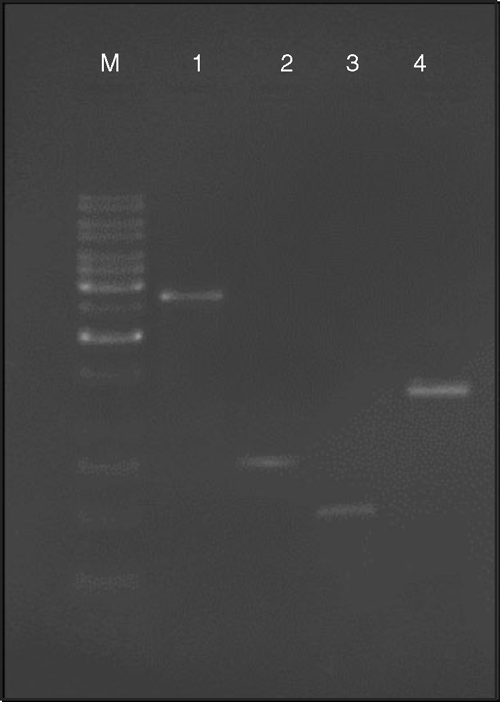
PCR was also used to confirm the presence of a begomovirus in the infected tissue. The amplification of a 2.7-kb fragment corresponding to full length DNA A and a 0.77-kb fragment corresponding to the coat protein gene of DNA A was achieved using primers designed by Usha and Jose (2000) and an ~0.55-kb amplicon was amplified using primers designed by Deng et al. (1994). These results confirmed the presence of Begomovirus DNA A in the plants. Primers specific to DNA β (Briddon et al. 2003) also confirmed the association of a satellite DNA β molecule with the disease (Fig. 3), yielding an amplicon of the expected size (1.3 kb). However, no amplification was observed with DNA isolated from healthy leaf tissues.
|
Whitefly transmission, NASH tests and positive PCR amplification confirmed the first record of a begomovirus with both DNA A and a satellite DNA β molecule being associated with yellow vein mosaic disease of U. lobata in India. The spread of this virus was assisted by the large densities of B. tabaci in eastern India and thus the management approach to this disease requires urgent attention.
Acknowledgements
Authors are grateful to the Director for his keen interest in the present investigation. The first author is also grateful to ICAR for financial assistance during the tenure of which this work was carried out.
Briddon RW,
Bull SE,
Amin I,
Idris AM, Mansoor S , et al.
(2003) Diversity of DNA β, a satellite molecule associated with some monopartite begomoviruses. Virology 312, 106–121.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Chatterjee A,
Roy A,
Padmalatha KV,
Malathi VG, Ghosh SK
(2005) Occurrence of a Begomovirus with yellow vein mosaic disease of mesta (Hibiscus cannabinus and Hibiscus sabdariffa). Australasian Plant Pathology 34, 609–610.
| Crossref | GoogleScholarGoogle Scholar |

Deng D,
Mc.Grath PF,
Robinson DJ, Harrison BD
(1994) Detection and differentiation of whitefly-transmitted geminiviruses in plants and vector insects by the polymerase chain reaction with degenerate primers. The Annals of Applied Biology 125, 327–336.

Saunders K,
Bedford ID,
Briddon RW,
Markham PG,
Wong SM, Stanley J
(2000) A unique virus complex causes Ageratum yellow vein disease. Proceedings of the National Academy of Sciences of the United States of America 97(12), 6890–6895.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Usha R, Jose J
(2000) Extraction of Geminiviral DNA from a highly mucilaginous plant (Abelmoschus esculentus). Plant Molecular Biology Reporter 18, 349–355.



