Effect of Ionic Liquids on the Hatching of Artemia salina Cysts
Minami Sakamoto A , Yusaku Ohama A , Shiori Aoki B , Keita Fukushi B , Tomoyoshi Mori B , Yukihiro Yoshimura B C and Akio Shimizu A CA Department of Environmental Engineering for Symbiosis, Faculty of Engineering, Soka University, 1-236 Tangi-Machi, Hachioji, Tokyo, 192-8577, Japan.
B Department of Applied Chemistry, National Defense Academy, 1-10-20, Hashirimizu, Yokosuka, Kanagawa, 239-8686, Japan.
C Corresponding authors. Email: muki@nda.ac.jp; shimizu@soka.ac.jp
Australian Journal of Chemistry 71(7) 492-496 https://doi.org/10.1071/CH18117
Submitted: 16 March 2018 Accepted: 27 May 2018 Published: 18 June 2018
Journal Compilation © CSIRO 2018 Open Access CC BY-NC-ND
Abstract
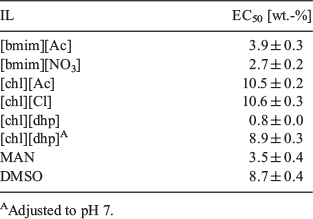
We investigated the effect of six ionic liquids (ILs), and dimethylsulfoxide (DMSO) as a typical molecular liquid (organic solvent), on the hatching of Artemia salina cysts. The effect of an IL on the hatching of Artemia salina strongly depends on the specific combination of cation and anion. Of the ILs tested, choline acetate and choline chloride had a significantly lower toxicity. The level of inhabitation followed the order [chl][dhp] > [bmim][NO3] > [MAN][NO3] > [bmim][Ac] > DMSO > [chl][Ac] ≥ [chl][Cl].
Introduction
Ionic liquids (ILs) are molten salts comprising a cation and an anion which exhibit unique physical and chemical properties such as negligible vapour pressure, inflammability, high chemical/thermal stability, high conductivity, and high solubility.[1] The low volatility of ILs makes them unlikely to contribute to atmospheric pollution and thus ILs are considered ‘green’ solvents relative to traditional solvents. However, due to their significant solubility, ILs may enter the environment through other routes, such as through industrial wastewater, becoming persistent pollutants that pass through typical wastewater treatment systems and into natural waters.[2]
Over the last decade ILs have demonstrated promise for applications in many areas, including the biological/biochemical sciences. For example, certain ILs stabilise the native structure of proteins while maintaining their bioactivity.[3–6] Recent studies on the structure and activity of several proteins in ILs at cryogenic temperature suggest that aqueous ILs have potential as an alternative cryoprotectant for proteins.[7] Furthermore, pharmaceutical applications of ILs have been established[8] and thus researchers are increasingly concerned about ILs moving through aquatic and terrestrial environments and potentially impacting organisms. An increasing number of toxicity studies with ILs on different model organisms have shed light on their possible toxic effects[9] and indicate that ILs can be as toxic, or even more toxic, than traditional solvents[10–16] and thus potentially harmful to the environment.
Artemia salina, or brine shrimp, is an invertebrate that plays an important role in energy flow through the food chain.[17] In particular, the larvae of brine shrimp are used as a model organism in preliminary tests to determine the toxicities of chemical and natural products in a laboratory bioassay (brine shrimp lethality test (BSLT),[18,19] mainly because BSLT is an easily performed, rapid, convenient, low cost test.
The toxic effects of ILs vary considerably depending on the type of IL, the test conditions, and the morphology of the model organism.[20,21] This variation is partly due to the different sensitivities of organisms. There have been some studies on the toxicity of ILs on larvae of Artemia salina.[22–24] For example, imidazolium, pyridinium, and choline cations with bromide and several amino acids prepared from biomaterials were used in the investigations, in which the toxicity depends on both the cation and anion, and the amino acid-derived ILs appeared to show very low toxicity.[22] However, to our knowledge, little is known about the effect of ILs on embryonic development, aside from a few studies.[25] A comprehensive understanding of the fate of ILs in the environment is clearly needed to understand their effect on developmental embryology and on the marine ecology. It is therefore important to be able to differentiate environmentally benign from harmful ILs based on their toxicological properties, which in turn would play a crucial role in designing non-toxic ILs. As mentioned above, BSLT is a rapid and convenient assessment method for toxicities of chemicals. Thus, we used Artemia salina to investigate the toxicity of ILs to the hatching success in this study. The present study focussed on evaluating the effect of different concentrations of ILs and a commonly used organic solvent on the hatching of Artemia salina cysts (embryos).
Experimental
Materials and Reagents
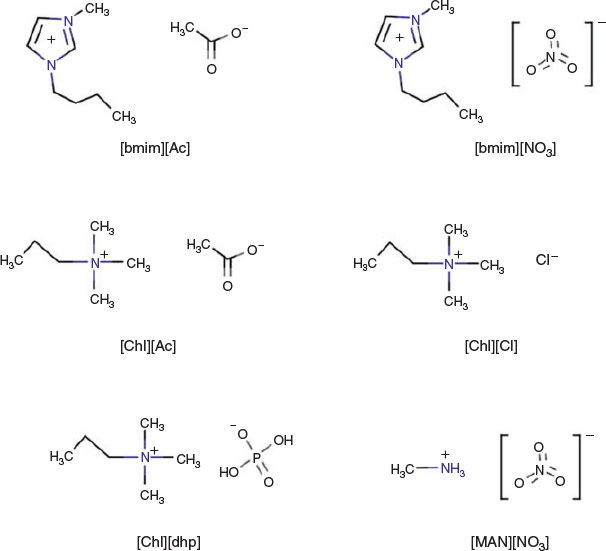
As ILs, 1-butyl-3-methylimidazolium acetate (hereafter abbreviated as [bmim][Ac]; purity > 98 %, Sigma Aldrich Co.), 1-butyl-3-methylimidazolium nitrate ([bmim][NO3]; purity > 95 %, Sigma Aldrich Co.), methylammonium nitrate ([MAN][NO3]; purity > 97 %, Iolitec GmbH), choline acetate ([chl][Ac]; purity > 95 %, Iolitec GmbH), choline chloride ([chl][Cl]; purity > 99 %, Sigma Aldrich Co.), and choline dihydrogen phosphate ([chl][dhp]; purity > 98 %, Iolitec GmbH) were used without further purification. Fig. 1 shows the structures of the ILs used.
|
All mixtures containing different concentrations of ILs were prepared by mixing the required amount of IL and artificial sea water (GEX, Tokyo, Japan, total amount of the sea water in the mixed solutions was adjusted to be 3.5 wt.-%) in x (wt.-% IL).
For comparison, dimethylsulfoxide (DMSO) (Special grade, Wako Pure Chemical) was used as a typical molecular liquid (organic solvent).
Hatching Test
To determine the effect of ILs on the hatching of Artemia salina cysts (eggs), ~50 Artemia salina cysts (Sanders Great Salt Lake, Brine Shrimp Co. L.C.) were hatched in artificial sea water containing an IL after 72 h at 28°C,[26] and the number of larvae hatched were counted. Experiments at each solvent concentration were repeated at least three times. Hatchability was calculated using Eqn 1:
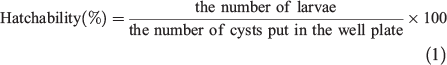
The obtained hatchability was corrected using Eqn 2
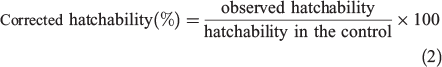
The corresponding half maximal effective concentrations (EC50) were calculated from the response curve of hatchability percentage versus concentration using the sigmoidal function.
In addition, visual observations (morphology) of embryonic development for 72 h (i.e. from cysts to larvae) were performed. Moreover, we checked the pH of each mixed test solution with a pH meter (model F-51, Horiba Co. Ltd).
Results and Discussion
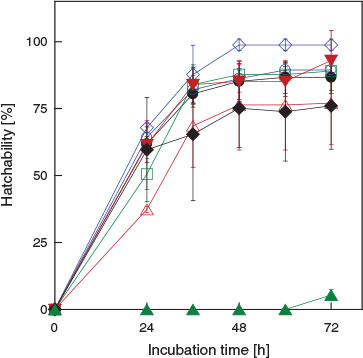
Fig. 2 shows the change in hatchability in the presence of ILs as a function of incubation time. The results show that over 48 h are required to attain the maximum hatchability and thus we used an incubation time of 72 h for the hatchability study.
|
Fig. 3 shows the corrected hatchability of Artemia salina following incubation with various concentrations of the tested solvents. Table 1 shows the calculated EC50 values for the tested ILs and for DMSO. Overall, the hatchability decreased with increasing IL concentration. The data presented in Fig. 3 show that [chl][Cl] and [chl][Ac] were much less toxic to Artemia than the other ILs. ILs containing the [chl] cation showed a marked difference in toxicity when the anion was changed from acetate to dhp, and the hatching of Artemia salina was clearly inhibited in the presence of [chl][dhp]. Even a slight increase in the concentration of [chl][dhp] dramatically decreased hatchability. We found that ~2; wt.-% of [chl][dhp] caused the Artemia salina cysts to hatch later. The ILs [bmim][NO3], [bmim][Ac], and [MAN][NO3] had similar toxicities. Overall, the tested ILs had a low toxicity, even [chl][dhp], and followed the order [chl][dhp] > [bmim][NO3] > [MAN][NO3] > [bmim][Ac] > DMSO > [chl][Ac] ≥ [chl][Cl]. We stress again that [chl][[Ac] and [chl][Cl] showed significantly lower toxicity than the other IL solvents. It should be pointed out that the hatchability curves of some ILs such as [chl][Cl] in Fig. 2 are higher than for the sea water. As was stated by Singh et al.[27] we may ascribe this to choline being a biocompatible compound and [chl][Cl] is commercially provided as a chicken feed additive.
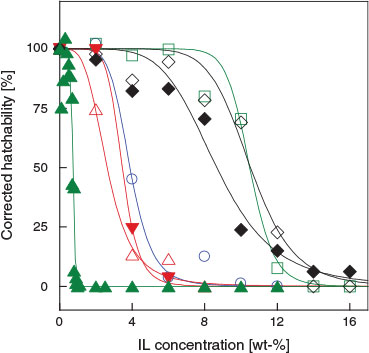
|
|
It is interesting to compare the results obtained for DMSO, a solvent commonly used in bioassays[2] such as the BSLT and toxicity activity tests. We found that the EC50 of DMSO was 8.47 ± 0.36 wt.-%, and thus DMSO did not inhibit the hatching of Artemia salina significantly, indicating that DMSO might be much less toxic for the cysts than several of the ILs tested. The maximum tolerable concentration for DMSO was ~14 wt.-%, comparable to that of [chl][Cl] and [chl][Ac].
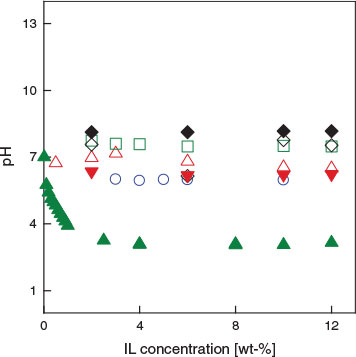
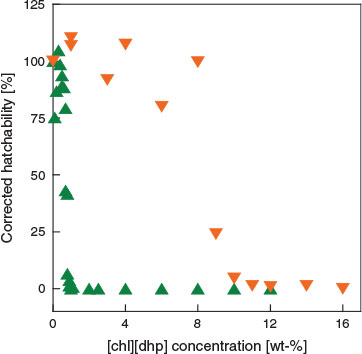
Hatchability might be affected by the pH of the solution because pH is generally a major regulator of metabolism during transitions in organisms.[28] We therefore checked the pH values of the test solutions and the results are shown in Fig. 4. Low pH appeared to lower hatchability, such as in the case of [chl][dhp]. We then performed additional experiments on how much pH inhibits hatching in the case of [chl][dhp]. Fig. 5 compares the corrected hatchability of pH-controlled (pH 7) to the not controlled solutions. We can see that a high hatchability was maintained up to around 8 wt.-% in the pH-controlled solution and a similar EC50 value was obtained for pH-controlled [chl][chp] and [chl][Cl] (Table 1). That is, we can safely conclude that the difference in the results between [chl][dhp] and [chl][Cl] is because of different acid–base characteristics. In view of the EC50 results among the tested ILs, it seems that the toxicity of ILs to the hatching success of Artemia salina is more dependent on the cationic character.
|
|
The toxicity of an IL has been reported to strongly correlate with its lipophilicity, which may affect the interaction of the IL with the surface of the model organism[29,30] showing that an IL with a longer alkyl chain length, 1-dodecyl-3-methylimidazolium tetrafluoroborate [C10mim][BF4] (EC50 = 13 μM), strongly inhibited the development of Artemia salina larvae, possibly due to increased interaction with the organism. Thus, the alkyl chain is one of the primary factors that affects the toxicity of solvents by causing a change in polarity.[31–36] For example, Ranke et al.[11] suggested that imidazolium compounds could increase membrane permeability, and Pretti et al.[37] reported that ILs alter the physical properties of the lipid bilayer and enhance its permeability to external ions. Moreover, chaotropic anions, such as NO3− used in the present study, affect the chaotropicity of ILs by enhancing the surfactant-like behaviour of cations and thus chaotropicity is an independent cause of the toxicity of ILs.[38,39] Pierandrea et al.[40] pointed out that Artemia salina prefers salty waters that contain weakly kosmotropic/chaotropic species such as chloride ions that sit in the middle of the Hofmeister series. In contrast, stronger kosmotropes such as fluoride ions and especially stronger chaotropes such as thiocyanate and perchlorate ions are severely toxic. As mentioned in the introduction, there is growing interest in biochemical applications of ILs. 1-Ethyl-3-methylimidazolium acetate ([emim][Ac]) and [bmim][Ac] were recently shown to be effective solvents for dissolving microcrystalline cellulose.[41] The cell walls of cysts may be particularly sensitive to acetate anions because their cell walls are chitin-based, and the structure of chitin is similar to that of cellulose. Strong kosmotropes significantly weaken the hydrogen bonds between water and the amide bonds in chitin whereas strong chaotropes can deplete water from the hydrophobic hydration layer around chitin.[42] This peculiar physico-chemical mechanism may operate synergistically with the chemical binding of these anions at the chitin/water interface, resulting in a potent toxic effect on Artemia. Davila-Rodriguez et al.[43] showed that several anions adsorb onto chitin and its derivatives with a selectivity trend that follows the Hofmeister sequence. Therefore, perturbations such as expansion or swelling may cause an increase in the fluidity of the cell walls, although the true mechanism requires further investigation.
Finally, we conducted visual observations, especially of larval deformities, through the stages from embryonic development to the larval stage during exposure to ILs and the results are shown in Fig. 6. Larvae in artificial sea water are shown as controls. The total exposure time was 72 h and no morphological changes were observed among the tested ILs. For comparison, it is interesting to note that Li et al.[25] reported the toxic effect of 1-methyl-3-octylimidazolium bromide ([C8mim][Br]) on the early embryonic development stage of the frog Rana nigromaculata, although the used IL was different from the present study. The significant malformation in the spinal area and abdomen was observed in the larvae of the Rana nigromaculata. It seems that imidazolium cations with longer alkyl chain length affect the embryonic development.

|
Conclusions
In summary, the toxicities of several ILs were evaluated using embryos of the brine shrimp Artemia salina and the results show that toxicity is affected by the specific combination of cation and anion in the IL. All the tested ILs showed relatively low toxicity towards hatching Artemia cysts. ILs with a choline cation showed significantly lower toxicity and thus are likely less toxic to aquatic and terrestrial environments. Of the tested solvents, [chl][Ac] and [chl][Cl] (and the pH-controlled (pH7)[chl][dhp]) were the least toxic to hatching Artemia salina embryos and therefore may be promising solvents for a wide range of uses in future applications of ILs. The information obtained in the present study might help in the design of further toxicological risk assessment experiments with ILs, as presently there is insufficient information regarding the toxic mechanism(s) responsible for IL-induced effects on organisms and the environment. Our laboratory will study the effects of long-term exposure on different development stages of Artemia salina larvae, together with other complementary studies, in the future.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgements
The authors thank Prof. T. Takekiyo of the National Defence Academy for experimental help and fruitful discussions during this investigation.
References
[1] R. Hayes, G. G. Warr, R. Atkin, Chem. Rev. 2015, 115, 6357.| Crossref | GoogleScholarGoogle Scholar |
[2] M. Amde, J. F. Liu, L. Pang, A. Review, Environ. Sci. Technol. 2015, 49, 12611.
| Crossref | GoogleScholarGoogle Scholar |
[3] K. Fujita, H. Ohno, Biopolymers 2010, 93, 1093.
| Crossref | GoogleScholarGoogle Scholar |
[4] T. Takekiyo, K. Yamazaki, E. Yamaguchi, H. Abe, Y. Yoshimura, J. Phys. Chem. B 2012, 116, 11092.
| Crossref | GoogleScholarGoogle Scholar |
[5] H. Weingärtner, C. Cabrele, C. Herrman, Phys. Chem. Chem. Phys. 2012, 14, 415.
| Crossref | GoogleScholarGoogle Scholar |
[6] A. A. Tietze, F. Bordusa, R. Giernoth, D. Imhof, T. Lenzer, C. Mrestani-Klaus, I. Neudorf, K. Oum, D. Reith, A. Stark, ChemPhysChem 2013, 14, 4044.
| Crossref | GoogleScholarGoogle Scholar |
[7] Y. Yoshimura, T. Takekiyo, T. Mori, Chem. Phys. Lett. 2016, 664, 44.
| Crossref | GoogleScholarGoogle Scholar |
[8] K. S. Egorova, E. G. Gordeev, V. P. Ananikov, Chem. Rev. 2017, 117, 7132.
| Crossref | GoogleScholarGoogle Scholar |
[9] T. P. Pham, C. W. Cho, Y. S. Yun, Water Res. 2010, 44, 352.
| Crossref | GoogleScholarGoogle Scholar |
[10] R. J. Bernot, M. A. Brueseke, M. A. Evans‐White, G. A. Lamberti, Environ. Toxicol. Chem. 2005, 24, 87.
| Crossref | GoogleScholarGoogle Scholar |
[11] J. Ranke, K. Mölter, F. Stock, U. Bottin-Weber, J. Poczobutt, J. Hoffmann, B. Ondruschka, J. Filser, B. Jastorff, Ecotoxicol. Environ. Saf. 2004, 58, 396.
| Crossref | GoogleScholarGoogle Scholar |
[12] J. Ranke, M. Cox, A. Müller, C. Schmidt, D. Beyersmann, Toxicol. Environ. Chem. 2006, 88, 273.
| Crossref | GoogleScholarGoogle Scholar |
[13] K. M. Docherty, J. C. F. Kulpa, Green Chem. 2005, 7, 185.
| Crossref | GoogleScholarGoogle Scholar |
[14] T. P. Pham, C. W. Cho, J. Min, Y. S. Yun, J. Biosci. Bioeng. 2008, 105, 425.
| Crossref | GoogleScholarGoogle Scholar |
[15] A. S. Wells, V. T. Coombe, Org. Process Res. Dev. 2006, 10, 794.
| Crossref | GoogleScholarGoogle Scholar |
[16] K. J. Kulacki, G. A. Lamberti, Green Chem. 2008, 10, 104.
| Crossref | GoogleScholarGoogle Scholar |
[17] S. Sanchez-Fortun, F. Sanz-Barrera, M. V. Barahona-Gomariz, Bull. Environ. Contam. Toxicol. 1995, 54, 76.
| Crossref | GoogleScholarGoogle Scholar |
[18] A. L. Parra, R. S. Yhebra, I. G. Sardiñas, I. Buela, Phytomedicine 2001, 8, 395.
| Crossref | GoogleScholarGoogle Scholar |
[19] L. Lewan, M. Andersson, P. Morales-Gomez, Altern. Lab. Anim. 1992, 20, 297.
[20] M. G. Montalbán, J. M. Hidalgo, M. Collado-González, F. G. Díaz Baños, G. Víllora, Chemosphere 2016, 155, 405.
| Crossref | GoogleScholarGoogle Scholar |
[21] M. G. Montalbán, G. Víllora, P. Licence, Ecotoxicol. Environ. Saf. 2018, 150, 129.
| Crossref | GoogleScholarGoogle Scholar |
[22] W. Gouveia, T. F. Jorge, S. Martins, M. Meireles, M. Carolino, C. Cruz, T. V. Almeida, M. E. M. Araújo, Chemosphere 2014, 104, 51.
| Crossref | GoogleScholarGoogle Scholar |
[23] V. Tsarpali, S. Dailianis, Ecotoxicol. Environ. Saf. 2015, 117, 62.
| Crossref | GoogleScholarGoogle Scholar |
[24] M. Vraneš, A. Tot, S. Jovanović-Šanta, M. Karaman, S. Dožić, K. Tešanović, S. Gadžurić, RSC Adv. 2016, 6, 96289.
| Crossref | GoogleScholarGoogle Scholar |
[25] X. Y. Li, J. Zhou, M. Yu, J. J. Wang, Y. C. Pei, Ecotoxicol. Environ. Saf. 2009, 72, 552.
| Crossref | GoogleScholarGoogle Scholar |
[26] B. N. Meyer, N. R. Ferrigni, J. E. Putnam, L. B. Jacobsen, D. E. Ichols, J. L. McLaughlin, Planta Medica 1982, 45, 31.
| Crossref | GoogleScholarGoogle Scholar |
[27] B. S. Singh, H. R. Lobo, G. S. Shankarling, Catal. Commun. 2012, 24, 70.
| Crossref | GoogleScholarGoogle Scholar |
[28] R. H. Jennings, D. M. Whitaker, Biol. Bull. 1941, 80, 194.
| Crossref | GoogleScholarGoogle Scholar |
[29] F. Stock, J. Hoffmann, J. Ranke, R. Stormann, B. Ondruschka, B. Jastorff, Green Chem. 2004, 6, 286.
| Crossref | GoogleScholarGoogle Scholar |
[30] S. Geethaa, P. J. Thavamany, S. P. Chiew, O. M. Thong, J. Adv. Pharm. Technol. Res. 2013, 4, 179.
| Crossref | GoogleScholarGoogle Scholar |
[31] M. Vraneš, A. Tot, S. Jovanović-Šanta, M. Karaman, S. Dožić, K. Tešanović, V. Kojićc, S. Gadžurića, RSC Adv. 2016, 6, 96289.
[32] S. Stolte, M. Matzke, J. Arning, A. Böschen, W. R. Pitner, U. Welz-Biermann, B. Jastorff, J. Ranke, Green Chem. 2007, 9, 1170.
| Crossref | GoogleScholarGoogle Scholar |
[33] M. T. D. Cronin, T. W. Schultz, Sci. Total Environ. 1997, 204, 75.
| Crossref | GoogleScholarGoogle Scholar |
[34] A. P. van Wezel, A. Opperhuizen, Crit. Rev. Toxicol. 1995, 25, 255.
[35] J. Ranke, S. Stolte, R. Stormann, J. Arning, B. Jastorff, Chem. Rev. 2007, 107, 2183.
| Crossref | GoogleScholarGoogle Scholar |
[36] J. Ranke, A. Müller, U. Bottin-Weber, F. Stock, S. Stolte, J. Arning, R. Stormann, B. Jastorff, Ecotoxicol. Environ. Saf. 2007, 67, 430.
| Crossref | GoogleScholarGoogle Scholar |
[37] C. Pretti, C. Chiappe, D. Pieraccini, M. Gregori, F. Abramo, G. Monni, L. Intorre, Green Chem. 2006, 8, 238.
| Crossref | GoogleScholarGoogle Scholar |
[38] M. Matzke, S. Stolte, K. Thiele, T. Juffernholz, J. Arning, J. Ranke, U. Welz-Biermann, B. Jastorff, Green Chem. 2007, 9, 1198.
| Crossref | GoogleScholarGoogle Scholar |
[39] P. Mester, M. Wagner, P. Rossmanith, Ecotoxicol. Environ. Saf. 2015, 111, 96.
| Crossref | GoogleScholarGoogle Scholar |
[40] P. Lo Nostro, B. W. Ninham, E. Carretti, L. Dei, P. Baglioni, Chemosphere 2015, 135, 335.
| Crossref | GoogleScholarGoogle Scholar |
[41] J. Wang, Y. Zheng, S. Zhang, in Clean Energy Systems and Experiences (Ed. K. Eguchi) 2010, pp. 71–84 (Sciyo: Roosbeek, Belgium).
[42] Y. Cho, Y. Zhang, T. Christensen, L. B. Sagle, A. Chikoti, P. S. Cremer, J. Phys. Chem. B. 2008, 112, 13765.
| Crossref | GoogleScholarGoogle Scholar |
[43] J. K. Davila-Rodriguez, V. A. Escobar-Barrios, J. R. Rangel-Mendez, J. Fluor. Chem. 2012, 140, 99.
| Crossref | GoogleScholarGoogle Scholar |


 , artificial sea water;
, artificial sea water;  , [bmim][Ac];
, [bmim][Ac];  , [bmim][NO3];
, [bmim][NO3];  , [chl][Ac];
, [chl][Ac];  , [chl][Cl];
, [chl][Cl];  , [chl][dhp];
, [chl][dhp];  , [MAN][NO3];
, [MAN][NO3];  , DMSO.
, DMSO. , [bmim][Ac];
, [bmim][Ac];  , [bmim][NO3];
, [bmim][NO3];  , [chl][Ac];
, [chl][Ac];  , [chl][Cl];
, [chl][Cl];  , [chl][dhp];
, [chl][dhp];  , [MAN][NO3];
, [MAN][NO3];  , DMSO.
, DMSO. , [bmim][Ac];
, [bmim][Ac];  , [bmim][NO3];
, [bmim][NO3];  , [chl][Ac];
, [chl][Ac];  , [chl][Cl];
, [chl][Cl];  , [chl][dhp];
, [chl][dhp];  , [MAN][NO3];
, [MAN][NO3];  , DMSO.
, DMSO. , pH, not controlled;
, pH, not controlled;  , pH, adjusted to be 7 by 50 mM phosphate buffer in 3.5 wt.-% NaCl salt water.
, pH, adjusted to be 7 by 50 mM phosphate buffer in 3.5 wt.-% NaCl salt water.