Polyphenylalanine as a self-adjuvanting delivery system for peptide-based vaccines: the role of peptide conformation
Mariusz Skwarczynski A # , Guangzu Zhao A # , Victoria Ozberk B , Ashwini Kumar Giddam B , Zeinab G. Khalil B , Manisha Pandey B , Waleed M. Hussein A , Reshma J. Nevagi A , Michael R. Batzloff B , Robert J. Capon
A # , Guangzu Zhao A # , Victoria Ozberk B , Ashwini Kumar Giddam B , Zeinab G. Khalil B , Manisha Pandey B , Waleed M. Hussein A , Reshma J. Nevagi A , Michael R. Batzloff B , Robert J. Capon  C , Michael F. Good B and Istvan Toth A C D *
C , Michael F. Good B and Istvan Toth A C D *
A School of Chemistry & Molecular Biosciences, The University of Queensland, St Lucia, Qld 4072, Australia.
B Institute for Glycomics, Griffith University, Gold Coast, Qld 4222, Australia.
C Institute for Molecular Bioscience, The University of Queensland, St Lucia, Qld 4072, Australia.
D School of Pharmacy, The University of Queensland, Woolloongabba, Qld 4102, Australia.
Handling Editor: Mibel Aguilar
Australian Journal of Chemistry 76(8) 429-436 https://doi.org/10.1071/CH22167
Submitted: 29 July 2022 Accepted: 3 October 2022 Published: 8 December 2022
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Peptide-based vaccines are composed of minimal microbial components that are required to stimulate immune responses. Peptide antigens are easy to produce, relatively cheap and non-toxic. They are also able to activate the immune system in a well-controlled manner. However, peptides themselves are poor immunogens and have to be co-administered with an adjuvant (immune stimulator) to produce desired immune responses. Unfortunately, many adjuvants are toxic, poorly effective or not compatible with peptide antigens. Recently, we demonstrated that, upon conjugation to a peptide antigen, poly(hydrophobic amino acids) can self-assemble into nanoparticles and induce strong humoral immune responses. Here, we examine the ability of polyphenylalanine to act as a self-adjuvanting moiety when conjugated to a peptide antigen derived from Group A Streptococcus M-protein. The polyphenylalanine moiety was further lipidated to alter the conjugate conformation and its ability to form nanoparticles. The lipidated analogue triggered the production of a high level of antibodies in immunized mice. The antibodies produced were highly opsonic against tested GAS clinical isolates.
Keywords: adjuvant, amino acid polymers, epitope conformation, Group A Streptococcus, nanoparticles, opsonic antibodies, peptide self-assembly, peptide vaccine.
Introduction
Peptide epitopes are minimalistic antigens that can be utilized to induce very specific immune responses without the risk of inflammation, allergic or autoimmune responses.[1,2] However, they are poorly immunogenic on their own. Consequently, they must be co-administered with an immune stimulating agent (adjuvant) and/or an appropriate delivery system to protect them from degradation. The only widely approved human adjuvant, alum, is not effective enough to induce a robust immune response against peptides.[3,4] Many other commercially available adjuvants are based on microbial components or their synthetic derivatives. The clinical applications of these adjuvants have been limited and they are usually poorly defined and present risks. Most current adjuvants considered safe for human use are lipid-based mixtures (i.e. MF59, AS01, AS03, AS04); these are generally only approved as components of specific vaccines.[5,6] A variety of very effective experimental adjuvants exist; however, their safety profile is limited. For example, lipid-based complete Freund’s adjuvant (CFA) is one of the most powerful adjuvants ever discovered. CFA stimulates strong immune responses against peptide antigens, but its toxicity prevents its application in human vaccines.[7] The development of a well-defined adjuvant that does not cause inflammation, allergic responses or other adverse effects is needed.
Group A Streptococcus (GAS) is a Gram-positive bacteria, and the common cause of pharyngitis. It is also responsible for invasive infections, such as streptococcal toxic shock syndrome and post-infection complications, such as rheumatic fever (RF) and rheumatic heart disease (RHD).[8] Recent estimates suggest that 33–70 million people worldwide have RHD, resulting in 0.3–1.4 million deaths per year.[9,10] Both RHD and RF are autoimmune diseases related to cross-recognition of bacterial antigens and human heart tissue. Historically, vaccines developed against GAS were based on whole bacteria or the major virulent factor of GAS: M-protein. However, whole bacteria triggered autoimmune responses in clinical trials, and M-protein was suggested to be the main concern.[11] In addition, the presence of immunologically redundant biological components or biological impurities in such vaccines could also cause other side effects (e.g. allergic responses). These disadvantages may all be overcome by the development of fully synthetic peptide-based vaccines and an appropriate adjuvanting system to stimulate strong immune responses.[12] Indeed, all vaccine candidates against GAS recently tested in clinical trials have been peptide-based.[13]
Previously, we demonstrated that conjugation of peptide epitopes with lipids or hydrophobic polymers produced amphiphilic conjugates that can self-assemble into nano- or microparticles.[14,15] Upon conjugation to non-autoimmunogenic B-cell epitope derived from GAS M-protein, poly(tert-butyl acrylate) was able to stimulate the production of high antibody titers,[16] even after single immunization in mice.[17] The antibodies produced were able to opsonize clinically isolated GAS strains.[18] The applied dendritic polymer had a relatively low polydispersity index (1.09);[16] however, the polymer was not biodegradable and neither its chemical composition (number of units) or stereochemistry were fully defined. In contrast, we recently proposed a new delivery system based on fully biocompatible, biodegradable and defined polymers built from hydrophobic amino acids.[19] Upon conjugation with hydrophilic peptide antigen, the hydrophobic unit formed an amphiphilic compound that self-assembled into a mixture of small nanoparticles (10–30 nm) and chain-like aggregates of nanoparticles with sizes reaching into the micrometer range. The GAS B-cell epitope-poly(hydrophobic amino acid) conjugates induced significant humoral immune responses; however, only the compound bearing a 15-leucine unit induced the production of superior IgG titers.[19–21] The antibodies produced were opsonic against GAS clinical isolates. Interestingly, mice treated with the compound bearing 10 phenylalanines did not produce opsonic antibodies, in contrast to its 10-leucine analogue, despite both inducing the same IgG titers.
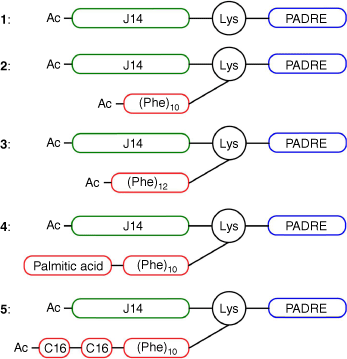
Here, we prepared a short series of polyphenylalanine-antigen conjugates and their analogues (Fig. 1), then evaluated how structural modifications influenced their physicochemical properties and immunogenicity.
|
Results and discussion
Nano/microparticles are usually far more potent in triggering immune reposes than soluble antigens.[14,15,22] Thus, vaccine candidates 2–5 were designed to have amphiphilic properties (Fig. 1). The candidates were able to self-assemble under aqueous conditions to form nanoparticles, in a similar manner to lipopeptides and polyacrylate-peptide conjugates.[23–26] Vaccine candidates 2–5 were comprised of a hydrophilic B-cell epitope from GAS M-protein (J14, KQAEDKVKASREAKKQVEKALEQLEDKVK) and a universal human T-helper epitope (PADRE, AKFVAAWTLKAAA) as antigen (1) and a hydrophobic polyphenylalanine unit as a delivery system. Polyphenylalanine was additionally lipidated (conjugates 4 and 5) to further promote self-assembly into particles. All conjugates were synthesized using stepwise Boc-SPPS. A polyphenylalanine moiety was also introduce to the compounds using Boc-SPPS, as click chemistry (copper-catalyzed 1,3-dipolar cycloaddition of polyphenylalanine-azide to PADRE-J14-alkyne) failed due to the extreme insolubility of the azide-modified polyphenylalanine unit during the HPLC purification process. Similarly, the production of conjugates with more than 12 copies of phenylalanine failed due to solubility issues.
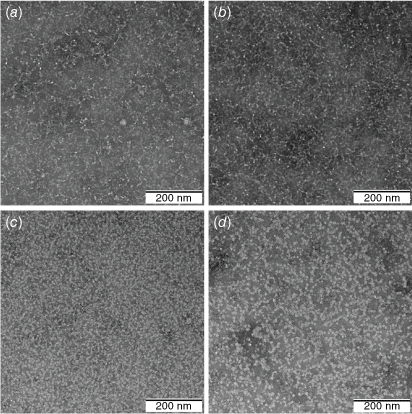
Conjugates 2–5 were self-assembled in PBS to form small nanoparticles (~5 nm for 2–4; ~10 nm for 5), as well-as chain-like aggregates of the nanoparticles (Fig. 2). A similar tendency to self-assemble was observed with polyleucine antigen conjugates.[19–21] Particles were highly polydisperse due to aggregates formation. Consequently, particle size distribution measured by dynamic light scattering (DLS) did not fully correspond with that observed through transmission electron microscopy (TEM) (Supplementary Fig. S1).
|
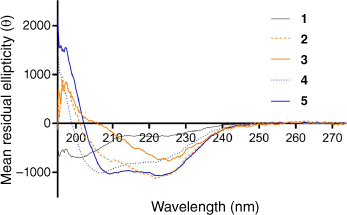
Peptide epitope conformation is crucial for generating antibodies that are able to recognize the native protein. As the GAS M-protein is helical, the J14 epitope should maintain the helical properties of its parent protein. Indeed, we previously reported that the polyleucine-PADRE-J8 conjugate adopted a predominantly helical conformation and induced strong antigen-specific humoral immunity.[19,20] J8 and J14 are close analogues derived from the same helical region of GAS M-protein. They differ in sequence only by four amino acids, and both have been widely used for GAS vaccine design.[13,27,28] Here, the J14 epitope adopted a mixture of random coil and helical conformations with minima at 201 nm and around 220 nm (Fig. 3). This correlated well with previously reported conformations when helix-stabilizing trifluoroethanol was not used for measurements.[29,30] Interestingly, J14 epitope-based conjugates 2 and 3 most likely lost helical properties, showing only single minima at 222 and 225 nm, respectively, closely resembling a β-sheet conformation. Indeed, this type of circular dichroism (CD) spectra (and minima) have been observed for β-sheet-rich proteins.[31,32] Conformational differences between polyleucine and polyphenylalanine could be expected as leucine has a tendency to adopt a helical conformation, while phenylalanine adopts a strand conformation (β-sheet).[33] Incorporation of a single lipid moiety into peptide 2 significantly restored the helical properties of J14 and the CD spectra of compound 4 displayed two minima, at 207 and 222 nm. Finally, compound 5, bearing two lipids, adopted typical α-helical conformation with minima at 223 nm (stronger) and 209 nm (weaker). Thus, lipidation significantly improved the helical properties of the peptide epitope, similarly as previously reported.[34–37]
|
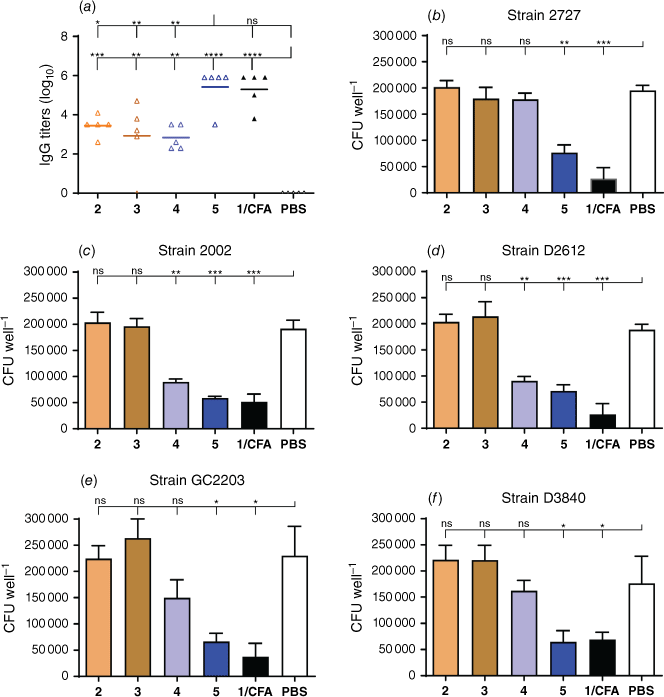
We previously demonstrated that conjugation of poly(hydrophobic amino acids), especially polyleucine, conjugated to peptide epitope can induce the production of high levels of GAS antigen-specific IgG titers in mice at a dose of 100–150 µg per mouse, following two or three immunizations.[19,20] Polyleucine conjugates were also very effective at generating humoral immunity against hookworm infection.[38–40] Here, the polyphenylalanine-based conjugates failed to induce strong humoral immune responses at a dose of 30 µg per mouse after four immunizations (Fig. 4a). However, antigen-specific immune responses were restored once conjugate 2 was lipidated. The resulting conjugate 5 was as effective as CFA-adjuvanted antigen in inducing IgG titers against J8 epitope. Importantly, the antibodies produced were opsonic against several clinical GAS isolates (Fig. 4b–f). As mentioned above, to generate opsonic antibodies against GAS, epitope conformation must be helical. Thus, the conformational properties of conjugate 2 and 3, rather than the reduced dose, were responsible for the loss of efficacy. Antibodies produced in mice following immunization with helical conjugate 5 were clearly opsonic; they were partially opsonic following immunization with partly helical conjugate 4. The antibodies generated by non-helical conjugates 2 and 3 were not at all opsonic on bacteria, even though 2–4 generated the same level of IgG titers. The larger size of particles formed by 5 (~10 nm) may also partially explain its higher immunogenicity when compared with other conjugates (~5 nm), as nanoparticles in the size range of 10–50 nm are typically the strongest inducers of humoral immunity.[14] In addition, the two lipidic moieties presented in conjugate 5 could have acted as an additional adjuvant; lipidation often enhances the immunogenicity of peptides.[27,28]
Conclusion
We demonstrated that polyphenylalanine can act as a self-adjuvanting moiety in peptide-based vaccines. However, further modifications of the moiety were required when the peptide antigen required helical properties. Interestingly, upon double-lipidation, polyphenylalanine conjugated to GAS-derived peptide antigen adopted the desired helical conformation and induced the production of high levels of opsonic antibodies, comparable to antigen adjuvanted with powerful CFA-adjuvant.
Experimental
Materials
Boc and Fmoc-protected l-amino acids were purchased from Novabiochem (Laufelfingen, Switzerland) and Mimotopes (Melbourne, Australia). pMBHA resin was obtained from Peptide International Inc. (Kentucky, USA). Trifluoroacetic acid (TFA), N,N′-dimethylformamide (DMF), dichloromethane (DCM), methanol, piperidine and HPLC-grade acetonitrile were purchased from Merck (Merck KGaA, Darmstadt, Germany). Todd–Hewitt broth (THB) was obtained from Oxoid and horse blood was purchased from Serum Australis. Phenol-free IMDM Glutamax medium was purchased from Gibco (California, USA). All other reagents were obtained from Sigma-Aldrich (Castle Hill, NSW, Australia). Microwave-assisted Fmoc and Boc-SPPS were performed on a CEM Discovery reactor (CME Corporation, Matthews, NC, USA). Hydrofluoric acid (HF) cleavage was achieved using an AKel-F HF apparatus (Peptide Institute, Osaka, Japan). ESI-MS was performed on a Perkin-Elmer-Sciex API3000 machine with Analyst 1.4 software (Applied Biosystems/MDS Sciex, Toronto, Canada). RP-HPLC separation was achieved using Agilent 1100 series equipment on gradient mode with solvent B (90% MeCN; 9.9% H2O; 0.1% TFA) over solvent A (99.9% H2O; 0.1% TFA) on a Vydac analytical C4-column (214TP54; 10 µm, 4.6 × 250 mm), 0–100% for 40 min. Compound purification was achieved using preparative RP-HPLC Shimadzu (Kyoto, Japan) instruments (LC-20AT, SIL-10A, CBM-20A, SPD-20AV, FRC-10A) in linear gradient mode. Dynamic light scattering (DLS) measurements were taken using a Nanosizer instrument (Zetasizer Nano Series ZS, Malvern Instruments, Worcestershire, UK) at a back-scattering angle of 173° at 25°C using Zetasizer 6.2 software. Particle imaging was achieved with a JEM-1010 transmission electron microscope (JEOL Ltd., Tokyo, Japan). Endotoxin free Millipore water (Merck, VIC, Australia) was used for all formulations and immunizations. CD spectra were measured using a Jasco J-710 CD spectropolarimeter (Jasco Corporation, Japan).
Synthesis of peptide 1 (J14-K-PADRE)
Peptide 1 was synthesized as described previously.[18]
Synthesis of conjugates 2–5
Peptides 2–5 (Fig. 1) were synthesized by microwave-assisted standard Boc-solid-phase peptide synthesis (SPPS),[41] similar to previously reported methods.[19] However, once the 10 phenylalanines were coupled, the palmitic acid and two (2-(R/S)-[(tert-butoxycarbonyl)amino]hexadecanoic acids[41] were conjugated using standard coupling conditions (HATU, DIPEA) to produce conjugates 4 and 5, respectively. Conjugates 2–5 were purified by RP-HPLC and analyzed by ESI-MS (Supplementary Fig. S2).
Compound 2. Yield: 30%. Molecular weight: 6325.5. ESI-MS [M + 3H]3+ m/z 2110.5 (calc. 2109.5), [M + 4H]4+ m/z 1583.0 (calc. 1582.4), [M + 5H]5+ m/z 1266.7 (calc. 1266.1), [M + 6H+]6+ m/z 1055.9 (calc. 1055.3), [M + 7H]7+ m/z 905.3 (calc. 904.6), [M + 8H]8+ m/z 792.5 (calc. 791.7), [M + 9H]9+ m/z 704.5 (calc. 703.8). tR = 26.6 min, purity ≥ 95%.
Compound 3. Yield: 24%. Molecular weight: 6619.8. ESI-MS [M + 4H]4+ m/z 1656.5 (calc. 1656.0), [M + 5H+]5+ m/z 1325.5 (calc. 1325.0), [M + 6H]6+ m/z 1104.8 (calc. 1104.3), [M + 7H]7+ m/z 947.5 (calc. 946.7), [M + 8H]8+ m/z 828.6 (calc. 828.5), [M + 9H]9+ m/z 736.9 (calc. 736.5), [M + 10H]10+ m/z 663.6 (calc. 663.0). tR = 27.9 min, purity ≥ 95%.
Compound 4. Yield: 21%. Molecular weight: 6563.9. ESI-MS [M + 4H]4+ m/z 1641.9 (calc. 1641.9), [M + 5H]5+ m/z 1314.0 (calc. 1313.8), [M + 6H]6+ m/z 1095.1 (calc. 1095.0), [M + 7H]7+ m/z 938.7 (calc. 938.7), [M + 8H]8+ m/z 821.6 (calc. 821.5), [M + 9H]9+ m/z 730.4 (calc. 730.3). tR = 24.8 min, purity ≥ 95%.
Compound 5. Yield: 19%. Molecular weight: 7050.2. ESI-MS [M + 4H+]4+ m/z 1763.9 (calc. 1763.6), [M + 5H]5+ m/z 1411.0 (calc. 1411.1), [M + 6H]6+ m/z 1176.0 (calc. 1176.0), [M + 7H]7+ m/z 1008.1 (calc. 1008.2), [M + 8H]8+ m/z 882.2 (calc. 882.3), [M + 9H]9+ m/z 784.3 (calc. 784.4), [M + 10H]10+ m/z 705.9 (calc. 706.0). tR = 29.7 min, purity ≥ 95%.
Size and morphology of nanoparticles 2–5
Conjugates 2–5 were self-assembled by simple dissolution of solid material in PBS (1 mg mL–1, 25°C) followed by sonication for 5 min at 25°C. The nanoparticles were characterized for average particle size and polydispersity index (PDI) by DLS. All measurements were performed at least five times. Morphological studies were performed using TEM at an accelerating voltage of 100 kV. A single drop of solution was placed on glow-discharged carbon-coated copper grids for 2 min, followed by the removal of excess of liquid, then negative staining with 1% phosphotungstic acid (pH 7).
Secondary structure analysis
The secondary structure of conjugates 1–5 (1 mg mL–1 in PBS) was analyzed by CD spectroscopy. The parameters were: 5 nm bandwidth; 50 nm min–1 scan rate; 2 s response time; 1 nm intervals over the wavelength range of 195–260 nm; in nitrogen atmosphere at 25°C. The data reported is the mean of six measurements. Mean residue molar ellipticity (deg × cm2 × dmol−1) was calculated using the formula [θ] = mdeg/(l × c × n), where: l is the path length (1 mm), c is the peptide concentration (M) and n is the number of residues in the peptide.
Immunization and serum collection
All animal protocols were approved by the Griffith University Animal Ethics Committee, GU Ref No: GLY/07/14 and carried out following the NHMRC Australia guidelines for generating, breeding, caring for and using genetically modified and cloned animals for scientific purposes (2007). The 4–6-week-old female C57BL/6 inbred mice (Animal Resource Centre, Perth, Western Australia, n = 5 mice per group) were immunized subcutaneously with 30 μg of peptide vaccine candidates 2‒5 dissolved in 50 μL of PBS, followed by equal booster doses on days 21, 28 and 35 post-primary immunization. A negative control group received 50 μL of PBS and a positive control group received 30 μg of peptide 1 emulsified in a total volume of 50 μL of CFA-PBS (1:1). On days −1, 20, 27 and 34, blood (10 μL) was collected from each mouse by tail snip and dissolved in 90 μL PBS. On day 49, mice were sacrificed by CO2 asphyxiation, and blood was collected via cardiac puncture. Collected blood was allowed to clot for at least 30 min at 37°C. Serum was collected following centrifugation of the blood samples for 10 min at 1000g. The collected serum was then stored at −20°C for antibody titer determination.
Antibody titer determination via ELISA
Indirect enzyme-linked immunosorbent assays (ELISA) were used to determine J14-specific IgG antibody titers. Polycarbonate plates were coated with 100 μL well–1 of J14 peptide (pH 9.6, 0.5 mg mL–1 in carbonate coating buffer), and incubated at 4°C overnight. After washing the plates five times with PBS-Tween 20 buffer, 150 μL of 5% skim milk PBS-Tween 20 was then added. The plates were incubated at 37°C for 2 h. The plates were washed in a similar manner between incubations. Sera samples (200 μL of 1:100 dilution) were added to the plate, followed by serial dilution down the plate with 0.5% skim milk PBS-Tween 20 buffer. All plates were incubated for 1.5 h at 37°C, then washed. Peroxidase-conjugated goat anti-mouse IgG (100 μL well–1, 1:3000 diluted in 0.5% skim milk PBS-Tween 20) was added, and the plates were again incubated for 1.5 h at 37°C and washed. OPD substrate was added at 100 μL well–1. The plates were incubated for 30 min in a dark environment at room temperature. Plates were read at 450 nm on a POLARstar Omega microplate reader (BMGLabtech, Victoria, Australia) and antibody endpoint titers were determined as the lowest dilution factor that gave an absorption value of more than three standard deviations (s.d.) above the mean absorbance of the respective control wells.
Bactericidal assessment
Serum collected from immunized mice was used to evaluate the bactericidal efficacy of the antibodies against clinically isolated GAS strains (Royal Brisbane Hospital): 2002 and 2727 (human abscess – lymph gland), GC2203 wound swab, D3840 (naso-pharynx swabs) and D2612 (naso-pharynx swabs). The bacteria were prepared by streaking on THB agar supplemented with 5% yeast extract, followed by incubation (37°C, 24 h). A single colony from the bacterium was transferred to THB (5 mL) supplemented with 5% yeast extract and incubated for 24 h at 37°C to give approximately 4.6 × 106 colony forming units (CFU) mL–1. The culture was serially diluted to 10−2 in PBS and an aliquot (10 µL) was mixed with heat-inactivated serum (10 µL) and horse blood (80 µL). Heat-inactivated sera were prepared by incubation in a 50°C water bath for 30 min. The bacteria were then incubated in the presence of sera in a 96-well plate at 37°C for 3 h. Ten microlitres of the suspension was plated on Todd–Hewitt agar plates supplemented with 5% yeast extract and 5% horse blood and incubated at 37°C for 24 h. Bacterial survival rate was analyzed based on CFUs counted on the incubated Todd–Hewitt agar plates. Assays were performed in triplicate from three independent cultures.
Statistical analysis
Statistical analysis was performed with the help of GraphPad Prism® 7 (GraphPad Software, Inc., California, USA), with P < 0.05 considered statistically significant. J14-specific IgG or IgA titers were described as the lowest dilution that offered an absorbance of greater than three s.d. above the mean absorbance of the negative control wells (wells coated with serum from mice injected with PBS). A one-way ANOVA followed by Tukey’s post hoc test was applied for statistical analysis of the antibody titers, with P < 0.05 considered statistically significant. The opsonic activity of the antibodies’ (anti-peptide) sera (% reduction in mean CFU) was calculated as [1 − (CFU in the presence of anti-peptide sera)/(mean CFU in the presence of PBS)] × 100. Two-way ANOVA followed by Tukey’s post hoc test was applied for opsonization statistical analysis.
Supplementary material
Particle size distribution analysed by dynamic light scattering and ESI‐MS spectra for peptides 2–5 are provided in the Supplementary Material. Supplementary material is available online.
Data availability
The data that support this study are available in the article and accompanying online supplementary material.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This work was supported by the Australian Research Council (grant no. DP21010280) and the National Health and Medical Research Council (NHMRC Program grant no. APP1132975).
Acknowledgements
The authors acknowledge the facilities and the scientific and technical assistance of the Australian Microscopy & Microanalysis Research Facility at the Centre for Microscopy and Microanalysis, The University of Queensland.
References
[1] AW Purcell, J McCluskey, J Rossjohn, More than one reason to rethink the use of peptides in vaccine design. Nat Rev Drug Discov 2007, 6, 404.| More than one reason to rethink the use of peptides in vaccine design.Crossref | GoogleScholarGoogle Scholar |
[2] M Skwarczynski, I Toth, Peptide-based synthetic vaccines. Chem Sci 2016, 7, 842.
| Peptide-based synthetic vaccines.Crossref | GoogleScholarGoogle Scholar |
[3] N Petrovsky, JC Aguilar, Vaccine adjuvants: Current state and future trends. Immunol Cell Biol 2004, 82, 488.
| Vaccine adjuvants: Current state and future trends.Crossref | GoogleScholarGoogle Scholar |
[4] F Azmi, AAH Ahmad Fuaad, M Skwarczynski, I Toth, Recent progress in adjuvant discovery for peptide-based subunit vaccines. Hum Vaccin Immunother 2014, 10, 778.
| Recent progress in adjuvant discovery for peptide-based subunit vaccines.Crossref | GoogleScholarGoogle Scholar |
[5] Nevagi RJ, Toth I, Skwarczynski M. In: Koutsopoulos S, editor. Peptide Applications in Biomedicine, Biotechnology and Bioengineering. Oxford, United Kingdom: Woodhead Publishing; 2018. pp. 327–358.
[6] Firdaus FZ, Skwarczynski M, Toth I. Methods in Molecular Biology. 2022. pp. 145–178.
[7] HF Stils Jr, Adjuvants and antibody production: Dispelling the myths associated with Freund’s complete and other adjuvants. ILAR J 2005, 46, 280.
| Adjuvants and antibody production: Dispelling the myths associated with Freund’s complete and other adjuvants.Crossref | GoogleScholarGoogle Scholar |
[8] MD Seckeler, TR Hoke, The worldwide epidemiology of acute rheumatic fever and rheumatic heart disease. Clin Epidemiol 2011, 3, 67.
| The worldwide epidemiology of acute rheumatic fever and rheumatic heart disease.Crossref | GoogleScholarGoogle Scholar |
[9] JA Paar, NM Berrios, JD Rose, M Cáceres, R Peña, W Perez, et al. Prevalence of Rheumatic Heart Disease in Children and Young Adults in Nicaragua. Am J Cardiol 2010, 105, 1809.
| Prevalence of Rheumatic Heart Disease in Children and Young Adults in Nicaragua.Crossref | GoogleScholarGoogle Scholar |
[10] DA Watkins, CO Johnson, SM Colquhoun, G Karthikeyan, A Beaton, G Bukhman, et al. Global, Regional, and National Burden of Rheumatic Heart Disease, 1990–2015. N Engl J Med 2017, 377, 713.
| Global, Regional, and National Burden of Rheumatic Heart Disease, 1990–2015.Crossref | GoogleScholarGoogle Scholar |
[11] AC Steer, MR Batzloff, K Mulholland, JR Carapetis, Group A streptococcal vaccines: facts versus fantasy. Curr Opin Infect Dis 2009, 22, 544.
| Group A streptococcal vaccines: facts versus fantasy.Crossref | GoogleScholarGoogle Scholar |
[12] AC Steer, JR Carapetis, JB Dale, JD Fraser, MF Good, L Guilherme, et al. Status of research and development of vaccines for Streptococcus pyogenes. Vaccine 2016, 34, 2953.
| Status of research and development of vaccines for Streptococcus pyogenes.Crossref | GoogleScholarGoogle Scholar |
[13] A Azuar, W Jin, S Mukaida, W Hussein, I Toth, M Skwarczynski, Recent advances in the development of peptide vaccines and their delivery systems against Group A Streptococcus. Vaccines 2019, 7, 58.
| Recent advances in the development of peptide vaccines and their delivery systems against Group A Streptococcus.Crossref | GoogleScholarGoogle Scholar |
[14] M Skwarczynski, I Toth, Recent advances in peptide-based subunit nanovaccines. Nanomedicine (London, UK) 2014, 9, 2657.
| Recent advances in peptide-based subunit nanovaccines.Crossref | GoogleScholarGoogle Scholar |
[15] M Skwarczynski, I Toth, Peptide-Based Subunit Nanovaccines. Curr Drug Deliv 2011, 8, 282-.
| Peptide-Based Subunit Nanovaccines.Crossref | GoogleScholarGoogle Scholar |
[16] M Skwarczynski, M Zaman, CN Urbani, IC Lin, Z Jia, MR Batzloff, et al. Polyacrylate dendrimer nanoparticles: a self-adjuvanting vaccine delivery system. Angew Chem Int Ed Engl 2010, 49, 5742.
| Polyacrylate dendrimer nanoparticles: a self-adjuvanting vaccine delivery system.Crossref | GoogleScholarGoogle Scholar |
[17] AA Ahmad Fuaad, Z Jia, M Zaman, J Hartas, ZM Ziora, IC Lin, et al. Polymer–peptide hybrids as a highly immunogenic single-dose nanovaccine. Nanomedicine (London) 2014, 9, 35.
| Polymer–peptide hybrids as a highly immunogenic single-dose nanovaccine.Crossref | GoogleScholarGoogle Scholar |
[18] S Chandrudu, S Bartlett, ZG Khalil, Z Jia, WM Hussein, RJ Capon, et al. Linear and branched polyacrylates as a delivery platform for peptide-based vaccines. Ther Deliv 2016, 7, 601.
| Linear and branched polyacrylates as a delivery platform for peptide-based vaccines.Crossref | GoogleScholarGoogle Scholar |
[19] M Skwarczynski, G Zhao, JC Boer, V Ozberk, A Azuar, JG Cruz, et al. Poly(amino acids) as a potent self-adjuvanting delivery system for peptide-based nanovaccines. Sci Adv 2020, 6, eaax2285.
| Poly(amino acids) as a potent self-adjuvanting delivery system for peptide-based nanovaccines.Crossref | GoogleScholarGoogle Scholar |
[20] A Azuar, Z Li, MA Shibu, L Zhao, Y Luo, AO Shalash, et al. Poly(hydrophobic amino acid)-Based Self-Adjuvanting Nanoparticles for Group A Streptococcus Vaccine Delivery. J Med Chem 2021, 64, 2648.
| Poly(hydrophobic amino acid)-Based Self-Adjuvanting Nanoparticles for Group A Streptococcus Vaccine Delivery.Crossref | GoogleScholarGoogle Scholar |
[21] A Azuar, MA Shibu, N Adilbish, N Marasini, H Hung, J Yang, et al. Poly(hydrophobic amino acid) Conjugates for the Delivery of Multiepitope Vaccine against Group A Streptococcus. Bioconjug Chem 2021, 32, 2307.
| Poly(hydrophobic amino acid) Conjugates for the Delivery of Multiepitope Vaccine against Group A Streptococcus.Crossref | GoogleScholarGoogle Scholar |
[22] TD Nandedkar, Nanovaccines: recent developments in vaccination. J Biosci 2009, 34, 995.
| Nanovaccines: recent developments in vaccination.Crossref | GoogleScholarGoogle Scholar |
[23] K Schulze, T Ebensen, S Chandrudu, M Skwarczynski, I Toth, C Olive, et al. Bivalent mucosal peptide vaccines administered using the LCP carrier system stimulate protective immune responses against Streptococcus pyogenes infection. Nanomed-Nanotechnol Biol Med 2017, 13, 2463.
| Bivalent mucosal peptide vaccines administered using the LCP carrier system stimulate protective immune responses against Streptococcus pyogenes infection.Crossref | GoogleScholarGoogle Scholar |
[24] A Chan, WM Hussein, KA Ghaffar, N Marasini, A Mostafa, S Eskandari, et al. Structure–activity relationship of lipid core peptide-based Group A Streptococcus vaccine candidates. Bioorg Med Chem 2016, 24, 3095.
| Structure–activity relationship of lipid core peptide-based Group A Streptococcus vaccine candidates.Crossref | GoogleScholarGoogle Scholar |
[25] WM Hussein, S Mukaida, F Azmi, S Bartlett, C Olivier, MR Batzloff, et al. Comparison of Fluorinated and Nonfluorinated Lipids in Self-Adjuvanting Delivery Systems for Peptide-Based Vaccines. ACS Med Chem Lett 2017, 8, 227.
| Comparison of Fluorinated and Nonfluorinated Lipids in Self-Adjuvanting Delivery Systems for Peptide-Based Vaccines.Crossref | GoogleScholarGoogle Scholar |
[26] TY Liu, WM Hussein, AK Giddam, Z Jia, JM Reiman, M Zaman, et al. Polyacrylate-based delivery system for self-adjuvanting anticancer peptide vaccine. J Med Chem 2015, 58, 888.
| Polyacrylate-based delivery system for self-adjuvanting anticancer peptide vaccine.Crossref | GoogleScholarGoogle Scholar |
[27] S Bartlett, M Skwarczynski, I Toth, Lipids as Activators of Innate Immunity in Peptide Vaccine Delivery. Curr Med Chem 2020, 27, 2887.
| Lipids as Activators of Innate Immunity in Peptide Vaccine Delivery.Crossref | GoogleScholarGoogle Scholar |
[28] W Zhong, M Skwarczynski, I Toth, Lipid Core Peptide System for Gene, Drug, and Vaccine Delivery. Aust J Chem 2009, 62, 956.
| Lipid Core Peptide System for Gene, Drug, and Vaccine Delivery.Crossref | GoogleScholarGoogle Scholar |
[29] WA Hayman, ER Brandt, WA Relf, J Cooper, A Saul, MF Good, Mapping the minimal murine T cell and B cell epitopes within a peptide vaccine candidate from the conserved region of the M protein of group A streptococcus. Int Immunol 1997, 9, 1723.
| Mapping the minimal murine T cell and B cell epitopes within a peptide vaccine candidate from the conserved region of the M protein of group A streptococcus.Crossref | GoogleScholarGoogle Scholar |
[30] MM Georgousakis, A Hofmann, MR Batzloff, DJ McMillan, KS Sriprakash, Structural optimisation of a conformational epitope improves antigenicity when expressed as a recombinant fusion protein. Vaccine 2009, 27, 6799.
| Structural optimisation of a conformational epitope improves antigenicity when expressed as a recombinant fusion protein.Crossref | GoogleScholarGoogle Scholar |
[31] M Bouchard, J Zurdo, EJ Nettleton, CM Dobson, CV Robinson, Formation of insulin amyloid fibrils followed by FTIR simultaneously with CD and electron microscopy. Protein Sci 2000, 9, 1960.
| Formation of insulin amyloid fibrils followed by FTIR simultaneously with CD and electron microscopy.Crossref | GoogleScholarGoogle Scholar |
[32] C-A Yang, Y-H Chen, S-C Ke, Y-R Chen, H-B Huang, T-H Lin, et al. Correlation of copper interaction, copper-driven aggregation, and copper-driven H2O2 formation with Aβ40 conformation. Int J Alzheimers Dis 2010, 2011, 607861.
| Correlation of copper interaction, copper-driven aggregation, and copper-driven H2O2 formation with Aβ40 conformation.Crossref | GoogleScholarGoogle Scholar |
[33] SN Malkov, MV Živković, MV Beljanski, MB Hall, SD Zarić, A reexamination of the propensities of amino acids towards a particular secondary structure: classification of amino acids based on their chemical structure. J Mol Model 2008, 14, 769.
| A reexamination of the propensities of amino acids towards a particular secondary structure: classification of amino acids based on their chemical structure.Crossref | GoogleScholarGoogle Scholar |
[34] AAH Ahmad Fuaad, MS Pearson, DA Pickering, L Becker, G Zhao, AC Loukas, et al. Lipopeptide Nanoparticles: Development of Vaccines against Hookworm Parasite. ChemMedChem 2015, 10, 1647.
| Lipopeptide Nanoparticles: Development of Vaccines against Hookworm Parasite.Crossref | GoogleScholarGoogle Scholar |
[35] AAH Ahmad Fuaad, R Roubille, MS Pearson, DA Pickering, AC Loukas, M Skwarczynski, et al. The use of a conformational cathepsin D-derived epitope for vaccine development against Schistosoma mansoni. Bioorg Med Chem 2015, 23, 1307.
| The use of a conformational cathepsin D-derived epitope for vaccine development against Schistosoma mansoni.Crossref | GoogleScholarGoogle Scholar |
[36] F Azmi, AAH Ahmad Fuaad, AK Giddam, MR Batzloff, MF Good, M Skwarczynski, et al. Self-adjuvanting vaccine against group A streptococcus: application of fibrillized peptide and immunostimulatory lipid as adjuvant. Bioorg Med Chem 2014, 22, 6401.
| Self-adjuvanting vaccine against group A streptococcus: application of fibrillized peptide and immunostimulatory lipid as adjuvant.Crossref | GoogleScholarGoogle Scholar |
[37] M Skwarczynski, AAH Ahmad, Fuaad, L Rustanti, ZM Ziora, M Aqil, MR Batzloff, et al. Group A streptococcal vaccine candidates based on the conserved conformational epitope from M protein. Drug Deliv Lett 2011, 1, 2.
| Group A streptococcal vaccine candidates based on the conserved conformational epitope from M protein.Crossref | GoogleScholarGoogle Scholar |
[38] S Bartlett, M Skwarczynski, X Xie, I Toth, A Loukas, RM Eichenberger, Development of natural and unnatural amino acid delivery systems against hookworm infection. Prec Nanomed 2020, 3, 471.
| Development of natural and unnatural amino acid delivery systems against hookworm infection.Crossref | GoogleScholarGoogle Scholar |
[39] AO Shalash, L Becker, J Yang, P Giacomin, M Pearson, WM Hussein, et al. Oral Peptide Vaccine against Hookworm Infection: Correlation of Antibody Titers with Protective Efficacy. Vaccines 2021, 9, 1034.
| Oral Peptide Vaccine against Hookworm Infection: Correlation of Antibody Titers with Protective Efficacy.Crossref | GoogleScholarGoogle Scholar |
[40] AO Shalash, L Becker, J Yang, P Giacomin, M Pearson, WM Hussein, et al. Development of a peptide vaccine against hookworm infection: Immunogenicity, efficacy, and immune correlates of protection. J Allergy Clin Immunol 2022, 150, 157.
| Development of a peptide vaccine against hookworm infection: Immunogenicity, efficacy, and immune correlates of protection.Crossref | GoogleScholarGoogle Scholar |
[41] Skwarczynski M, Toth I. In: Mark SS, editor. Bioconjugation Protocols. Humana Press; 2011. pp. 297–308.