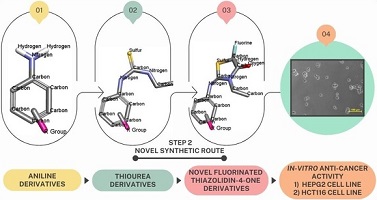
A series of thiourea intermediates were prepared by addition reaction between various aniline derivatives and ethyl isothiocyanate. These intermediates were further cyclised with the help of ethyl bromofluoroacetate to yield fluorinated thiazolidin-4-one derivatives. The anticancer activities of these novel fluorinated moieties against human liver and colon cancer cell lines have been investigated. (Image credit: Shreyash Kadam.)

Australian Journal of Chemistry
Volume 77 Number 9 2024
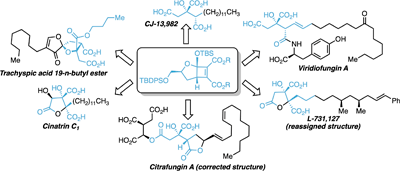
This review outlines the synthesis of alkyl citrate natural products using cyclobutene diester precursors. This highly stereoselective approach gives the citrate core with the correct oxidation state and allows for the synthesis of a large selection of these interesting natural products. Furthermore, stepwise oxidation provides access to the higher oxidised alkyl citrates from a common intermediate. (Image credit: Nikolai Rossouw.)
CH24088 Abstract | CH24088 Full Text | CH24088PDF (1.6 MB) Open Access Article
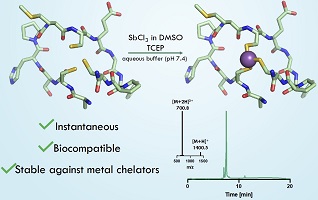
Cyclic peptide drugs are important therapeutics. Previously, bismuth and arsenic were introduced to create peptide bicycles by binding three cysteines. Now, antimony complements this set of elements to form stable bicyclic peptides. These remain stable in the presence of a common metal chelator and glutathione. Bismuth outcompetes antimony as the core atom in peptide bicycles. (Image credit: Lani Davies.)
This article belongs to the 10th Anniversary Collection of RACI and AAS Award papers.
CH24094 Abstract | CH24094 Full Text | CH24094PDF (986 KB) | CH24094Supplementary Material (349 KB) Open Access Article
Organic radical polymers are being developed for applications such as energy storage, catalysis and spintronics. Quantification of radical content can be nuanced, with a variety of techniques available for characterisation. This primer provides an overview and discusses the challenges of implementation to macromolecules containing pendant radicals. (Image credit: Theo A. Ellingsen.)
This article belongs to the 10th Anniversary Collection of RACI and AAS Award papers.
CH24085 Abstract | CH24085 Full Text | CH24085PDF (1.7 MB) | CH24085Supplementary Material (869 KB) Open Access Article
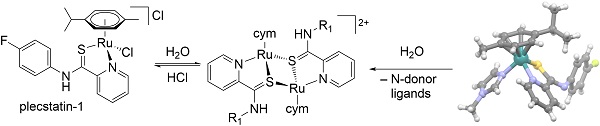
Replacing the chlorido ligand in [Ru(η6-p-cymene)(p-fluoropyridinecarbothioamide)Cl]PF6 with the N-heterocycles 1-methylimidazole, 1-methylbenzimidazole and pyridine gave complexes with similar antiproliferative activities in human cancer cells at low micromolar concentrations. This can be explained by dimerisation in aqueous solution and formation of the same di-Ru complexes after cleaving of the chlorido or N-heterocycle ligands. (Image credit: Saawan Kumar.)
This article belongs to the 10th Anniversary Collection of RACI and AAS Award papers.
CH24080 Abstract | CH24080 Full Text | CH24080PDF (1.2 MB) | CH24080Supplementary Material (1.2 MB) Open Access Article