Robert Vyent Stick: A Colourful Character
Spencer J. Williams AA School of Chemistry and Bio21 Molecular Science and Biotechnology Institute, University of Melbourne, Parkville, Vic. 3010, Australia. Email: sjwill@unimelb.edu.au
Australian Journal of Chemistry 62(6) 503-509 https://doi.org/10.1071/CH09047
Submitted: 22 January 2009 Accepted: 24 February 2009 Published: 10 June 2009
This Special Issue on carbohydrates emanated from the recent symposium held on September 18, 2008, in honour of the retirement of Professor Robert (Bob) Vyent Stick (Fig. 1). This introductory essay provides an overview of Bob’s career and is divided into two parts: first, a summary of Bob’s career and research interests; and second, an interview with Bob that provides insights into his career and the changing nature of research and teaching in universities in Australia.

|
In Australia the name Bob Stick is synonymous with a redhead from the (Wild) West who is an expert in most areas of carbohydrate chemistry. From the earliest days of Bob’s independent career he has maintained a strong focus on synthetic carbohydrate chemistry. In the early years this was largely concerned with synthetic methodology for the manipulation of carbohydrates; later, this work was guided by a desire to develop ever more potent inhibitors of glycoside hydrolases, driven by the use that these inhibitors have in structural studies of such enzymes. These studies resulted in over 140 publications and recognition on the world stage, through serving as president of the International Carbohydrate Organization and release of two major texts on carbohydrates. Bob’s contribution to the field was honoured by the award of Doctor of Science (University of Queensland, 2003). Bob is also a gifted lecturer and undergraduate educator whose entertaining presentation of the subject is complemented by a string of live demonstrations.[1]
Early Years
Bob’s earliest work (with F. Norm Lahey) concerned the synthesis of the chromanoid bioactive natural products acronycine and evodionol.[2] This work led to the total synthesis of these natural products and some analogues, and was published in the Australian Journal of Chemistry, thereby initiating a life-long association and devotion to this journal. Following these studies Bob graduated with the University Medal from the University of Queensland and secured a post-doctoral position with one of the then eminent intellectual forces in the world of carbohydrate chemistry, Professor Raymond Lemieux, at the University of Alberta. Lemieux has been described as the ‘Emil Fischer’ of the latter half of the 20th century, and Bob arrived at one of the most productive points in Lemieux’s career. Bob was set to work on a truly innovative solution to a major problem in carbohydrate chemistry, the synthesis of 1,2-cis α-glycosidic bonds. The method that emerged, the halide ion catalyzed glycosidation, is a simple, general, and quite brilliant solution to the problem, and the insight into glycosylation reactions provided by Lemieux at the time remains as topical as ever to contemporary issues of glycosylation.[3]
With the support of Lemieux, Bob was able to secure a subsequent post-doctoral position with Professor Sir Derek Barton at Imperial College in London. Here, Bob worked on the reactions of cyclic thionocarbonates, and was able to apply some of these reactions to carbohydrates, thereby cementing his interests in the field. It was always an inspiration to be in the laboratory of Sir Derek Barton, who at the time was interested in the ‘invention’ of new reactions, and much previously uncharted chemical land was being surveyed in the Barton Group. Bob also became acquainted with various expatriate Australians (Richard Haynes, Richard Russell, Chris Pincombe, Rob Allen, John Corrie, Keith Watson, and John Turner) and good friends with a few Brits (Anthony Barrett, Dave Widdowson, Willie Motherwell, and Peter Freeman).
Independent Research Career at the University of Western Australia
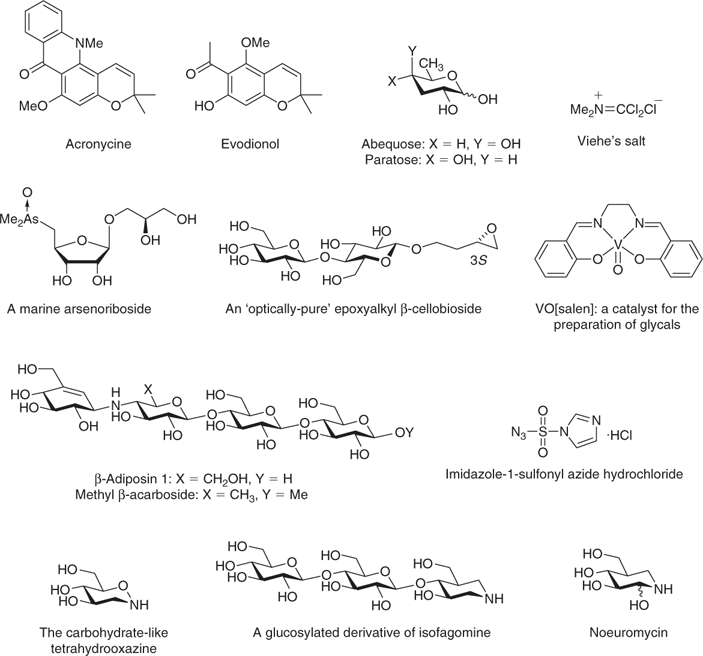
Bob became enamored of various free-radical processes being developed in Barton’s laboratory, and upon securing an appointment at the University of Western Australia (UWA), his first PhD students (Chris Copeland and Joe Patroni; Table 1) were set to work applying free-radical chemistry to the deoxygenation of various carbohydrates. This work involved applying a new reaction developed in the Barton laboratory to carbohydrates, the Barton–McCombie reaction. Carbohydrate alcohols were converted into xanthates, and the stereochemical course of reduction with tributyltin hydride was followed. This work included demonstration of the stereoselectivity of the process (using tributyltin deuteride),[4] and several syntheses of hitherto difficult to prepare sugars (e.g. abequose, paratose) were achieved (Fig. 2).[5]

|
A Few Good Reactions
Inspired by Sir Derek’s lead in the development of new reactions, Bob has maintained a keen interest in developing and/or applying new reagents to carbohydrate chemistry. The earliest of this work concerned the application of Viehe’s salt to carbohydrate chemistry,[6] and has followed with applications of a great number of reagents and/or improved procedures, including benzylidenation reactions using benzaldehyde dimethyl acetal (in DMF and later chloroform),[7] preparation of sugar amino acids using the Corey–Link reaction,[8] discovery of an efficient vanadium catalyst for the preparation of glycals by reductive elimination,[9] the preparation and reactions of various thio-, seleno-, and tellurosugars[10] and, most recently, the development of imidazole-1-sulfonyl azide hydrochloride, a shelf-stable diazotransfer reagent that is a real alternative to triflyl azide.[11]
Marine Arsenosugars
Bob was well placed at UWA to take an interest in the new field of arsenosugars. A few years earlier a group led by Jack Cannon had published the isolation of the first natural (and non-toxic) organoarsenic compound, arsenobetaine (Me3As+CH2CO2–),[12] and the Western Australian Marine Research Laboratories in North Perth were interested in defining the biosynthesis of organoarsenic compounds. Two scientists, John Edmonds and Kevin Francesconi, approached Bob asking for his assistance with some arsenosugars they had isolated, and so a long-running research collaboration was born. The earliest of this work reported the synthesis (R)-2′,3′-dihydroxypropyl 5-deoxy-5-dimethylarsinyl-β-D-riboside[13] and over the course of the next 20 years the structures of several new marine arsenicals were divulged and several other arsenic-containing compounds were to succumb to total synthesis.
Hitting the Sweet Spot
Following a sabbatical with Professor Bertram Fraser-Reid in 1983, Bob developed an interest in the use of carbohydrates as inhibitors of carbohydrate processing enzymes. The earliest of this work was undertaken by Evelyn Rodriguez, and dealt with the synthesis of epoxyalkyl glycosides.[14] The electrophile in the aglycon allows reaction with nucleophilic residues in the active site of glycoside hydrolases; these compounds thus act as time dependent inactivators of these enzymes. This work led to a collaboration and friendship with Professor Bruce Stone, an outstanding Australian biochemist,[15] and the resultant compounds were used to identify key catalytic residues involved in the enzyme-catalyzed reaction.[16] Bruce Stone was also responsible for the invention of an unnatural product, β-acarbose. Acarbose is an extraordinarily potent inhibitor of amylases, enzymes which catalyze the hydrolysis of starch. As starch is a diastereoisomer of cellulose, it was imagined that the ‘all β’ diastereoisomer of acarbose, β-acarbose, would be an inhibitor of the corresponding hydrolases that act on cellulose, cellulases. This synthetic challenge defined the Stick laboratory for almost a decade and culminated in the synthesis of a wide variety of related molecules and the development of a range of innovative methodologies.[17] The interest in glycosidase inhibitors was continued in the synthesis of novel inhibitors including isofagomine[18] and noeuromycin[19] (compounds invented by Mikael Bols), the invention and synthesis of a carbohydrate-like tetrahydrooxazine,[20] and other carba, imino, and aza sugars.
Chemoenzymatic Carbohydrate Chemistry
Any carbohydrate chemist who has been faced with the reality of the difficulties of oligosaccharide synthesis will appreciate the benefits offered by chemoenzymatic approaches to the synthesis of the glycosidic bond. And so it was that an interest in transglycosidation reactions using glycoside hydrolases as synthetic catalysts was born. The earliest work was conducted by Jon Fairweather and concerned the use of cellulases to prepare various epoxyalkyl C-glycosides that were unobtainable by purely chemical approaches,[21] and led to other elegant work glucosylating various inhibitor core fragments. This work was soon to change in a remarkable new direction owing to Bob’s undertaking of an extended 2-year sabbatical in the laboratory of Professor Stephen Withers at the University of British Columbia starting in 1998. At this time Withers had just invented a completely new concept in the enzymatic synthesis of the glycosidic linkage, namely the use of specially engineered mutant glycosidases (glycosynthases) as high yielding glycosidation catalysts.[22] Withers’ invention was and still is one of the most practical and easily applied approaches for the simple synthesis of oligosaccharides, and, in collaboration with Withers, Bob was able to transplant the technology back to Australia. Here it was used for a conceptually brilliant strategy – the glycosynthase is a mutant glycosidase that has lost its ability to degrade glycosides and was put to use to synthesise inhibitors for the corresponding wild-type glycosidases. It was through the application of Withers’ glycosynthase technology that Bob was able to exploit fully the synthetic expertise that had been developed in his group for the synthesis of various monosaccharide glycosidase inhibitors. Thus, the previously mentioned isofagomine and tetrahydrooxazine were elongated to oligosaccharide inhibitors for oligosaccharide-processing enzymes.[23] These compounds were suddenly in great demand and led to the establishment of a very productive collaboration with an X-ray crystallographer and biochemist, Professor Gideon Davies. These compounds were used to define and dissect various facets of inhibitor binding to various enzymes and led to a series of high profile publications and numerous crystallographic determinations of enzyme-inhibitor complexes and definition of some unusual aspects of conformational change that occur during catalysis.[24]
The research conducted by the Stick group has been carried out by a small cohort of 20 graduate students and a handful of postdoctoral research associates. All members of the group will appreciate the outward-looking international vision of the laboratory, maintained through regular attendance at international conferences, and the high esteem in which Bob is held around the world. A major outcome of Bob’s interest in carbohydrates was the publication of a textbook, Carbohydrates: The Sweet Molecules of Life in 2001,[25] which has been described as ‘like a newly discovered dessert – after finishing it, the reader will certainly want more’.[26] The success of this text, which is in use in graduate carbohydrate chemistry courses internationally, has led to Bob’s development of a second edition (with the present author). Carbohydrates: The Essential Molecules of Life has just been published.[27]
An Interview with Bob Stick
SJW: Many scientists have inspiring teachers or key events that occurred when growing up that turned them on to science. What were your high-school years like and can you pinpoint anything that fueled your interest in science?
RVS: I was privileged to attend Brisbane State High School, the only state school in the Greater Public Schools group in Brisbane. There I spent my sub-junior, junior, sub-senior, and senior years (1959–1962) with as bright a bunch of lads (a co-ed but segregated school) as you could find. We spent the 4 years concentrating on math, chemistry, physics, and English, backed up at various stages with geography, German (we spoke very little but spent most of our time translating poetry into English!) and Latin (taught by ‘Brutus’). I struggled with languages, geography was dead boring, and physics never grabbed me. That left chemistry and math. We had a great chemistry teacher in Thos Porter (‘Spike’, after his crew cut) who performed lots of demonstrations for us, and this encouraged me to visit Selby's to procure my own chemicals and to do things at home. The present regulatory bodies would have kittens at the range of chemicals that one could buy ‘over the counter’ in those days.
SJW: What made you choose science, and chemistry in particular, for your tertiary education at UQ?
RVS: I was actually awarded a State Fellowship to do dentistry at the University of Queensland but by then I had the chemistry bug and instead took a similar fellowship with the State Department of Health, signing myself off as a bonded servant for 3 years post-graduation. How a small decision like this can turn one away from a life of luxury, fast cars, fast boats, and fast … First-year science (1963) at UQ required a study of chemistry, math, physics (ugh!), and something else (geology in my case) – what an extraordinarily sensible enrolment for a science student! The math course was a doddle, I was awarded a conceded pass in physics (not surprising in view of the fact that we often played bridge in the back row during lectures), gained a distinction in chemistry (the organic part was my worst mark), and a high distinction in geology. The then Professor of Geology gave me a stern talking to when I informed him that I would not be continuing my study of rocks and fossils in second year, and I was also able to rid myself of physics (thank you, o’ board of examiners, for that CP!). Second-year was more math, biochemistry (what a dark ages subject in those days but Burt Zerner at least appreciated the chemical nature of his subject), and the beloved chemistry. Physical chemistry seemed a necessary distraction and the strong background in math allowed one to walk out of the exam with a 100% mark in pocket; inorganic was very descriptive in the 60s, with only John Broomhead providing any synthetic input; and Maurice Sutherland and Ray Carman gave inspiring lectures on classical organic chemistry. It was all chemistry in third-year, stimulated by the arrival of Bill Kitching from Winstein’s laboratory in the States. However, my bond to the State Government had to be paid so it was off to the toxicology section of the Health laboratory in late 1965.
SJW: What was the BSc(Hons) program like in 1966? Can you recall your first exposure to research? Do you still think that there is a place for Honours in Australia?
RVS: Norm Lahey convinced me to return to UQ to complete Honours with him in organic chemistry; Ron Plowman, Professor of Inorganic Chemistry, was not pleased at this decision as he was sure that I would do better in his field. However, hours of sitting on a Carey spectrophotometer manually recording a spectrum of some complex did not turn me on. The Honours program at UQ then consisted of a lot of course work, with a laboratory project. I excelled at the former, still remembering the obscure paper on colchicine that only I had read and which formed the basis of the question that Professor Lahey set and, totally opposite, the complete bollocks I made of a mechanistic question on the Hoesch reaction, set by Bill Kitching. My laboratory project was diabolical, building a molecular still to separate the constituents of some obscure essential oil. I think that I doubled my knowledge of chemistry in that Honours year; it must surely be retained as a part of the BSc in some shape or form. Then back to bondage with the Queensland Government!
SJW: You did your PhD studies with Professor Lahey on the total synthesis of various chromanoid natural products. How has synthesis and analysis of products changed since this time?
RVS: It was Norm Lahey who again rescued me from the Health laboratory, convincing me to break my bond and accept one of two offered fellowships to do a PhD under his guidance. Norm was an exceptional natural products chemist, blessed with ‘green fingers’, but in his synthetic projects you were very much left to your own devices. Although Norm’s research group was quite small, it was great to be practically assisted by Huss Fazldeen, and to have Malcolm McCamish at hand for synthetic ideas. I shudder at the simplicity of the synthetic projects of those days, where one avoided chiral targets and any associated stereocontrol. We very much relied on IR and UV spectroscopy to characterize products, with NMR spectroscopy (60 MHz) slowly coming into its own. As with many a PhD student, I spent too much time playing bridge but I do fondly remember the long Friday night sessions at the UQ Club with Fazldeen, McCamish, Zerner, and Peter Wells; Burt was infamous for his opening bids of small slam. Peter Wells and I formed a very successful partnership at various State bridge events.
SJW: You moved to Canada to undertake your first postdoctoral stint with Prof Ray Lemieux. Why did you choose his laboratory and why Canada?
RVS: I submitted my Ph.D. in late 1970 and needed to follow my then wife to Edmonton, Alberta, where she had commenced an MSc program in geography. This reminded Norm of the first classical synthesis of sucrose by Ray Lemieux and he suggested I might work with him. So, after bagging carbohydrates during my undergraduate years, I found myself heavily involved in the stereoselective synthesis of some of the blood group antigens.
SJW: What was the environment like in Lemieux’s laboratory at that time?
RVS: Apart from Nagabhushan, Lemieux’s lieutenant, the laboratory was an intellectual desert and so it was not too difficult to shine; the work ethic was also poor. Lemieux was a giant in his field, but his management of people and the ability to publish rapidly when it was necessary probably cost him the ultimate (Nobel) prize; the rigid framework presented by pyranose structures allowed him to observe relationships and effects later claimed by people such as Karplus and Overhauser. However, Lemieux did describe the exo-anomeric effect, a crowning achievement. My departure from Lemieux’s laboratory was followed by the arrival of some excellent people such as Driguez, Koto, Hindsgaul, and Bock. Alan Jones, of Chemistry in Australia notoriety, was the NMR jock at the University of Alberta at the time and I must admit that I got him into a deal of hot water with Lemieux by inviting him (AJ) to one of the many (wild) parties that were held in my shared house. Perhaps this was later understandable when I went to my first Christmas party held at Lemieux’s house – you got one drink on arrival, and that was it!
SJW: After Canada you were fortunate to be offered a place in Sir Derek’s laboratory at Imperial College. After his award of a Nobel prize this must have been an exciting place to work – what influences did Barton and his laboratory have on you in that period?
RVS: I left Edmonton in late 1972, bound for a postdoc with Cookson (a self-funded researcher) in Southampton. At the last minute I received an invitation from Sir Derek Barton to join his laboratory and had little choice but to accept. The atmosphere in the Hofmann was incredible – DHRB still ruled the roost with an iron fist (although I was assured that he had mellowed a lot since his Nobel years) and held weekly group meetings where any weakness was seized upon immediately. The level of intellect and experience in the laboratory was amazing, with about 10 Australian postdocs ruling the roost and generally terrorizing the English PhD students; it was also great to benefit from the likes of Tony Barrett, Stuart McCombie, Phil Magnus, and (later) Steve Ley. The card game was now poker, usually on a Friday night, but a game usually started in the laboratory as soon as the word was spread that the ‘old man’ had left for one of his many consulting trips to the USA (Schering–Plough). I clearly remember being cleaned out by McCombie who held four kings against my full house. On Sunday there was usually a laboratory walk around some part of London, with a stop for lunch and a pint or two – imagine the mirth of the publican when asked to warm up a cold pork pie. Dave Widdowson was one of Barton’s lieutenants and became a close friend of most of the Australians, helping some of them repair their ‘heaps’ in the ‘Greenford garage’. The early 70s were the time of attrition under Ted Heath, with power restrictions being common. I remember Rar (Richard Russell) brushing by Alf, a laboratory technician and solid union man, threatening to knock his block off if he didn’t hand over a confiscated heater/magnetic stirrer.
SJW: What were the most enduring lessons you learnt during your post-doctoral years?
RVS: Always to question a result, to prove to yourself that you had the desired molecule and not a figment of your imagination. DHRB often had two people, in different institutions, working on the same problem; he had obviously been ‘burnt’ on previous occasions by dubious claims. I also learnt to tackle fairly simple research problems, keeping my eyes open for an unusual result; Dieter Wege at UWA followed this mantra for years, with spectacular rewards.
SJW: How did you get your academic appointment at UWA?
RVS: At the end of 1975 most of the Australians in the Hofmann were looking for an academic job back home and so we were all submitting applications to the same institutions. A bolt from the blue informed me that I had the offer of a lectureship in Chemistry at UWA – I immediately accepted! Many years later I learnt that it had come down to a choice between me and John Corrie; my experience with carbohydrates, ironically, won me the day. I was about to join a department rated, then, in the top three in Australia, ably led by Phil Jefferies, and one where just about everyone held an ARGC research grant.
SJW: How did you decide upon your initial research directions at UWA?
RVS: I never really had an interest in total synthesis; it requires enormous resources and skill, the likes of which were possessed by Lou Mander and very few others. I always liked small, simple but interesting projects, those where you could develop or expand upon a new method or suggest a mechanism for the formation of an unexpected product. My bottom line was for an average Honours student to produce a paper at the end of the year.
SJW: What do you enjoy most (and least) about your work?
RVS: In the 70s it was great to be at UWA – teaching loads were realistic, there were plenty of Honours and Ph.D. students, funding was adequate if not overly generous, and most of the staff in Organic Chemistry (there were nine of us!) worked well together. Then came Federal Government changes that saw a move from individual research to group research, the so-called Centres of Excellence. Financial support for individuals dropped away, so much so that we now have people spending 6–8 weeks on a proposal that has barely (~20%) a chance of success. What a waste of time (ask Nobel Laureate Barry Marshall for his opinion of this)! Our universities choose the best candidate for an academic position, putting them through a rigorous selection process, and then the funding bodies leave them penniless, struggling to survive. Why not fund all new appointees automatically for, say, 5 years, then decide on any subsequent funding according to their immediate track record? I have observed several C’s of E in action, highlighted by money-wasting logos, directors, administrative staff, furniture, and acrimonious outcomes. One Centre even asked ‘What do we need to do to get a paper into Nature?’ What twaddle! Much of what is promised is not research, it is development.
SJW: Much of your work has involved collaborations to use compounds prepared in your laboratory in biological studies. Do you have a favourite piece of work that you have been involved in?
RVS: Not really. It was always a great thrill to see a simple idea come to fruition. In my early years at UWA I essentially ran each Ph.D. project; later on, it was a pleasure to pull back and let the people involved take responsibility for their own work. In this way, ideas were generated that I would never have considered. I still did most of the paper writing but even this was relinquished with my last Ph.D. student. The collaborative work was excellent, making small molecule inhibitors of glycoside hydrolases and then, say, glycosylating them using Steve Withers’ glycosynthases; then off to Gideon Davies for the structural biology work. Much of this never attracted funding from the ARC, yet resulted in publications in many high impact factor (ugh!) journals. I loved it when this happened – for years I adequately funded my group research with ‘private’ money, generated from book publishers, lawyers, and industrial concerns.
SJW: You have always been very supportive of internationalizing the education of Ph.D. students in your laboratory, through attendance at international conferences, and strongly supporting them in obtaining postdoctoral positions overseas. Why is an international education so important and does it remain so?
RVS: It is not uncommon these days for a Ph.D. student to attend an overseas conference; what better opportunity to include a visit to a different laboratory? These short stays usually proved very beneficial as long as the work conducted in the overseas laboratory remained a part of the Ph.D. program. I always found it much better (even essential), after synthesizing a molecule of interest at UWA, for the Ph.D. student to take it personally to an overseas laboratory for, say, some biological testing or structural investigation. There was always the added benefit that such a visit often generated an invitation to a postdoctoral position.
SJW: Australian Universities have undergone radical change since the 1970s. How have the administrative burdens and competing non-research pressures on academics at UWA changed over your career?
RVS: The old answer of course is that you spend 50% of your time teaching, 50% doing research, and 50% doing administration. I think that most people survive by doing just two of these tasks well, and letting the other be picked up by more interested people. I never became interested in university politics but others did. However, over the past decade, student expectations (and the specter of on-line delivery) have increased enormously, to the extent that if you are teaching a large class on your own, you have time for little else – the weaker research students tend to flounder under this lack of daily supervision.
SJW: Anyone who has been fortunate to see you teach will appreciate that you have a real passion for lecturing and undergraduate education, which you have expanded upon in a recent article in Chemistry in Australia.[1] Are there any secrets to giving a good lecture? And is good teaching valued sufficiently by Universities?
RVS: I did the majority of my teaching to first-year students who were focussed on a major in the biological sciences. I always tried to challenge the class, training them to use their senses when watching a chemical demonstration, rather than falling back on previous knowledge. A classic example of this was to add a piece of sodium metal to water on an overhead projector and ask what was seen – one answer from a proud lad was ‘Hydrogen!’. It was always very gratifying when a student either switched to chemistry or continued with chemistry as another major. No longer can you hold the interest of first-year students with a functional group approach for organic chemistry; I have used 13C NMR spectroscopy to underpin such lectures for years. Why should we not teach undergraduates in the same manner that we do research? What better impact than to bring a vial of crystals into a lecture, telling the class that this compound was unknown until yesterday? Better still, show the class the 13C NMR spectrum and ask them to deduce something about the structure of the new compound! The whole nature of teaching will change in the next decade as on-line learning supersedes lectures, and students balance their working lives with campus commitments. Fortunately, universities value teaching highly; gone are the days when you were hired on your potential as a researcher, with any innate teaching ability being a bonus.
SJW: Your entire independent career has been undertaken in a remote part of the world, Perth. What are the benefits and disadvantages of working in such a geographically isolated location?
RVS: There are very few, if any, academic benefits to being isolated. It was always my policy to attend the RACI organic division meetings, and the national conventions. These meetings later became secondary to the International Carbohydrate Symposia and Eurocarb meetings. UWA had a generous sabbatical leave program and I used these yearlong stays in the USA, Canada, and Europe to great advantage; long-service leave was another very helpful opportunity for professional development.
SJW: What will be your lasting legacy to Australian science?
RVS: A tough question. Perhaps some would say ‘that lecture’ in Brisbane in the early 90s! On the other hand, I like to think that I have given over 10000 students a taste of (biological) organic chemistry, and sent several gifted Ph.D. students back to academia. There is no doubt that my research group at UWA had an international reputation.
SJW: Throughout your career you have been a stalwart supporter of the Australian Journal of Chemistry, with the bulk of your career output (over 90 papers) residing there. Why do we need an Australian chemistry journal?
RVS: Without one, we have no mouthpiece on the international stage, we would be swamped by some local Asian publication. That said, if the AJC is to exist, it has to be good – a string of great editors, from Bob Schoenfeld to Alison Green, has seen to that. I do not have a lot of time for these young bucks who think that all of their work is so great that it warrants publication in journals with a high impact factor (another disease that we have allowed to prosper). The AJC is better refereed and presented than a lot of journals considered its peer.
SJW: What should be the ultimate goals for scientists working in academic institutions?
RVS: To be scientifically honest and realistic to themselves, to recognize greatness in a student and to foster that gift, to be able also to recognize a weaker student who needs a lot of attention and help to complete a lower postgraduate qualification. I have little respect for the high-flying researcher who makes a lot of noise, brings in a lot of money, does obvious research, and pays little attention to his underlings.
SJW: What would you view as the crowning moments of your 35 years at UWA?
RVS: Three things come to mind. First, obtaining a DSc in 2003 from the University of Queensland meant a lot to me, being the culmination of a life’s work and recognition of the effort put in on my behalf by a band of talented graduate students. Second, chairing the XXIst International Carbohydrate Symposium was an enormous honour for me, and leap of faith in me by Bruce Stone. We made a booking, actually the very first booking, at the still unconstructed Cairns Convention Centre in 1994 when the site was just an oil storage depot. Eight years later, 700 delegates turned up in Cairns for what several described as ‘the best ICS ever’ (will London do as well in 2012?). This Symposium delivered enormous international recognition for Australia. Third, one of my happiest memories at UWA had nothing to do with excellence but the story of a true Aussie (Italian) battler by the name of Lorenzo Peci. Enzo started his chemistry at UWA with a 1-year catch-up course before completing first-year biological chemistry; he then worked his way through second and third-year, leaning towards organic chemistry. Unable to qualify for entry into Honours, Enzo completed a Masters Preliminary with me – to see Enzo’s face after graduation was something that I will never forget.
Prologue: An academic career should not be considered unless you have a passion for research and teaching. Heavy teaching loads can be tolerated only if you enjoy a performance, and the not uncommon consequence of seeing a student’s face light up when something drops into place or a simple experiment is successful. Research must be met head on, with more ‘bad’ news than good (generally) – do simple things and keep your eyes open. Administration should really not be an issue for a young academic as any responsible Head should give such matters to senior staff. So, my advice for success is to teach enthusiastically and well, attract lots of graduate students, and then get into the laboratory with them so that their enthusiasm can be harnessed to produce great research.
[1]
R. V. Stick,
Chem. Aust. 2007, 74, 6.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
| Crossref | GoogleScholarGoogle Scholar |
CAS |
| Crossref | GoogleScholarGoogle Scholar |
CAS |
| Crossref | GoogleScholarGoogle Scholar |
CAS |
| Crossref | GoogleScholarGoogle Scholar |
CAS |
| Crossref | GoogleScholarGoogle Scholar |
CAS |
| Crossref | GoogleScholarGoogle Scholar |
CAS |
| Crossref | GoogleScholarGoogle Scholar |
CAS |
| Crossref | GoogleScholarGoogle Scholar |
CAS |
| Crossref | GoogleScholarGoogle Scholar |
CAS |
| Crossref | GoogleScholarGoogle Scholar |
CAS |
| Crossref | GoogleScholarGoogle Scholar |
CAS |
| Crossref | GoogleScholarGoogle Scholar |
CAS |
| Crossref | GoogleScholarGoogle Scholar |
CAS |
| Crossref | GoogleScholarGoogle Scholar |
CAS |
| Crossref | GoogleScholarGoogle Scholar |
CAS |
| Crossref | GoogleScholarGoogle Scholar |
CAS |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
CAS |
| Crossref | GoogleScholarGoogle Scholar |
CAS |
| Crossref | GoogleScholarGoogle Scholar |
CAS |
| Crossref | GoogleScholarGoogle Scholar |
CAS |
| Crossref | GoogleScholarGoogle Scholar |
CAS |
| Crossref | GoogleScholarGoogle Scholar |
CAS |
| Crossref | GoogleScholarGoogle Scholar |
CAS |
| Crossref | GoogleScholarGoogle Scholar |
CAS |
| Crossref | GoogleScholarGoogle Scholar |
CAS |
| Crossref | GoogleScholarGoogle Scholar |
CAS |
|
CAS |

[27]