Alkali Metal Hydride Complexes: Well-Defined Molecular Species of Saline Hydrides*
Lea Fohlmeister A and Andreas Stasch A BA School of Chemistry, 17 Rainforest Walk, Clayton Campus, Monash University, Melbourne, Vic. 3800, Australia.
B Corresponding author. Email: Andreas.Stasch@monash.edu

Lea Fohlmeister studied chemistry at the Humboldt University of Berlin, Germany, and received her chemistry diploma in 2009. In the same year, she was awarded a scholarship from the German Academic Exchange Service (DAAD) to undertake doctoral research studies overseas, which she conducted and successfully completed between 2010 and 2014 under the supervision of Professor Cameron Jones at Monash University, Melbourne, Australia. Since 2014, she has been working as a post-doctoral research fellow with Dr Andreas Stasch at Monash University, focussing on the preparation and reactivity of main group element hydride complexes. |

Andreas Stasch studied chemistry in Göttingen, Germany, and completed his chemistry diploma (2000) and Ph.D. (Dr. rer. nat., 2003) under the guidance of Professor Herbert W. Roesky. He then undertook post-doctoral research with Professor Cameron Jones from 2004 to 2006 at Cardiff University, UK, which included a visit to Monash University, Melbourne, Australia, working with Professor Peter Junk in 2005. He has been an ARC-funded Research Fellow at Monash University since 2007 and an ARC Future Fellow since 2012, working on unusual organometallic and inorganic molecular compounds, including low oxidation state complexes, metal hydride systems, and metal-metal bonded compounds. He was awarded the Alan Sargeson Lectureship by the Inorganic Chemistry division of the RACI in 2010 and the RACI Organometallic Award in 2014. |
Australian Journal of Chemistry 68(8) 1190-1201 https://doi.org/10.1071/CH15206
Submitted: 23 April 2015 Accepted: 15 May 2015 Published: 22 June 2015
Abstract
The first examples of well-defined alkali metal hydride complexes have been synthesised and characterised in recent years, and their properties and underlying principles for their generation and stabilisation are emerging. This article gives an account of the hydrides of the alkali metals (Group 1 metals) and selected ‘-ate’ complexes containing hydrides and alkali metals, and reviews the chemistry of well-defined alkali metal hydride complexes including their syntheses, structures, and characteristics. The properties of the alkali metal hydrides LiH, NaH, KH, RbH, and CsH are dominated by their ionic NaCl structure. Stable, soluble, and well-defined LiH and NaH complexes have been obtained by metathesis and β-hydride elimination reactions that require suitable ligands with some steric bulk and the ability to coordinate to several metal ions. These novel hydride complexes reward with higher reactivity and different properties compared with their parent ionic solids.
Introduction
Hydrogen is the simplest and smallest chemical element and the most abundant in the universe. It can combine with most elements in the periodic table to form stable binary compounds and can formally act as a proton (H+), a radical (H•) or a hydride (H–) in its compounds.[1–3] Because of its exothermic reaction with oxygen (ΔH = –572 kJ mol–1 for one O2),[1] hydrogen is important as a dense energy carrier and hydrogen storage applications have been intensely investigated.[4,5] Metal hydrides are relevant for many applications, including in synthetic transformations, as catalysts, as reducing agents, and as hydrogen storage materials. Owing to the large number of metallic elements and their location in different parts of the periodic table, binary metal hydrogen compounds can have very different properties.[1–3] The electronegativity of hydrogen (2.20 on the Pauling scale) is relatively high – higher than that of most metals and metalloids – and thus it can be viewed as a hydride unit when joined with highly electropositive metals. Generally speaking, p-block metal hydrides[6] are typically considered to be covalent hydrides, and many elements from the early d- and f-block of the periodic table form metallic or interstitial hydrides, though others, often late transition metals, have intermediate properties. Because of the highly electropositive nature of the s-block metals and their electronegativity difference with hydrogen (~1.2–1.3 units), their binary hydrides are classified as ionic or saline, with the exception of the more covalent or polymeric hydride of beryllium.[1–3,6,7]
The Alkali Metal Hydrides
The alkali metal hydrides MH (M = Li to Cs) are high-melting, white, ionic solids. They can be prepared by reacting hydrogen with the molten alkali metal at high temperatures (e.g. 600–700°C for Li, 250–300°C for Na, and ~350°C for K, Rb, and Cs),[1,8] or high pressures for Li,[9] and they generally show a slightly grey appearance from small amounts of metal contamination. Under ambient conditions, all alkali metal hydrides crystallise in the cubic rock-salt structure with alternating six-coordinate M+ and H– ions. At higher pressures, the MH compounds convert to the CsCl structure with eight-coordinate hydrides, which has been the focus of many theoretical investigations. The necessary pressure for this phase transition decreases with increasing atomic number of the metal centre, 29.3, 4.0, 2.2, 0.83 GPa for NaH, KH, RbH, CsH respectively,[10] and although this structural change has been predicted for all alkali metal hydrides, it has not been observed for LiH to date. The pressure required for this structural phase transition was calculated to be ~200 GPa.[11] Another pressure-induced phase transition to the orthorhombic CrB structure has been predicted for the heavier alkali hydrides KH, RbH, and CsH,[10,12] and has been experimentally found for RbH[13] and CsH.[14] This phase transition does not occur for the hydrides of the lighter metals Li and Na, because their valence band states cannot be influenced by ds hybridisation as there are no suitable d states energetically accessible.[10,12]
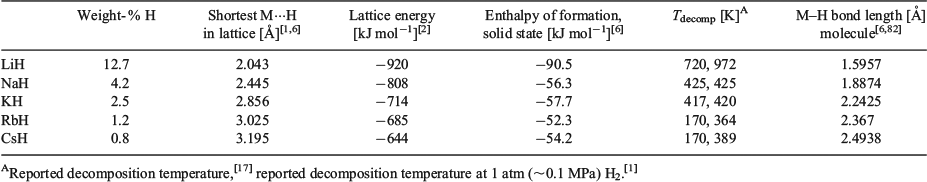
Some of the structural and physical properties of the alkali metal hydrides are listed in Table 1 and will be briefly discussed. The table focusses on the properties of alkali metal hydrides in their condensed phase having an NaCl structure, with the exception of the M–H bond length for the diatomic molecule in the gas phase (last column).
|
As expected, with ascending atomic number and ionic radii of the alkali metals down the group, the weight percentage of hydrogen in the Group 1 metal hydrides decreases, and the shortest M···H contacts in the cubic rock-salt lattice of the Group 1 hydrides increase. All alkali metal hydrides possess fairly high lattice energies, which are larger for the lighter metals. The strongest lattice is formed by hydrides and Li cations, which have the most compatible ion sizes. This trend explains why the reactivity of MH can increase from NaH to CsH, whereas LiH is more inert. The high lattice energies also offer an explanation for why well-defined alkali metal hydride complexes generally cannot be synthesised from their parent metal hydrides, because their lattices are far too stable (see the sections LiH Complexes and NaH Complexes). Generally, the bonding situation in these ionic metal hydrides depends on electronegativity differences between M and H, and on the cation size of M+. The difference in electronegativity for the Group 1 metals is not very pronounced and thus is less important in alkali metal hydrides, but the size difference of M+ from Li+ to Cs+ is noteworthy as it increases descending down the group.[15] The smaller Li+ cation polarises the two 1s electrons in the hydride ion more effectively than its larger heavier congeners, which results in a somewhat more covalent Li–H bond character (it is still mainly ionic) than in the almost purely ionic heavier alkali metal hydrides. As a result, LiH is reported to be slightly soluble in inert organic solvents such as diethyl ether.[1,8] Other sources report no solubility for these metal hydrides in common solvents, but that they dissolve without decomposition in molten NaOH, or alkali metal halides.[1–3]
The reaction of the elemental alkali metals with hydrogen to give the solid metal hydrides is overall an exothermic process as illustrated by the enthalpies of formation for MH in Table 1, with LiH formation being more exothermic compared with the very similar enthalpies found for NaH to CsH. These values are largely dominated by the gain in stabilisation that is achieved when the metal hydrides solidify and form the very stable NaCl lattice, releasing large lattice energies, which significantly outweigh the atomisation enthalpies for M(s) and 0.5H2(g), the ionisation energy for M(g), and the electron affinity for H(g) combined. On reaction of the alkali metals with hydrogen, the density of the solid increases considerably because the alkali metal cations are significantly smaller compared with their atoms, and the packing of MH is more space-efficient. The enthalpies of formation for the gaseous metal hydrides are endothermic. At high temperatures, all alkali metal hydrides except LiH (mp 686.5°C)[1] decompose to the elements before melting, with generally lower decomposition temperatures descending down the group. The melting point for NaH has been estimated as ~800–900°C.[1]
The average M–H bond lengths in the diatomic molecules in Table 1 are experimental values that were determined in gas-phase studies at low pressures. These types of studies are the only means to obtain experimental data for the free mononuclear hydrides, which are typically generated by thermal stimulation or via discharge reactions of the metal vapour and dihydrogen. Though the species formed in this way are short-lived, they can survive long enough at sufficiently low pressures to have their emission and absorption spectra recorded using advanced spectroscopic techniques. From these ground- and excited-state spectra, the vibrational and rotational constants of the molecules can then be approximated and other physical data such as internuclear distances can be assigned and compared with results from theoretical calculations with good agreement.[11,16] Because the alkali metal hydrides, especially LiH, are very small and simple ionic solids or diatomic molecules, a large number of theoretical studies have investigated numerous compositions and properties of these systems, including stoichiometric (LiH)n, hydrogen-rich or Li-rich clusters or phases that are beyond the scope of this report, but selected examples have been included throughout this article and often accompany experimental studies.
In particular, the light s-block metal hydrides are of interest with respect to their hydrogen storage potential,[4,5,17–19] as they possess a high weight percentage of H atoms; LiH (12.7 % H) and NaH (4.2 % H) are being studied for the alkali metals. Significant hydride–hydride interactions including bond paths with bond critical points have been found for the solid-state structure of LiH, but not for NaH.[20,21] Theoretical studies on the decomposition of the rhombic dimers (MH)2, M = Li, Na, K, into the elements revealed a more facile H2 formation for Na and K compared with Li, due to the larger size and poorer orbital overlap with hydrogen for the heavier ions.[20,22] This qualitative trend is in line with the observed decomposition temperatures of the alkali metal hydrides; see Table 1. Problematic is the high temperature required for hydrogen release in these lighter hydrides (see Table 1), which is in part a result of the high lattice energies of the lighter MH phases, plus the reversibility of this reaction. All these properties are more favourable for the heavier alkali metal hydrides, though these show an undesirable low hydrogen weight percentage. Several strategies have been employed to improve the hydrogen storage properties of metal hydrides. These include materials with large surface areas, nanoscale or ‘destabilised’ materials, the use of catalysts, and the study of a large number of mixed element hydride systems with various additives.[23–28] Nanoscale metal hydride cores have been suggested as materials with improved predicted hydrogen storage properties based on computational studies, because significant structural differences are found in comparison with the bulk metal hydride phases. For clusters of (LiH)n, however, these studies suggest that the structural changes from the bulk phase are too small for significant improvements of the decomposition into the elements.[29]
Whereas the industrial-scale synthesis of the bulk alkali hydrides via direct reaction of the elements at high temperatures is state of the art for LiH, NaH, and KH, the resulting crystalline solids often show reasonably poor reactivity. They are, however, common and commercially available laboratory bases and hydride sources, often used for deprotonation reactions in organic synthesis, and they react with protic reagents such as water violently under H2 evolution. LiH is the least reactive and is stable in dry air. Commercially available NaH and KH are typically delivered in and protected with mineral oil.
The commercial synthesis of RbH and CsH has not been realised at all because the rate of reaction is too slow. Very pure samples of these compounds can be prepared in the laboratory by reaction of the molten metal with hydrogen that is produced by thermal decomposition of UH3, using a temperature gradient (250–150°C).[30] The higher temperature helps to break up the passivating hydride layer on the surface of the metal and product deposition then occurs in the cooler part of the reaction vessel, though this method is only suitable for generating milligram amounts. A more efficient protocol was developed later by ball milling the respective alkali metal at room temperature under an atmosphere of H2 at 0.5–0.7 MPa. This procedure can be used to make very pure gram-scale quantities of MH (M = Na–Cs).[31]
Highly reactive, finely divided powders of MH (M = Li, Na, K) were obtained from reactions of nBuLi(TMEDA), tBuONa(TMEDA), and tBuOK(TMEDA) (TMEDA = N,N,N′N′-tetramethylethylenediamine) with hydrogen in hexane.[32] The resultant MH powders were able to deprotonate and reduce carbonyl and thiocarbonyl compounds, alcohols, and nitriles among others.[33] Similarly, treating tert-butyllithium in an aliphatic solvent at ~7–20 MPa hydrogen pressure at room temperature affords active LiH.[34] Instead of dihydrogen, cyclohexadienes were used with alkyllithium reagents to produce LiH of high reactivity in situ.[35] Also, the catalysed reaction of some alkenes with lithium metal affords a range of organolithium compounds and LiH as a by-product.[36] It has furthermore been long known that β-hydride elimination from organo alkali metal compounds with suitable alkyl substituents leads to initially highly reactive MH that can precipitate from solution and is best investigated for alkyllithium compounds.[37] More recently, photochemically induced decomposition of tBuLi/tBuOLi mixtures led to soluble tBuOLi/LiH aggregates, which will be covered in the section LiH Complexes. Chemists have further developed other strategies to utilise and activate LiH in synthetic protocols, for example by using transition metal-based additives.[34a,38] Another method describes that alkali metal intercalation compounds with graphite chemi- and physisorb hydrogen, to yield intercalated MH and can form ‘triple layers’ of ions represented as K+–H––K+.[39]
Three consecutive studies in 2007 detailed the results from matrix isolation experiments[40–42] on LiH,[40] NaH,[41] and KH[42] systems trapped in different gas matrices at very low temperatures. The desired species were obtained by laser ablation of alkali metal atoms, which reacted with H2 and were embedded in matrices of solid hydrogen (all three metals), neon (Li, Na) or argon (Li) and studied by IR spectroscopic methods in combination with computational investigations. These studies resulted in the detection of MH, (H2)n···MH and (MH)2 for Li, Na, and K, and (MH)3,4 for Li and Na only, including their deuterium isotopomers, with the heavier clusters being increasingly obtained after irradiation. Irradiation failed to produce higher KH clusters and it has been concluded that excited K atoms are capable of activating H2, but not excited K2 molecules under these experimental conditions, unlike the lighter alkali metals. The (MH)2–4 molecules A–C (Fig. 1) all show ring structures with alternating M and H atoms and highly ionic bonding further increasing down the group. The (MH)4 (M = Li, Na) molecules are cyclic tetramers, although the cubic structure D is calculated to be lower in energy, and it has been concluded that the latter structural motif cannot be formed under the experimental matrix conditions. The accompanying theoretical studies also took thermodynamic considerations into account and generally showed that aggregation to larger systems is energetically favoured.

|
Alkali metal polydrides with unusual stoichiometry are predicted to form phases of MHn, n > 1 for Li,[43,44] Na,[45,46] K,[47] Rb,[48] and Cs,[49] which can contain the weakly bound H3– anion[50,51] at high pressures. Very recently, a first experimental study[46] reported the phases NaH3 and NaH7 found at above 40 GPa and 2000 K, which could be studied by a combination of synchrotron X-ray diffraction and Raman spectroscopy and were formed in a laser-heated diamond anvil cell. So far, Li polyhydrides have not been observed under these conditions,[9,46] although this was predicted previously.[43] The alkali metal polyhydrides are somewhat related to the recently prepared NaCl compounds with unusual stoichiometries, e.g. NaCl3.[52]
Finally, we would like to make the reader aware of some pseudo-compounds reported in the peer-reviewed literature that relate to alkali metal hydrides of unusual stoichiometry. It has been claimed that ‘KHI’ and related ‘MHX’ (M = alkali metal ion, X = halide) materials[53] have been made that contain the hydrogen as a ‘different’ hydride, based on the hydrino model,[54] which claims to contain hydrogen below the energetic ground state and is linked to some cold fusion science. Blue (or green) crystals of ‘KHI’ were apparently obtained from KI, a catalytic amount of potassium metal, and hydrogen under pressure and at high temperatures. The material was studied with various techniques, though unfortunately no X-ray structural investigation was reported. New hydride forms as well as a mention of remarkable K2+ were discussed for an explanation of this unusual MHX stoichiometry.[53]
Mixed Metal and Metalloid ‘-ate’ Complexes Involving Hydridic Hydrogen and Alkali Metals
Well-defined hydride complexes purely of Group 1 metals are very rare and are in fact only known for lithium and sodium so far (see the sections LiH Complexes and NaH Complexes). Most isolated compounds involving alkali metals and hydridic hydrogen centres are mixed-element systems and are best described as ‘ate’-type complexes in which the strongest interaction of the hydride ligand is with the non-alkali metal centre or metalloid.[1–3,6,7] This makes the majority of these ‘ate’ complexes covalent hydride complexes. The most prominent examples in this compound class are perhaps LiAlH4, NaBH4, and other related commercial derivatives such as L-selectride®, N-selectride,® and K-selectride® (lithium, sodium, potassium tri-sec-butyl(hydrido)borate), or derivatives with sterically demanding ligands.[55] These ‘ate’ complexes can be synthesised from the corresponding MH and a suitable Lewis-acidic borane derivative. Even fairly inert LiH reacts with Lewis-acidic element halides in suitable solvents such as ethers under hydride–halide metathesis plus possibly ‘ate’ complex formation.[1–3]
These mixed-element boranates and alanates are very potent reducing agents (for example towards unsaturated organic substrates)[56] and hydride-transfer reagents reminiscent of soluble molecular hydrides, and their structures are better viewed as Li+[AlH4]–, Na+[BH4]–, and M+[HB(sBu)3]– (M = Li, Na, K). The aforementioned M[HBR3] (R = sBu or other alkyl group) compounds are particularly popular as organic reducing agents as they are soluble in ethers, form neutral and volatile BR3 side products, and thus can be seen as carriers of stoichiometric amounts of ‘MH’. They are also convenient hydride sources in organometallic chemistry, e.g. for the synthesis of novel element hydride complexes, for similar reasons.[57] In general, reactions of EXn (E = element, X = halide) with M[HBR3] proceed typically rapidly, giving EHn or derivatives, and the only by-products, MX and BR3, do often not interfere with the synthetic procedure and can be easily removed because they are insoluble (MX) or volatile and highly soluble (BR3). In addition, owing to their high hydrogen content (e.g. 18.5 wt- % H for LiBH4), they have increasingly been studied as potential hydrogen-storage materials; also see the section The Alkali Metal Hydrides. Accordingly, a wide variety of mixed-element hydride materials have been studied as more promising hydrogen storage materials compared with purely binary metal hydrides.[23–27]
A range of mixed metal/metalloid hydride complexes are of relevance here because they possess fragments similar to those found for alkali metal hydride moieties, contain chemically related metals, or are linked to the formation of alkali metal hydride complexes.
An impressive example of a mixed metal hydride complex was published in 2005.[58] [[{(Me3Si)2N}2AlH2Li]3(LiH)] 1, see Fig. 2, obtained from [(tBuOAlH2)2] and [Li{N(SiMe3)2}] in toluene, possesses a solid-state structure that consists of a distorted central Li4H4 cube, which is surrounded by three {HAlN(SiMe3)2} moieties. One of the central hydride centres is exclusively coordinated to Li ions, whereas the other three H atoms in the central cube all bind to a lithium and an aluminium centre. Treatment of compound 1 with substoichiometric water leads to liberation of hydrogen gas and displacement of the all-lithium-bound hydride ion with OH– to form Li4H3(OH)[HAl{N(SiMe3)2}2]3. The other three hydride ligands in the central cube remain unaffected, suggesting that they are kinetically more protected by the three coordinating {HAlN(SiMe3)2} fragments.

|
‘Ate’ complexes of chemically more ‘innocent’ silanes (cf. the silane method in the section LiH Complexes, see below) have been prepared and characterised in some instances for potassium, for example in the form of derivatives of K[Ph3SiH2] including its donor adduct with 18-crown-6 (18c6), [K(18c6)][Ph3SiH2] 2,[59] (see Fig. 3) and related species.[60] These highly reactive complexes can formally deliver a stoichiometric quantity of KH. Importantly, solid KH can in some instances be used to generate the hydridosilicates.[59–61] Reactions of these silanes and KH also involve exchange processes of the organic substituents on silicon, and reduction to R3SiK species and polysilanes.[62]

|
A molecule with 1,3-P,Al-substitution has been used as a frustrated Lewis pair to solubilise MH (M = Li, Na, K) in THF forming hydridoaluminate complexes that can be used catalytically to activate the bulk metal hydrides for enhanced reactivity.[63] Other methods have been employed to yield solubilised alkali metal hydride equivalents. The reaction of alkyllithium compounds with pyridine initially affords 1,2-addition products.[64] These soluble hydropyridyllithium complexes can deliver LiH equivalents, which has been demonstrated by the reduction of benzophenone.
A series of mixed metal hydride complexes exclusively incorporating s-block metal centres include the heterobimetallic hydride complexes, [M2Mg2(NiPr2)4(μ-H)2·(toluene)2],[65] with an Mg(µ-H)2 Mg core that is encapsulated by four amide ligands and two alkali metal centres M (M = Na or K). These compounds were synthesised by heating a toluene solution of ‘NaMg(NiPr2)3’ or ‘KMg(NiPr2)3’ respectively, which were prepared in situ from nBuNa or BzK (Bz = benzyl), n,s-Bu2Mg, and HNiPr2 in a 1 : 1 : 3 ratio. At a later point, a solvent-free version of the sodium complex was also isolated by refluxing a hexane solution of ‘NaMg(NiPr2)3’ in the presence of bis(benzene)chromium.[66] Only the solvent-free compound [Na2Mg2(NiPr2)4(μ-H)2], which is stabilised by intermolecular Na–C(iPr) agostic interactions to form a one-dimensional chain, and unreacted bis(benzene)chromium could be isolated from the reaction mixture. It is thought that the hydride ligand in all three heterobimetallic compounds is formed via β-hydride elimination from a diisopropylamide group in ‘(M)Mg(NiPr2)3’ and then migrates to an Mg centre before dimerisation takes place.[65b] A hydrocarbon solution of the potassium derivative was shown to reduce benzophenone to benzhydrol, supporting the fact that these compounds can be viewed as soluble magnesium hydride complexes with strong reducing properties.
More recently, the synthesis of two alkali metal/magnesium hydride clusters that were prepared from the reaction of [M{N(SiMe3)2}2MgBu] (M = Na, K) with phenylsilane were reported.[67] In one case, the molecular heterobimetallic K/Mg complex, [K2Mg2{N(SiMe3)2}4(μ-H)2], was obtained, which displays a similar molecular structure to the previously mentioned compound [K2Mg2(NiPr2)4(μ-H)2·(toluene)2][65] (see above) with a central Mg(µ-H)2Mg core, stabilised by four amide ligands and two potassium ions. Its formation, however, was the result of a σ-bond metathesis between Mg-alkyl/amide and Si–H bonds using the silane method (see the section LiH Complexes), and not a β-hydride elimination from an N-bound alkyl group. When the precursor [Na{N(SiMe3)2}2MgBu] was reacted with phenylsilane, a different product was isolated. X-ray diffraction analysis revealed the formation of the heterobimetallic compound, [Mg6Na6{N(SiMe3)2}8H10] 3, which incorporates 10 hydride ligands; see Fig. 4. The central unit of the structure can again be viewed as an Mg2H2 core, but in this instance, the magnesium atoms are six-coordinate, binding to a further four hydride ligands each, not unlike the bulk structure of MgH2. The two central hydrides also coordinate to two Na centres, while all amide ligands are located on the outside of the Mg6Na6H10 framework, each binding to a four-coordinate Mg and Na atom.

|
LiH Complexes
In recent years, chemists have succeeded in the generation, isolation, and characterisation of several well-defined LiH complexes using various routes and methods. Following on from long-known β-hydride eliminations of organolithium compounds (see the section The Alkali Metal Hydrides),[37] exposing mixtures of tBuOLi/tBuLi, including their 6Li and/or 2H-labelled isotopomers, in cyclopentane to heat and daylight afforded resonances in 1D and 2D multinuclear NMR spectroscopy experiments that suggested the generation of [Li10H(OtBu)9] (2J(6Li,1H) 2.2 Hz, 2J(6Li,2H) 0.34 Hz) and [Li12H(OtBu)11] (2J(6Li,1H) 1.7 Hz, 2J(6Li,2H) 0.25 Hz).[68] A high alkoxide/alkyl ratio of ROLi : RLi ≥ 7 : 3 was required to prevent the irreversible precipitation of LiH and this form of hydrocarbon-soluble LiH showed a higher reactivity compared with commercial LiH. It was further suggested that the ‘decamer’ [Li10H(OtBu)9] is the photochemical decomposition product and the ‘dodecamer’ [Li12H(OtBu)11] is the thermal decomposition product.[69] Subsequently, the same set of studies led to the crystalline ‘superaggregate’ [Li33H17(OtBu)16] 4 (see Fig. 5) from similar experiments with prolonged irradiation using an Hg lamp at –40°C.[69] Compound 3 shows some structural motifs reminiscent of cubic LiH/LiOtBu fragments and randomly ordered ionic contacts with tert-butoxide ligands occupying the anionic corner positions.

|
A series of polyhedral cluster compounds of the general formula [L6Li8H][A] (A = organometallic complex anion) was published between 1999 and 2006,[70] and this chemistry has been reviewed.[71] In these complexes, a central, ‘interstitial’ hydride is surrounded by eight Li cations, with two to eight reasonably close contacts of the Li+ cations to the central hydride. The Li ions are coordinated by six monoanionic, N,N′-chelating ligands (L, see Fig. 6), and see Fig. 7 for the cationic complex [(hpp)6Li7H]+ (hppH = 1,3,4,6,7,8-hexahydro-2H-pyrimido[1,2-a]pyrimidine) 5. The anion (A) consists of organometallic complex anions containing Li, B, Al, and/or Zn. The hydrides originated from the use of tert-butyl lithium in the synthesis, though the reaction of [(hpp)Li] with Li[HBEt3] could also be used.[71] A neutron diffraction study was carried out, which confirmed the location of the central hydride in one example.[70b]

|

|
In 2012, a different strategy was employed to synthesise well-defined LiH complexes using the ‘silane method’. The sterically demanding phosphinoamine Ph2PN(H)Dip (Dip = 2,6-iPr2C6H3) was treated with two equivalents of n-butyllithium to afford the heteroleptic alkyl complex [(DipNPPh2)2Li4(nBu)2] 6; see Fig. 8. This complex can be rapidly and conveniently converted to the hydride complex [(DipNPPh2)4Li8H4] 7 with commercially available phenylsilane in an aromatic solvent; see Fig. 8.[72] The structure of 7 can be derived from that of 6 by replacing the n-butyl groups with hydrides, followed by dimerisation via the hydride ligands. In 6 and 7, both N and P donors are involved in Li coordination. The Li···H contacts within the central cube are ~2.0 Å, similar to those in solid LiH, though shorter Li···H contacts of ~1.8 Å are found to the ‘outer’ Li ions. Complex 7 shows a broad resonance at 4.18 ppm in its 1H NMR spectrum for the hydride resonances. NMR spectroscopic studies revealed a higher symmetry of 7 at elevated temperatures likely due to fluxional behaviour. Above ~60°C, slow irreversible decomposition of 7 to presumably polymeric and largely insoluble [(DipNPPh2)Li] and, by implication, LiH occurs. Complex 7 decomposes at 100°C in solution within minutes, but is sufficiently stable at room temperature.
The availability of hydrocarbon-soluble 7 allowed reactivity studies to be undertaken and 1H, 7Li, and 31P NMR spectroscopy aided rapid in situ reaction monitoring.[72,73] The lithium hydride complex [(DipNPPh2)4Li8H4] 7 reacts at room temperature and below in hydrolithiation reactions with benzophenone and dicyclohexylcarbodiimide respectively; see Scheme 1 for a simplified summary. Similarly, complex 7 reacts with azobenzene, likely in a hydrolithiation reaction, but the resulting product is acidic enough to react with a second LiH unit to furnish a substituted dilithium hydrazine-1,2-diide (Scheme 1).[73] The complex was not reactive enough to hydrolithiate diphenylacetylene or 1,1-diphenylethylene, presumably owing to the relatively low Lewis acidity of the metal ion (cf. B3+, Al3+). Previously, the multiple-bond addition reactions of monomeric LiH with ethyne and ethene respectively have been investigated by computational studies[74] and were predicted to proceed, though they show a significant activation barrier that is somewhat lowered by the presence of the Li+ cation as a Lewis acid. In a special case, in situ-generated LiH succeeded in reducing the C≡C bond of ynolates.[35] The reactivity studies of 7 were complicated owing to competing reactions of the stabilising phosphinoamide ligand, DipNPPh2–, with several unsaturated organic substrates either in P or N addition reactions. This latter diversity is likely governed by a combination of electronic (hard and soft Lewis acids and bases) and steric effects.[73]
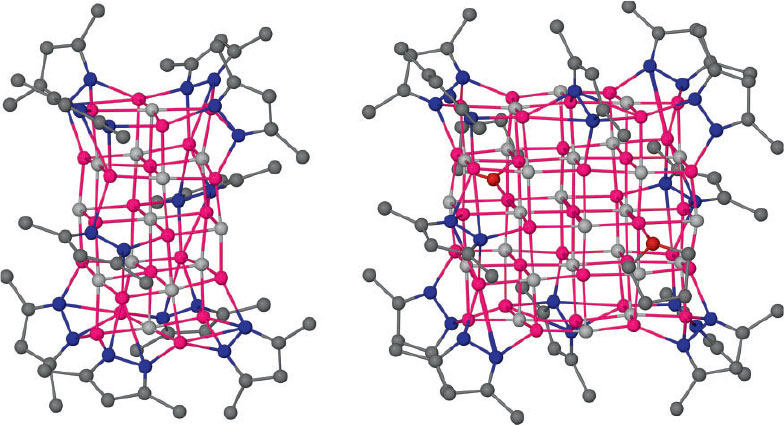
Substituted pyrazolates were investigated as more inert ligands for the stabilisation of LiH complexes. Treating 3,5-di-tert-butyl-1H-pyrazole (pzH) with more than one equivalent of n-butyllithium in aromatic or aliphatic solvents, followed by addition of phenyl- or diphenylsilane to convert the excess alkyllithium groups to hydrides resulted in initially clear solutions with dissolved ‘LiH’ units.[75] These solutions can turn milky for high hydride to pyrazolate ratios likely because colloidal LiH is formed. From these experiments, the soluble, well-defined LiH cluster complexes [(pz)6Li7H] 8, [(pz)10Li22H12] 9, and [(pz)12Li37H25] 10 could be isolated and structurally characterised. The overall structure of complex 8 is related to previously characterised ‘interstitial’ cluster cations (cf. [(hpp)6Li7H]+ 5, Fig. 7), and the molecular structure of 9 is shown in Fig. 9. Treating crude [(pz)12Li37H25] 10 with THF or adding small amounts of THF to the abovementioned clear LiH/Li(pz) solutions (ratio ≈ 2.5) reproducibly formed [Li(thf)4][(pz)12Li37(thf)2H26] 11, which could be structurally characterised; see Fig. 9. The molecular structure of 11 (and related 10) can be rationalised as a cubic lattice cut-out of 5 × 5 × 3 ions of alternating cations (Li+) and anions (H– or pz–) with approximate core dimensions of 0.9 × 0.8 × 0.45 nm. In 11, all eight corners are occupied by μ3-bridging pz ligands, and on every second edge is located a μ4-bridging pz ligand. The latter are arranged on neighbouring edges of the larger 3 × 3 faces and not on opposing ones. This leaves one quadrant of these faces less sterically shielded so that they accommodate exactly one coordinating THF molecule. The complex core has a region of 3 × 3 × 1 (5 Li+, 4 H–) ions that have identical surroundings (six nearest neighbours) as those in bulk LiH.
Complex 11 shows very broad resonances at 3.4–4.0 ppm in the 1H NMR spectrum for the LiH fragments, and broad overlapping peaks in the region from 0.8 to 4.3 ppm in its 7Li{1H} NMR spectrum for the numerous Li environments. Irreversible LiH elimination from [Li(thf)4][(pz)12Li37(thf)2H26] 11 does occur at elevated temperatures in solution, but appears to be significantly slowed down by the sterically demanding ligands. Heating solutions of 11, or storing it in THF for long times, does eventually produce [(pz)6Li7H] 8 and solid LiH, which is probably the endpoint of the decomposition.[75]
We found that other pyrazolates also produced soluble LiH solutions using the same method (L. Fohlmeister, A. Stasch, unpubl. data), including those obtained from commercially available 3,5-dimethyl-1H-pyrazole, though, so far, no well-defined complexes could be isolated for pyrazolate ligands other than those of pz, i.e. 8–11. This ligand class appears to be very successful in solubilising several equivalents of LiH per solubilising ligand, e.g. see complexes 10 and 11.
These latter syntheses have shown that the ‘silane method’ offers a fast and convenient route to soluble LiH units in hydrocarbon solvents at or below room temperature from commercially available reagents and suitable ligands. This route is now established[7,76] for a range of electropositive metal complex fragments, because commercially available silanes such as phenyl- and diphenylsilane can be used as hydride sources and nucleophilic alkyl and amide substituents can be conveniently converted. It can be summarised according to Scheme 2: alkyl- or amidometal fragments undergo a metathesis reaction with the silane via a hydridosilicate intermediate (cf. the more stable potassium complexes such as [K(18c6)][Ph3SiH2] 2), which eliminates an MH fragment and produces a substituted silane). For the synthesis of a rare CaH complex, a silane reactivity order of Ph2SiH2 ≈ PhSiH3 > Ph3SiH >> Et3SiH has been reported.[77]
|
|
The role of the stabilising ligand is to coordinate and solubilise the MH entities and to prevent irreversible MH precipitation (e.g. LiH) by suppressing equilibria with significant structural changes of these complexes having labile and ionic metal–ligand interactions. Significant slowing of these equilibria can lead to experimentally observed metastable solutions. These decomposition reactions can be expressed as in Scheme 3 for neutral donor ligands (Do, top) or monoanionic ligands (L–, bottom). Only isolated compounds of the latter concept have been reported (e.g. as in 4, 5, 7–11), although related, reactive simple donor-stabilised MgH2 solids have been accessed via this silane method.[76] For [(DipNPPh2)4Li8H4] 7, NMR studies at elevated temperatures in solution suggested that the decomposition starts parallel with structural changes to the molecule (~55–60°C) as judged by more symmetrical NMR spectra on 7.[72] Thus, the phosphinoamide ligand in 7 is a suitable stabilising system for Li that pushes the conditions for the decomposition reactions according to Scheme 3, bottom, to well above room temperature in hydrocarbon solvents. The ability of a ligand to stabilise MH fragments under certain conditions (including temperature and solvent choice) will likely depend on many factors such as metal–ligand bond strengths, donor atom orientation, and steric bulk of the ligands, as well as metal ion coordination numbers. All successfully employed ligands to date show some steric bulk and contribute several donor electron pairs to coordinate to at least three Li cations and as such can stabilise cluster-type complexes. The decomposition reactions according to Scheme 3 for specific conditions may halt when a stable system has been reached that the ligand can stabilise. For the pzLiH system, for example, and as studied on [Li(thf)4][(pz)12Li37(thf)2H26] 11, the endpoint of the decomposition in solution at elevated temperatures appears to be [(pz)6Li7H] 8, cf. the related ‘interstitial’ cluster compounds such as 5. These latter cluster species with a central hydride ion appear to be kinetically protected from further LiH elimination; too many coordination bonds need to be broken or rearranged to remove the remaining LiH. Overall, the stability depends on the ratio of a certain stabilising ligand to hydride fragments, as for example studied for LiOtBu/LiH.[68,69]

|
NaH Complexes
Sodium with its larger ionic radius compared with lithium is expected to be more difficult to incorporate into a molecular NaH complex. Remarkably, an ‘inverse sodium hydride’ complex had been reported before any true molecular sodium hydride complex was published. The extremely sensitive, golden crystalline complex of a protonated ‘adamanzane’ cage (a saturated species with four tertiary amine functionalities and an encapsulated proton) with the highly reactive sodide anion, Na–, was obtained using Na metal, ammonia or amines, and ether solvents at low temperatures.[78]
Very recently, the first NaH complex was prepared and structurally characterised. Employing the same synthetic strategy and sterically demanding pyrazolate ligand (pz) used to obtain the Li complexes 8–11, the reaction of [Na(pz)], [Na(nBu)], and diphenylsilane in aromatic solvents afforded [(pz)6Na7H] 12; see Fig. 10.[79] The complex contains a central hydride ligand that is approximately tetrahedrally surrounded by four Na cations. Three of these four tetrahedron faces are capped by another Na ion each and the cluster is completed by three μ3-bridging and three μ4-bridging pyrazolates. The Na···H distances in 12 range from 2.25(2) to 2.38(3) Å.

|
Complex 12 shows one set of resonances for the pyrazolate ligands in its 1H NMR spectrum confirming the flexible nature of these complexes, plus a slightly broadened NaH resonance at 5.17 ppm. Complex 12 could not be prepared from commercially available solid NaH. The reaction could not, so far, be extended to potassium. A similar experiment for the synthesis of 12, using [K(pz)], [K(nBu)] instead of the sodium reagents plus diphenylsilane, only led to the isolation of crystalline polymeric [K(pz)].[79]
Conclusions and Outlook
A series of well-defined LiH complexes and one NaH complex have been prepared and structurally characterised in recent years. These range from complexes with a single hydride ion in the centre of a cluster arrangement for Li and Na to nanometre-sized LiH cluster compounds with cores of up to (Li37H26)11+. The complexes were obtained using organometallic synthesis in solution, via β-hydride elimination or hydride metathesis (silane method) reactions. Suitable stabilising ligands are required to obtain complexes that can be handled at ambient temperature without decomposition. The newly formed compounds generally show a higher reactivity compared with their bulk alkali metal hydrides, likely because no lattice energy has to be invested for these soluble complexes. Some LiH complexes were found to undergo hydrolithiation reactions with activated unsaturated substrates at or below room temperature.
With the general concepts for synthesis and stabilisation in place, novel types of well-defined and soluble alkali metal hydride complexes will without doubt be reported in the future, including those of the heavier alkali metals. In this field of chemistry, the use of new ligands and the development of novel ligand types will be needed to successfully isolate hydride complexes of the heaviest alkali metals, considering their very large ionic radii. Access to these new compounds and easy protocols for in situ generation of those species in solution, even as metastable complexes or reactive intermediates, will allow their chemistry to be extensively investigated and new synthetic protocols to be widely used. The study of the further reactivity of these well-defined alkali metal hydride complexes is still in its infancy.
Complexes of the s-block elements are highly popular as (Lewis acid) catalysts for a wide variety of applications.[7,80] These include various transformations where metal hydride fragments are involved, such as catalytic hydroelementation reactions (e.g. hydrosilylations and hydroborations of unsaturated organic substrates), rearrangements, and hydrogenation reactions. The majority of these catalytic transformations for the s-block elements are performed with Group 2 metal ions.[7,61,80] With the developed convenient silane method being used in many research groups around the world, more novel metal hydride complexes including those with alkali metal ions will be forthcoming and applied to stoichiometric and catalytic transformations. The cheap, abundant, non-toxic, and even biocompatible nature of some alkali metals such as Na and K will without doubt be further investigated by researchers for greener and more sustainable chemistry solutions. Very recently, an intriguing silylation reaction of unactivated aromatic CH bonds with stoichiometric silanes and catalytic KOtBu was described.[81] The authors suggest a radical mechanism for this transformation, but soluble KH fragments and/or relevant ‘-ate’ complexes could also be considered to play a role in this reaction. However, commercially available solid KH has been found to be inactive as a catalyst for this reaction. The combination of popular and widely used silane reagents and alkali metal bases and organometallics will likely produce more surprising outcomes in activation reactions.
More knowledge on the important compound classes discussed in this article and the chance to study the many aspects of well-defined molecular complexes will also bring benefits to other applications including hydrogen storage technologies. The newly made complexes will allow studies to be undertaken on soluble and well-defined molecular compounds under ambient conditions rather than solely on their parent solids. Other important achievements for this science could be realised in the future. This could for example include alkali metal hydride complexes that deviate from simple M+H– compositions and introduce novel compounds with unusual metal- or hydrogen-rich stoichiometries. The first exciting experimental work on sodium polyhydride compounds,[46] studied under extreme conditions though, does show that novel materials with unusual stoichiometries and properties are possible.
Acknowledgements
We thank the Australian Research Council for project support and a fellowship for AS. The Australian Synchrotron and their MX1 and MX2 beamlines are acknowledged for collection time of some of the crystal structures presented herein.
References
[1] A. F. Holleman, E. Wiberg, N. Wiberg, Lehrbuch der Anorganischen Chemie, 102nd edn 2007 (de Gruyter: Berlin, New York).[2] C. E. Housecroft, A. G. Sharpe, Inorganic Chemistry, 4th edn 2012 (Pearson: New York, NY).
[3] N. N. Greenwood, A. Earnshaw, Chemistry of the Elements, 2nd edn 1997 (Elsevier: Oxford).
[4] L. Schlapbach, A. Züttel, Nature 2001, 414, 353.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXovFGitrk%3D&md5=d3ba598e7798d51896617c254b35298bCAS | 11713542PubMed |
[5] J. Graetz, Chem. Soc. Rev. 2009, 38, 73.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsFWjtLzF&md5=900a2b49022f97991ecb8e86e2999edbCAS | 19088966PubMed |
[6] S. Aldridge, A. J. Downs, Chem. Rev. 2001, 101, 3305.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXot1emsLY%3D&md5=ee8da770746e71c6bba72af321880c05CAS | 11840988PubMed |
[7] S. Harder, Chem. Commun. 2012, 11165.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsFKgsr7J&md5=b8c63d0c964a83996269091fcebaaf26CAS |
[8] P. Rittmeyer, U. Wietelmann, Hydrides, Ullmann’s Encyclopedia of Industrial Chemistry 2000 (Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim).
[9] R. T. Howie, O. Narygina, C. L. Guillaume, S. Evans, E. Gregoryanz, Phys. Rev. B 2012, 86, 064108.
| Crossref | GoogleScholarGoogle Scholar |
[10] J. Hooper, P. Baettig, E. Zurek, J. Appl. Phys. 2012, 111, 112611.
| Crossref | GoogleScholarGoogle Scholar |
[11] G. S. Priyanga, A. T. A. Meenaatci, R. R. Palanichamy, K. Iyakutti, Comput. Mater. Sci. 2014, 84, 206.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXisFentrg%3D&md5=7e277bbe33342d55714650f80f280957CAS |
[12] R. Ahuja, O. Eriksson, J. M. Wills, B. Johansson, J. Phys. Condens. Matter 1998, 10, L153.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXitVKrtbs%3D&md5=dc8636ebdab791fd8d554f574483e177CAS |
[13] K. Ghandehari, H. Luo, A. L. Ruoff, S. S. Trail, F. J. DiSalvo, Mod. Phys. Lett. B 1995, 9, 1133.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXos1Snu74%3D&md5=19a842a6a737087f3679cdd161ac8985CAS |
[14] K. Ghandehari, H. Luo, A. L. Ruoff, S. S. Trail, F. J. DiSalvo, Phys. Rev. Lett. 1995, 74, 2264.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXksVaku7w%3D&md5=34d6adcca1def5fad63cdd7e2ac04a7aCAS | 10057884PubMed |
[15] J.-B. Liu, W. H. E. Schwarz, J. Li, Chem. – Eur. J. 2013, 19, 14758.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsF2nu7%2FO&md5=11918e4230fdd13f7a0e613def7f4e1eCAS | 24105988PubMed |
[16] M. Vasiliu, S. Li, K. A. Peterson, D. Feller, J. L. Gole, D. A. Dixon, J. Phys. Chem. A 2010, 114, 4272.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXislyksbg%3D&md5=abadda7c73eae974dfb042f1b9d8674dCAS | 20201583PubMed |
[17] W. Grochala, P. P. Edwards, Chem. Rev. 2004, 104, 1283.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXht12gtrw%3D&md5=0e51a3e8da4b6ad8484b0c96eaba99a2CAS | 15008624PubMed |
[18] B. Sakintuna, F. Lamari-Darkrim, M. Hirscher, Int. J. Hydrogen Energy 2007, 32, 1121.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXlvValsb8%3D&md5=ce1a104297c37d84e70aa2d56ad0e6ddCAS |
[19] S.-i. Orimo, Y. Nakamori, J. R. Eliseo, A. Züttel, C. M. Jensen, Chem. Rev. 2007, 107, 4111.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtVahsrbL&md5=c0e98cfceeb7738b4483b6b061f31780CAS |
[20] D.J. Wolstenholme, J.L. Dobson, G.S. McGrady, Dalton Trans. 2015, 44, 9718.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXms1ygurY%3D&md5=760f960a9d2cd504c44abd7fbddfdbe7CAS | 25915435PubMed |
[21] P. Sirsch, F. N. Che, J. T. Titah, G. S. McGrady, Chem. – Eur. J. 2012, 18, 9476.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XovFejsLg%3D&md5=57a374b15231cc2d35316445dd141d44CAS | 22730237PubMed |
[22] D. J. Wolstenholme, M. M. D. Roy, M. E. Thomas, G. S. McGrady, Chem. Commun. 2014, 3820.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXktF2kt7g%3D&md5=e4edfe9aa59dceaa2c0f6f9425252ad1CAS |
[23] V. Bérubé, G. Radtke, M. Dresselhaus, G. Chen, Int. J. Energy Res. 2007, 31, 637.
| Crossref | GoogleScholarGoogle Scholar |
[24] J. J. Vajo, G. L. Olson, Scr. Mater. 2007, 56, 829.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXis1Gktrs%3D&md5=631ca13137343fd4625d0fe5871fc3c9CAS |
[25] A. M. Seayad, D. M. Antonelli, Adv. Mater. 2004, 16, 765.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXks1yksbw%3D&md5=511ce00df9b74ead07c7b1c7a238b10bCAS |
[26] I. P. Jain, P. Jain, A. Jain, J. Alloys Compd. 2010, 503, 303.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXptVWis7o%3D&md5=8fdb0f90021a1e68979a92c69ca26213CAS |
[27] L. H. Rude, T. K. Nielsen, D. B. Ravnsbæk, U. Bösenberg, M. B. Ley, B. Richter, L. M. Arnbjerg, M. Dornheim, Y. Filinchuk, F. Besenbacher, T. R. Jensen, Phys. Status Solidi A 2011, 208, 1754.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXpvFyjur0%3D&md5=2193a932f95006f383311d09ab2c31a1CAS |
[28] P. E. de Jongh, P. Adelhelm, ChemSusChem 2010, 3, 1332.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsFCkt77M&md5=f0b2cfcccd22d5148aeaf91063315accCAS | 21080405PubMed |
[29] L. K. Wagner, E. H. Majzoub, M. D. Allendorf, J. C. Grossman, Phys. Chem. Chem. Phys. 2012, 14, 6611.
| Crossref | GoogleScholarGoogle Scholar | 22456531PubMed |
[30] D. Guerard, N. E. Elalem, S. El Hadigui, L. Ansari, P. Lagrange, F. Rousseaux, H. Estrade-Szwarckopf, J. Conard, P. Lauginie, J. Less Common Met. 1987, 131, 173.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2sXltVOqtbo%3D&md5=2a298ae7684e7e163a735b3cde4555eeCAS |
[31] L. Elansari, L. Antoine, R. Janot, J. C. Gachon, J. J. Kuntz, D. Guérard, J. Alloys Compd. 2001, 329, L5.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXosFyit7Y%3D&md5=499c5963a28e50cb509b25e8c9850b9bCAS |
[32] P. A. A. Klusener, L. Brandsma, H. D. Verkruijsse, P. R. Schleyer, T. Friedl, R. Pi, Angew. Chem. Int. Ed. Engl. 1986, 25, 465.
| Crossref | GoogleScholarGoogle Scholar |
[33] (a) E. M. Zippi, Synth. Commun. 1994, 24, 2515.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXmsFCqs7k%3D&md5=f4b8c2bccdaeec267734c1efff596484CAS |
(b) R. Pi, T. Friedl, P. R. Schleyer, P. Klusener, L. Brandsma, J. Org. Chem. 1987, 52, 4299.
| Crossref | GoogleScholarGoogle Scholar |
[34] (a) E. C. Ashby, S. A. Noding, J. Org. Chem. 1980, 45, 1041.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3cXht1yqu7k%3D&md5=93dd3ee4d7d1c88ef4fde4d2ed3a4951CAS |
(b) E. C. Ashby, R. D. Schwartz, Inorg. Chem. 1971, 10, 355.
| Crossref | GoogleScholarGoogle Scholar |
[35] C. J. Kowalski, G. S. Lal, J. Am. Chem. Soc. 1986, 108, 5356.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL28Xltleisrk%3D&md5=57e621b9de29a1396dccf9ada20e6a85CAS |
[36] B. Bogdanovic, Angew. Chem. Int. Ed. Engl. 1985, 24, 262.
| Crossref | GoogleScholarGoogle Scholar |
[37] (a) P. R. Schleyer, A. J. Kos, E. Kaufmann, J. Am. Chem. Soc. 1983, 105, 7617.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2cXmsF2m&md5=0ffe4ed835a36b68d3037731ce12eefeCAS |
(b) M.-Y. Li, J. San Filippo, Organometallics 1983, 2, 554.
| Crossref | GoogleScholarGoogle Scholar |
(c) W. H. Glaze, T. L. Brewer, J. Am. Chem. Soc. 1969, 91, 4490.
| Crossref | GoogleScholarGoogle Scholar |
(d) W. H. Glaze, G. M. Adams, J. Am. Chem. Soc. 1966, 88, 4653.
| Crossref | GoogleScholarGoogle Scholar |
(e) R. A. Finnegan, H. W. Kutta, J. Org. Chem. 1965, 30, 4138.
| Crossref | GoogleScholarGoogle Scholar |
[38] Y. Fort, Tetrahedron Lett. 1995, 36, 6051.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXnslWrsLo%3D&md5=997aa08c08c2c09110465f7f21fa86edCAS |
[39] T. Enoki, S. Miyajima, M. Sano, H. Inokuchi, J. Mater. Res. 1990, 5, 435.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXhs1Gls70%3D&md5=b526aebcebf55d8bdd8e1e91b7ed95a3CAS |
[40] X. Wang, L. Andrews, J. Phys. Chem. A 2007, 111, 6008.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXmtVKlurc%3D&md5=656e203efc09f58d18bd2247a8241b94CAS | 17547379PubMed |
[41] X. Wang, L. Andrews, J. Phys. Chem. A 2007, 111, 7098.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXmvFejt7k%3D&md5=18b0c98697f7612fbb89a6360db9167fCAS | 17602543PubMed |
[42] X. Wang, L. Andrews, J. Phys. Chem. A 2007, 111, 12260.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXht1WltLnF&md5=86f7f4394d04ac4077eb305af2d55003CAS | 17958401PubMed |
[43] E. Zurek, R. Hoffmann, N. W. Ashcroft, A. R. Oganov, A. O. Lyakhov, Proc. Natl. Acad. Sci. USA 2009, 106, 17640.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtl2rur7J&md5=d9db1007736852e14fab84bd4fc2c033CAS | 19805046PubMed |
[44] R. Napan, E. L. Peltzer y Blanca, Int. J. Hydrogen Energy 2012, 37, 5784.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xjs1yjsLg%3D&md5=d25ea85d64a3513c8617a284bce5a688CAS |
[45] P. Baettig, E. Zurek, Phys. Rev. Lett. 2011, 106, 237002.
| Crossref | GoogleScholarGoogle Scholar | 21770539PubMed |
[46] V. V. Struzhkin, D.-Y. Kim, E. Stavrou, T. Muramatsu, H.-K. Mao, C. J. Pickard, R. J. Needs, V. B. Prakapenka, A. F. Goncharov, eprint arXiv:1412.1542, 2014. Available at http://arxiv.org/abs/1412.1542.
[47] J. Hooper, E. Zurek, J. Phys. Chem. C 2012, 116, 13322.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XnsFOms7s%3D&md5=416b162e3d99cd3c537c39c95c8e7d92CAS |
[48] J. Hooper, E. Zurek, Chem. – Eur. J. 2012, 18, 5013.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XjtFOgs7c%3D&md5=1536a8c7d1b6c76c17f9a3a8967d2151CAS | 22392860PubMed |
[49] A. Shamp, J. Hooper, E. Zurek, Inorg. Chem. 2012, 51, 9333.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xht1WqurnI&md5=86a6367cd792dbce3b8eab958b34a747CAS | 22897718PubMed |
[50] G. Chalasinski, R. A. Kendall, J. Simons, J. Phys. Chem. 1987, 91, 6151.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2sXmt1entr0%3D&md5=6410312d40179f60ac714c20ba3759daCAS |
[51] S. J. Grabowski, R. Hoffmann, ChemPhysChem 2012, 13, 2286.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XlvVShu7k%3D&md5=c2554a2fbd94e5a213172a22329c598cCAS | 22517729PubMed |
[52] W. Zhang, A. R. Oganov, A. F. Goncharov, Q. Zhu, S. E. Boulfelfel, A. O. Lyakhov, E. Stavrou, M. Somayazulu, V. B. Prakapenka, Z. Konôpková, Science 2013, 342, 1502.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhvFemu77O&md5=05b4e9ce3096e944ba2df3ff85a440cdCAS | 24357316PubMed |
[53] (a) R. L. Mills, J. New Mater. Electrochem. Syst. 2003, 6, 45.
| 1:CAS:528:DC%2BD3sXivVyktrg%3D&md5=c80ea7de6c504478bad80693c2726b17CAS |
(b) R. L. Mills, J. Dong, W. Good, A. Voigt, Int. J. Hydrogen Energy 2001, 26, 1199.
| Crossref | GoogleScholarGoogle Scholar |
(c) R. L. Mills, B. Dhandapani, M. Nansteel, J. He, T. Shannon, A. Echezuria, Int. J. Hydrogen Energy 2001, 26, 339.
| Crossref | GoogleScholarGoogle Scholar |
(d) R. L. Mills, B. Dhandapani, N. Greenig, J. He, Int. J. Hydrogen Energy 2000, 25, 1185.
| Crossref | GoogleScholarGoogle Scholar |
[54] (a) A. S. de Castro, Phys. Lett. A 2007, 369, 380.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtVOrtLbL&md5=61b9b5d21633462188efc594f733d6bfCAS |
(b) N. Dombey, Phys. Lett. A 2006, 360, 62.
| Crossref | GoogleScholarGoogle Scholar |
(c) A. K. Vijh, Int. J. Hydrogen Energy 2001, 26, 281.
| Crossref | GoogleScholarGoogle Scholar |
[55] R. J. Wehmschulte, P. P. Power, Polyhedron 2000, 19, 1649.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXmtF2jtbc%3D&md5=200ab325384f4d2b2096057ab3ccd9fbCAS |
[56] M. Hudlicky, Reductions in Organic Chemistry 1984 (Ellis Horwood Limited: Chichester).
[57] A. F. Richards, A. D. Phillips, M. M. Olmstead, P. P. Power, J. Am. Chem. Soc. 2003, 125, 3204.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXhsVygu7w%3D&md5=2e0fd8302a47566e79a83bbb6803764fCAS | 12630862PubMed |
[58] M. Veith, P. König, A. Rammo, V. Huch, Angew. Chem. Int. Ed. 2005, 44, 5968.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtVKqs7vL&md5=34fd8e498ea4ad451e99d28b124f3259CAS |
[59] (a) P. D. Prince, M. J. Bearpark, G. S. McGrady, J. W. Steed, Dalton Trans. 2008, 271.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsFSisA%3D%3D&md5=93344ec0ddbe39970b39bdd6a012334aCAS | 18097494PubMed |
(b) M. J. Bearpark, G. S. McGrady, P. D. Prince, J. W. Steed, J. Am. Chem. Soc. 2001, 123, 7736.
| Crossref | GoogleScholarGoogle Scholar |
[60] (a) R. Corriu, C. Guhin, B. Henner, Q. Wang, Inorg. Chim. Acta 1992, 198–200, 705.
| Crossref | GoogleScholarGoogle Scholar |
(b) R. J. P. Corriu, C. Gubrin, B. J. L. Henner, Q. Wang, Organometallics 1991, 10, 3574.
| Crossref | GoogleScholarGoogle Scholar |
[61] F. Buch, J. Brettar, S. Harder, Angew. Chem. Int. Ed. 2006, 45, 2741.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XksFWku7Y%3D&md5=33cf712aaed9648617af4b19616d6b42CAS |
[62] (a) M. Itoh, K. Inoue, J.-i. Ishikawa, K. Iwata, J. Organomet. Chem. 2001, 629, 1.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXkvFCiu7o%3D&md5=cdd69ff8d3c37214ebc0d9a33a6f7f14CAS |
(b) B. Becker, R. J. P. Corriu, C. Guerin, B. J. L. Henner, J. Organomet. Chem. 1989, 369, 147.
(c) R. J. P. Corriu, C. Guerin, J. Chem. Soc., Chem. Commun. 1980, 168.
| Crossref | GoogleScholarGoogle Scholar |
[63] C. Appelt, J. C. Slootweg, K. Lammertsma, W. Uhl, Angew. Chem. Int. Ed. 2012, 51, 5911.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XmtFCiurg%3D&md5=670c929a02556b58b94bfbf2294c297cCAS |
[64] (a) S. D. Robertson, A. R. Kennedy, J. J. Liggat, R. E. Mulvey, Chem. Commun. 2015, 5452.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhsFeisr3J&md5=2d5d9a441681bac9155c2e2ebcafeb00CAS |
(b) R. E. Mulvey, L. Dunbar, W. Clegg, L. Horsburgh, Angew. Chem. Int. Ed. Engl. 1996, 35, 753.
| Crossref | GoogleScholarGoogle Scholar |
[65] (a) P. C. Andrikopoulos, D. R. Armstrong, A. R. Kennedy, R. E. Mulvey, C. T. O’Hara, R. B. Rowlings, Eur. J. Inorg. Chem. 2003, 3354.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXotFSkurc%3D&md5=aa2b3fa786b7bb0a0b5e5e1015b76885CAS |
(b) D. J. Gallagher, K. W. Henderson, A. R. Kennedy, C. T. O’Hara, R. E. Mulvey, R. B. Rowlings, Chem. Commun. 2002, 376.
| Crossref | GoogleScholarGoogle Scholar |
[66] D. V. Graham, A. R. Kennedy, R. E. Mulvey, C. T. O’Hara, Acta Crystallogr. C 2006, 62, m366.
| Crossref | GoogleScholarGoogle Scholar | 16891710PubMed |
[67] D. J. Liptrot, M. S. Hill, M. F. Mahon, Chem. – Eur. J. 2014, 20, 9871.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhtFWns73N&md5=c2a53a66416dbb9a95c6bf8d2dd94c64CAS | 25042080PubMed |
[68] G. T. DeLong, D. Hoffmann, H. D. Nguyen, R. D. Thomas, J. Am. Chem. Soc. 1997, 119, 11998.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXnvVCms7w%3D&md5=f0b96f85fb33f68a75bcb4e8ba795fa4CAS |
[69] D. Hoffmann, T. Kottke, R. J. Lagow, R. D. Thomas, Angew. Chem. Int. Ed. 1998, 37, 1537.
| Crossref | GoogleScholarGoogle Scholar |
[70] (a) S. R. Boss, M. P. Coles, V. Eyre-Brook, F. Garcia, R. Haigh, P. B. Hitchcock, M. McPartlin, J. V. Morey, H. Naka, P. R. Raithby, H. A. Sparkes, C. W. Tate, A. E. H. Wheatley, Dalton Trans. 2006, 5574.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXit1Knsg%3D%3D&md5=78d71ec92019a2ee0ce09f2ef7dd3918CAS | 17225894PubMed |
(b) S. R. Boss, J. M. Cole, R. Haigh, R. Snaith, A. E. H. Wheatley, G. J. McIntyre, P. R. Raithby, Organometallics 2004, 23, 4527.
| Crossref | GoogleScholarGoogle Scholar |
(c) S. R. Boss, M. P. Coles, R. Haigh, P. B. Hitchcock, R. Snaith, A. E. H. Wheatley, Angew. Chem. Int. Ed. 2003, 42, 5593.
| Crossref | GoogleScholarGoogle Scholar |
(d) D. R. Armstrong, R. P. Davies, W. Clegg, S. T. Liddle, D. J. Linton, P. Schooler, R. Snaith, A. E. H. Wheatley, Phosphorus Sulfur Silicon Relat. Elem. 2001, 168, 93.
| Crossref | GoogleScholarGoogle Scholar |
(e) D. R. Armstrong, W. Clegg, R. P. Davies, S. T. Liddle, D. J. Linton, P. R. Raithby, R. Snaith, A. E. H. Wheatley, Angew. Chem. Int. Ed. 1999, 38, 3367.
| Crossref | GoogleScholarGoogle Scholar |
[71] J. Haywood, A. E. H. Wheatley, Dalton Trans. 2008, 3378.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXnslKht70%3D&md5=e043e084788cdef76349991dc38cb471CAS | 18580974PubMed |
[72] A. Stasch, Angew. Chem. Int. Ed. 2012, 51, 1930.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xnt1KisA%3D%3D&md5=d9986c79ac1208d4cadd476d75151e30CAS |
[73] A. Stasch, Dalton Trans. 2014, 7078.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXmtV2rsLY%3D&md5=2802ddcae1359e0f63b7a0661f02e534CAS | 24668322PubMed |
[74] K. N. Houk, N. G. Rondan, P. R. Schleyer, E. Kaufmann, T. Clark, J. Am. Chem. Soc. 1985, 107, 2821.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXhs1Krsr4%3D&md5=98951293473eb79ef160913219da21c6CAS |
[75] A. Stasch, Angew. Chem. Int. Ed. 2014, 53, 1338.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhvFart7vK&md5=5349b48f4c92ea5ef4817f4888a998acCAS |
[76] M. J. Michalczyk, Organometallics 1992, 11, 2307.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38Xis1Orurc%3D&md5=3ac63314114d413b45942e8106f6be00CAS |
[77] P. Jochmann, J. P. Davin, T. P. Spaniol, L. Maron, J. Okuda, Angew. Chem. Int. Ed. 2012, 51, 4452.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XksV2htLc%3D&md5=e81f3d89db2dc461594d23119e1131bbCAS |
[78] M. Y. Redko, M. Vlassa, J. E. Jackson, A. W. Misiolek, R. H. Huang, J. L. Dye, J. Am. Chem. Soc. 2002, 124, 5928.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XjtlGjurk%3D&md5=caa8d440cfb4e2ac88492aa6359bb6c2CAS | 12022811PubMed |
[79] A. Stasch, Chem. Commun. 2015, 51, 5056.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXis1yrtr4%3D&md5=50a1a5fc5a4da4d0d0538289940d0db6CAS |
[80] (a) J.-F. Carpentier, Y. Sarazin, Top. Organomet. Chem. 2013, 45, 141.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhslWqsrvE&md5=1560731160a9b54a95ccfb2c3be23388CAS |
(b) T. Tsubogo, Y. Yamashita, S. Kobayashi, Top. Organomet. Chem. 2013, 45, 243.
| Crossref | GoogleScholarGoogle Scholar |
(c) M. R. Crimmin, M. S. Hill, Top. Organomet. Chem. 2013, 45, 191.
| Crossref | GoogleScholarGoogle Scholar |
(d) A. L. Reznichenko, K. C. Hultzsch, Top. Organomet. Chem. 2013, 43, 51.
| Crossref | GoogleScholarGoogle Scholar |
(e) S. Harder, Chem. Rev. 2010, 110, 3852.
| Crossref | GoogleScholarGoogle Scholar |
(f) M. P. Coles, Curr. Org. Chem. 2008, 12, 1220.
| Crossref | GoogleScholarGoogle Scholar |
[81] A. A. Toutov, W.-B. Liu, K. N. Betz, A. Fedorov, B. M. Stoltz, R. H. Grubbs, Nature 2015, 518, 80.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXitFGju7c%3D&md5=d921afcfbd1ff41c4fe289a7e9558e36CAS | 25652999PubMed |
[82] K. P. Huber, G. Herzberg, Molecular Spectra and Molecular Structure: IV. Constants of Diatomic Molecules 1979 (Van Nostrand Reinhold Co.: New York, NY).
* The corresponding author, Andreas Stasch, is the winner of the 2014 RACI Organometallic Chemistry Award.