The Position of Solid Carbon Dioxide in the Triboelectric Series*
Jinyang Zhang A and Simone Ciampi A B
A B
A School of Molecular and Life Sciences, Curtin Institute of Functional Molecules and Interfaces, Curtin University, Bentley, WA 6102, Australia.
B Corresponding author. Email: simone.ciampi@curtin.edu.au
Australian Journal of Chemistry 72(8) 633-636 https://doi.org/10.1071/CH19239
Submitted: 28 May 2019 Accepted: 12 July 2019 Published: 1 August 2019
Abstract
The process of releasing liquid carbon dioxide from a fire extinguisher is accompanied by a strong static charging of the plastic material making up the extinguisher discharge horn. Firefighters often report an electric shock when operating CO2 extinguishers, but the origin of this electrostatic hazard is largely unknown. Here, we begin to investigate this phenomenon, and test the hypothesis of plastic samples being tribocharged on contact with rapidly flowing solid CO2. Using Faraday pail measurements, we show that non-conductive polymers gain a net static charge when brought in and out of contact with dry ice (solid CO2). These measurements of charge sign and magnitude give indirect evidence helping to place solid CO2 for the first time on the triboelectric series. Polydimethylsiloxane (PDMS), polytetrafluoroethylene (PTFE), and polyvinyl chloride (PVC) samples acquire a negative charge when rubbed against dry ice, whereas poly(methyl methacrylate) (PMMA), glass, and nylon surfaces become positively charged. Therefore, we suggest the position of dry ice in the triboelectric series to be close to that of materials with stable cations and unstable anions, possibly locating it between PMMA and PVC.
Introduction
Contact electrification[1] is a process in which two materials that are brought in and out of contact acquire a net charge of opposite sign. Although contact electrification is exploited in several important technologies, such as photocopying and laser printing,[2] electrostatic painting,[3] industrial separations,[4] and new forms of alternating current generation,[5] when uncontrolled it remains a detrimental, or even dangerous, phenomenon.[6] For examples, in industries such as textiles, manufacturing of explosives, and storage of grains and flour, static electricity is a well-known hazard.[6b,7] Contact charging is, however, not limited to contact between polymers, as the flow of water or of liquid and gaseous hydrocarbons[8] is known to generate static charge.[7a,9] Analogously, when a CO2 extinguisher is operated, the liquefied gas expands and rapidly moves across the surface of the discharge horn, generating a large amount of electrostatic charge.[10] Major accidents have been associated with static charging created by the release of CO2 extinguishers, such as an explosion in Germany in 1966.[11] Electrification induced by solid CO2 has been studied by several groups,[12] and as early as in 1954 Heidelberg and coworkers investigated charge generation during release of CO2 from a large fire-snuffing installation.[11] They reported a highly variable rate of charge generation, but to date, how different dielectrics respond to contact with solid CO2 and how the environment influences CO2-related tribocharging events are still largely unexplored.
Here, we have begun to quantify the magnitude and sign of static electricity that develops on polydimethylsiloxane (PDMS), polytetrafluoroethylene (PTFE), polyvinyl chloride (PVC), poly(methyl methacrylate) (PMMA), nylon, and glass surfaces that were rubbed against dry ice under either an air or argon atmosphere, and tried for the first time to tentatively place CO2 in the triboelectric series.
Results and Discussion
We first considered experiments in which solid CO2 is allowed to slide down an inclined plane made of a sheet of the polymer under test (Fig. 1a). This system is especially convenient in terms of reproducibly controlling the contact force. Solid CO2 is not found in any available triboelectric series,[13] and therefore, we performed experiments where dry ice was in contact with different polymers whose position in these charts is known.
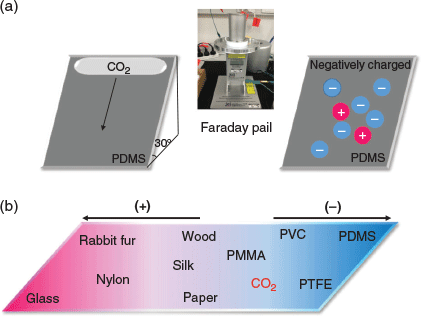
|
Fig. 1a illustrates the experimental set-up in which PDMS, PTFE, PVC, PMMA, nylon, and glass plates were rested on an inclined plane made of a wood surface tilted 30° away from the horizontal plane. Wood was used as to hold the samples because it is known to gain negligible charge after contact with any other polymer. The choices of the polymer materials were motivated as follows: (i) PDMS, PTFE, PVC, and PMMA are known to be close to the bottom (i.e. negative end) of the triboelectric series,[14] and (ii) glass and nylon are found towards the top (i.e. positive) end of the series. After contact with CO2, glass, PMMA, and nylon gain a positive charge, whereas PDMS, PTFE, and PVC become negatively charged. Our Faraday pail data therefore suggest that solid CO2 should be placed in a triboelectric series between PMMA and PVC (Fig. 1b). Furthermore, the magnitude of charging is strongly atmosphere-dependent: data comparing tribocharging in air and argon are shown in Fig. 2a and Fig. 2b, respectively. In 2018, Grzybowski and coworkers observed the magnitude of the charges developed on polymers by contact-electrification to be dependent not only on the material, but also on the nature of the atmosphere surrounding the polymer.[15] The dielectric strength of air (3 kV mm−1)[16] is six times than that of argon (0.5 kV mm−1),[17] and our data are consistent with an atmosphere of high dielectric strength favouring tribocharing. For the tribocharging mechanism, guided by our recent work demonstrating that charge carriers are polymer fragments,[18] we suggest that triboelectrification between solid CO2 and polymers can be ascribed to material transferred from the polymer to CO2, and this may be of relevance to gas–solid fluidized reactors.[19]
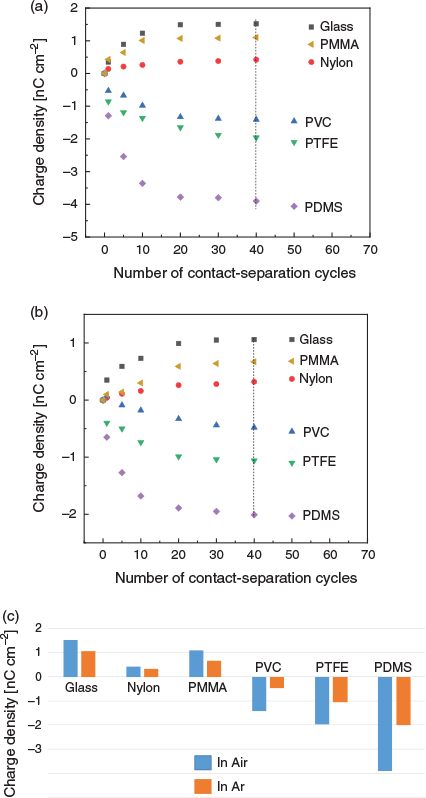
|
Our Faraday pail data show that for PDMS, PTFE, and PVC samples handled in argon, ~34, 52, and 90 % (respectively) of the tribocharge has dissipated 20 s after their separation from a CO2 surface (Fig. 3b). For these polymers, a faster decay is observed in air, where ~85, 91, and 96 % of the negative charges are lost over the same period of time (Fig. 3a). Charge dissipation, in both air and argon, is faster for PVC than for PTFE or PDMS (Fig. 3c), which is probably linked to the lower work function of the former.[18] We hypothesized that the differences in charge magnitude observed under argon or in the presence of atmospheric water could be due to the different abilities of these media to stabilize tribocharges.[20] Under ambient conditions, polymers are known to harbour surface water layers, and charges can potentially dissipate ‘laterally’ through surface conductance. Under a dry and inert atmosphere this mechanism of charge dissipation is likely to be inoperative or less efficient.
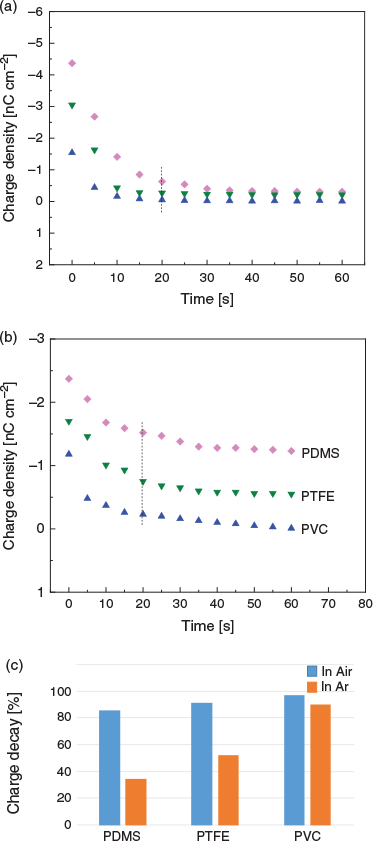
|
Conclusion
The conclusions of the present work are two-fold. First, solid CO2 can be tentatively placed between PMMA and PVC in a triboelectric series. This is important because it can guide the engineering and design of materials such as to either maximize or minimize their static charging on contact with solid CO2. For example, the Australian Standard concerning the manufacturing of CO2 extinguishers (AS/NZS 1841.6:2007) states that ‘a discharge horn shall be constructed of electronically non-conductive material’. CO2 extinguishers are primarily used on electrical fires, or on fires involving electrical equipment, hence the need of an insulating horn, but here we have shown that not all insulators have the same tendency to build up static electricity after contact with dry ice. Second, we have shown that contact electrification between CO2 and polymers is not just material-dependent, but also dependent on the environment: in an atmosphere of high dielectric strength, the density of surface charges generated upon contact is maximized. This work extends our growing understanding of static electricity and it may guide the development of experimental platforms for the study of how charged groups can influence chemical bonding and reactivity, an area that is beginning to attract significant interest in chemical catalysis.[21]
Experimental
Materials
Redistilled solvents and Milli-QTM water (>18 MΩ cm) were used for substrate cleaning. Samples of PTFE (McMaster-Carr, part no. 8545K26), PDMS (McMaster-Carr, part no. 87315K65), PVC (McMaster-Carr, part no. 87545K521), PMMA (McMaster-Carr, part no. 8536K141), nylon (nylon-66, RS Components Australia, part no. 514–607), and glass (microscope slides from Qorpak®) were cut to sheets 2 × 2 cm and were ~0.3 cm thick. Solid CO2 was purchased from BOC Australia.
Methods
Prior to being tribocharged, polymers were washed with water, methanol, and dichloromethane and then dried under a flow of nitrogen gas. The charge on the polymer was measured using a Faraday cup connected to an electrometer (JCI 140 static monitor and JCI 147 charge measurement units) operating on the 10−9-C scale. Charging data are reported as charge-to-geometric area ratios. A solid timber wedge was used to hold the plastic (nylon, PMMA, PVC, PTFE, and PDMS) and glass sheets at 30° from the horizontal. A small sample of dry ice (generally 3.5 g, rod-shaped) was rubbed against the inclined plane made by the plastic and glass samples by letting it slide, assisted by gravity, from the top to the bottom of the inclined plane (Fig. 1a). This operation of contacting and then separating the dry ice sample from the dielectric sheet was repeated a number of times. After the last contact–separation cycle, the plastic or glass sample was transferred to the Faraday pail to measure the magnitude and sign of its tribocharging. The number of contact–separation cycles (with one cycle defined as one slide down the tilted plane) was varied between 1 and 50 and the time that elapsed between the last cycle and the charge measurement was generally between 3 and 5 s. Experiments and charge measurements under an argon atmosphere were conducted using a glove box (Innovative Technology, PL-HE-2GB with PL-HE-GP1 inert gas purifier) with water and oxygen levels of less than 1 ppm.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgements
This work was financially supported by grants from the Australian Research Council (DP190100735 and DE160100732 (S.C.)).
References
[1] R. G. Horn, D. Smith, A. Grabbe, Nature 1993, 366, 442.| Crossref | GoogleScholarGoogle Scholar |
[2] J. W. Weigl, Angew. Chem. Int. Ed. Engl. 1977, 16, 374.
| Crossref | GoogleScholarGoogle Scholar |
[3] A. G. Bailey, J. Electrost. 1998, 45, 85.
| Crossref | GoogleScholarGoogle Scholar |
[4] A. Tilmatine, S. Bendimerad, M. Younes, L. Dascalescu, Int. J. Sustain. Eng. 2009, 2, 184.
| Crossref | GoogleScholarGoogle Scholar |
[5] S. Wang, L. Lin, Z. L. Wang, Nano Lett. 2012, 12, 6339.
| Crossref | GoogleScholarGoogle Scholar | 23130843PubMed |
[6] (a) N. Gibson, J. Electrost. 1997, 40–41, 21.
| Crossref | GoogleScholarGoogle Scholar |
(b) M. Glor, J. Electrost. 1985, 16, 175.
| Crossref | GoogleScholarGoogle Scholar |
(c) M. Glor, Powder Technol. 2003, 135–136, 223.
| Crossref | GoogleScholarGoogle Scholar |
[7] (a) J. Leonard, J. Electrost. 1981, 10, 17.
| Crossref | GoogleScholarGoogle Scholar |
(b) M. Nifuku, H. Enomoto, J. Loss Prev. Process Ind. 2001, 14, 509.
| Crossref | GoogleScholarGoogle Scholar |
(c) R. E. Nabours, IEEE Trans. Ind. Appl. 2004, 40, 1003.
| Crossref | GoogleScholarGoogle Scholar |
[8] J. T. Leonard, H. W. Carhart, J. Colloid Interface Sci. 1970, 32, 383.
| Crossref | GoogleScholarGoogle Scholar |
[9] L. G. Britton, Plant/Oper. Prog. 1992, 11, 56.
| Crossref | GoogleScholarGoogle Scholar |
[10] (a) V. Morgan, S. Collocott, R. Morrow, J. Electrost. 1981, 9, 201.
| Crossref | GoogleScholarGoogle Scholar |
(b) S. Collocott, V. Morgan, R. Morrow, Proc. Inst. Electr. Eng. 1980, 127, 119.
[11] E. Heidelberg, K. Nabert, G. Schön, Arbeitsschutz 1958, 11, 221.
[12] (a) A. Stäger, Ann. Phys. 1925, 381, 49.
| Crossref | GoogleScholarGoogle Scholar |
(b) A. Tietze, Z. Naturforsch. B 1957, 12, 82.
[13] (a) C. H. Park, J. K. Park, H. S. Jeon, B. C. Chun, J. Electrost. 2008, 66, 578.
| Crossref | GoogleScholarGoogle Scholar |
(b) A. Diaz, R. Felix-Navarro, J. Electrost. 2004, 62, 277.
| Crossref | GoogleScholarGoogle Scholar |
[14] D. M. Gooding, G. K. Kaufman, Encycl. Inorg. Bioinorg. Chem. 2011, 1.
[15] M. Siek, W. Adamkiewicz, Y. I. Sobolev, B. A. Grzybowski, Angew. Chem. Int. Ed. 2018, 130, 15605.
| Crossref | GoogleScholarGoogle Scholar |
[16] A. Russell, Philos. Mag. 1906, 11, 237.
| Crossref | GoogleScholarGoogle Scholar |
[17] W. Khechen, J. R. Laghari, IEEE Trans. Electr. Insul. 1989, 24, 1141.
| Crossref | GoogleScholarGoogle Scholar |
[18] J. Zhang, F. J. M. Rogers, N. Darwish, V. R. Gonçales, Y. B. Vogel, F. Wang, J. J. Gooding, M. C. R. Peiris, G. Jia, J.-P. Veder, M. L. Coote, S. Ciampi, J. Am. Chem. Soc. 2019, 141, 5863.
| Crossref | GoogleScholarGoogle Scholar | 30884944PubMed |
[19] (a) M. Manafi, R. Zarghami, N. Mostoufi, J. Electrost. 2019, 99, 9.
| Crossref | GoogleScholarGoogle Scholar |
(b) M. Hadisarabi, R. S. Gharebagh, R. Zarghami, N. Mostoufi, J. Electrost. 2019, 97, 108.
| Crossref | GoogleScholarGoogle Scholar |
[20] (a) H. Nakanishi, K. J. M. Bishop, B. Kowalczyk, A. Nitzan, E. A. Weiss, K. V. Tretiakov, M. M. Apodaca, R. Klajn, J. F. Stoddart, B. A. Grzybowski, Nature 2009, 460, 371.
| Crossref | GoogleScholarGoogle Scholar | 19606145PubMed |
(b) J. A. Wiles, M. Fialkowski, M. R. Radowski, G. M. Whitesides, B. A. Grzybowski, J. Phys. Chem. B 2004, 108, 20296.
| Crossref | GoogleScholarGoogle Scholar |
[21] (a) L. Zhang, Y. B. Vogel, B. B. Noble, V. R. Gonçales, N. Darwish, A. L. Brun, J. J. Gooding, G. G. Wallace, M. L. Coote, S. Ciampi, J. Am. Chem. Soc. 2016, 138, 9611.
| Crossref | GoogleScholarGoogle Scholar | 27373457PubMed |
(b) L. Zhang, E. Laborda, N. Darwish, B. B. Noble, J. H. Tyrell, S. Pluczyk, A. P. Le Brun, G. G. Wallace, J. Gonzalez, M. L. Coote, S. Ciampi, J. Am. Chem. Soc. 2018, 140, 766.
| Crossref | GoogleScholarGoogle Scholar |
(c) S. Ciampi, N. Darwish, H. M. Aitken, I. Díez-Pérez, M. L. Coote, Chem. Soc. Rev. 2018, 47, 5146.
| Crossref | GoogleScholarGoogle Scholar |
* Simone Ciampi is the winner of the 2017 RACI Alan Bond Medal.


