The financial implications of investigating false-positive and true-positive mammograms in a national breast cancer screening program
Jason Soon A * , Nehmat Houssami B C , Michelle Clemson D , Darren Lockie D , Rachel Farber B , Alexandra Barratt B , Adam Elshaug A E and Kirsten Howard A
A * , Nehmat Houssami B C , Michelle Clemson D , Darren Lockie D , Rachel Farber B , Alexandra Barratt B , Adam Elshaug A E and Kirsten Howard A
A Menzies Centre for Health Policy and Economics, School of Public Health, Faculty of Medicine and Health, University of Sydney, NSW, Australia.
B School of Public Health, Faculty of Medicine & Health, University of Sydney, NSW, Australia.
C Daffodil Centre, The University of Sydney – joint venture with Cancer Council NSW, NSW, Australia.
D Maroondah BreastScreen, Eastern Health, Vic., Australia.
E Centre for Health Policy, Melbourne School of Population and Global Health and the Melbourne Medical School, University of Melbourne, Vic., Australia.
Australian Health Review 47(2) 159-164 https://doi.org/10.1071/AH22120
Submitted: 13 May 2022 Accepted: 15 November 2022 Published: 8 December 2022
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of AHHA. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Objectives To determine the total annual screening and further-investigation costs of investigating false-positive and true-positive mammograms in the Australian population breast-screening program.
Methods This economic analysis used aggregate-level retrospective cohort data of women screened at a breast-screening clinic. Counts and frequencies of each diagnostic workup-sequence recorded were scaled up to national figures and costed by estimating per-patient costs of procedures using screening clinic cost data. Main outcomes and measures estimated were percentage share of total annual screening and further-investigation costs for the Australian population breast-screening program of investigating false-positive and true-positive mammograms. Secondary outcomes determined were average costs of investigating each false-positive and true-positive mammogram. Sensitivity analyses involved recalculating results excluding subgroups of patients below and above the screening age range of 50–74 years.
Results Of 8235 patients, the median age was 60.35 years with interquartile range of 54.17–67.17 years. A total of 15.4% (ranging from 13.4 to 15.4% under different scenarios) of total annual screening and further-investigation costs were from investigating false-positive mammograms. This exceeded the share of costs from investigating true-positives (13%).
Conclusions We have developed a transparent and non-onerous approach for estimating the costs of false-positive and true-positive mammograms associated with the national breast-screening program. While determining an optimal balance between false-positives and true-positive rates must rely primarily on health outcomes, costs are an important consideration. We recommend that future research adopts and refines similar approaches to facilitate better monitoring of these costs, benchmark against estimates from other screening programs, and support optimal policy development.
Keywords: breast cancer, breast neoplasms, BreastScreen Australia, cancer screening, cost of healthcare, digital breast tomosynthesis, false-positive results, health services, mammography, public health, women’s health.
Introduction
Screening mammography aims to identify women more likely to have breast cancer, and therefore requiring diagnostic testing to exclude or confirm malignancy. Its main benefit is early detection to reduce breast cancer mortality through earlier treatment with the additional benefit of reduced treatment intensity for screen-detected cancers.1,2 However there are also risks associated with screening such as the risk of false-positive mammograms, causing women to be recalled for further investigation. False-positive mammograms may increase anxiety among women with abnormal findings, even after cancer is later ruled out.3–5 Breast screening policies must strike a balance between reducing the number of women recalled who do not have cancer (false-positives) and the risk of missing (true-positive) cancers. While the goal of breast screening is to extend life and lessen morbidity, program costs and cost-effectiveness remain important considerations. Quantitative estimates of the costs associated with each of these risks therefore can help decision-makers better evaluate these trade-offs and maximise the health value of the program.
Evidence suggests that false-positive rates of screening mammography vary considerably between countries. For instance, a study of European breast-screening programs estimated false-positive rates of 5.4% for initial screens and a cumulative false-positive risk of 8–21% after 10 screens6 compared to recent estimates of a false-positive rate of approximately 11%7 and cumulative false-positive risk after 10 screens of almost 50% for US breast-screening.8
While there are studies investigating the costs of false-positive mammography investigations, few have reported total national costs of breast-screening programs or the cost shares of these investigations. One Swedish study9 estimated the variable costs per-person of false-positive mammography investigations at US$664–$973 (as estimated by Chubak et al.10 based on prevailing exchange rates). US estimates range from US$527 per-person in a 2010 study10 to US$852 in a 2015 study by Ong and Mandl,11 who also estimated an annual national cost of false-positive mammograms of US$2.8 billion.11
This paper addresses these gaps in the Australian literature by estimating the respective shares of the total annual costs of screening and further investigation (hereafter referred to as assessment) within Australia’s national breast-screening program from assessment costs for false-positive and true-positive mammograms. These assessment costs arise from additional tests women undergo after being recalled for a positive mammogram, and may include clinical breast examination with palpation, diagnostic mammography, ultrasound, percutaneous needle biopsy and open biopsy.
Methods
Setting and study design
The Australian national government funded breast-screening program, BreastScreen Australia, invites women aged 50–74 for screening mammograms every 2 years, and will also screen women aged 40–49 and 75 and over on request. BreastScreen Australia screens approximately 900 000 women per annum. Victoria is one of eight states/territories of Australia, representing approximately 26% of the Australian total population.12 Aggregate level retrospective cohort de-identified data on clients screened by a BreastScreen Australia screening clinic in Victoria were collected and analysed. This clinic is one of five fixed screening sites located in a metropolitan Reading and Assessment Service. Across the state of Victoria there are eight such services. The catchment area for this service undertook approximately one-sixth of the screenings in Victoria in 2016 while this clinic screened over one-fifth of the clients in this service in that same year.
This facility provides mammogram screening using two view, full-field digital mammography (with digital breast tomosynthesis mammography reserved for assessment). All images are double-read independently by radiologists. Each client is screened and then leaves immediately. Results are provided after her mammogram is reported, within 14 days.13 If the screen is reported as abnormal, the client is ‘recalled to assessment’ (i.e. further investigation). If normal, she is asked to return for her next screen in 2 years’ time (routine recall). Clients recalled to assessment are recorded as either having breast cancer detected, or as routine recall if cancer is not detected.
Services provided through BreastScreen are free to women at the point of service. We only report costs borne by the public health system (i.e. BreastScreen with the exception of diagnostic open biopsies which are assumed to be borne by public hospitals – see Section 1, Supplementary Material). We did not consider out-of-pocket costs from private screening which may account for approximately 26% of total breast-screening in Australia (although this may be an overestimate – see Section 2, Supplementary Material for methodology and caveats).
Population and subgroups
Participants were all clients screened by the service between 1 January 2016 and 31 December 2016 excluding:
Women with a history of breast cancer, so that only those undergoing routine screening were included and those returning for follow-up after previously diagnosed cancer were excluded, similar to the exclusion adopted by Ong and Mandl.11
Clients recalled to assessment but recorded as ‘incomplete assessment’ or ‘early review at assessment’ without record of final results, who comprised only 0.04% of the 2016 dataset.
Data were analysed in two client groups – those screened for the first time in 2016, and those undergoing a second or subsequent screen in 2016. This ensured that the different diagnostic frequency patterns of first and subsequent-screen clients were fully captured rather than averaged out.
False-positives were defined as screening results in clients recalled to assessment who ultimately received a ‘routine recall’ recommendation (subject to exceptions detailed in Section 3, Supplementary Material). True-positives were defined as screening results in clients recalled to assessment ultimately recorded as having breast cancer detected.
Estimating resources and costs
Counts and frequencies of each diagnostic-workup sequence recorded for clients were quantified separately for those undergoing their first screen and those undergoing a subsequent screen.
Per-patient costs of each screening and assessment procedure (and associated administrative, consent and reporting costs) comprising each recorded sequence were estimated. Cost data were provided by the screening clinic which supplied the client data. Full information on component cost estimates is documented in Supplementary Table S2.
These cost estimates were applied to the frequency analysis, producing an estimate of the total annual costs of breast cancer screening and assessment in 2016 undertaken in the screening clinic. Cost components were partitioned into false-positive and true-positive results to estimate false-positive and true-positive assessment costs as shares of total screening and assessment costs. These reported costs are incremental costs of screening/assessment and exclude provision for fixed costs such as overheads. Estimates were reported in 2020 Australian dollars, scaled up and converted accordingly using Reserve Bank of Australia data on the Australian Consumer Price Index. All analysis was undertaken using Microsoft Excel.
This diagnostic-workup sequence frequency data was extrapolated to 2016 national figures for all national BreastScreen Australia clients by calculating the percentage of clients recalled to assessment in each 5 year age-group that underwent each distinct diagnostic workup sequence; and then applying these age-based percentages to the number of national BreastScreen Australia clients recalled to assessment in 2016 by first and subsequent screening rounds in each age-group. The per-patient costs of each screening and assessment procedure were then applied to this age-based frequency data to derive national cost estimates for 2016. This approach adjusts the frequency data to take account of differences between national data and our screening clinic data which might affect overall false-positive rates (and costs) such as the share of screens which are first round screens, the recall to assessment rate and differences in age distribution.
Sensitivity analysis
Results were calculated excluding patients: (i) outside the screening range of 50–74 years of age; (ii) below 50 years of age; and (iii) above 74 years.
Ethics approval
Ethics approval for use of the data in this study was obtained from Eastern Health (reference number LR19/031).
Results
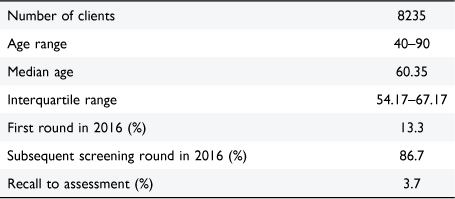
Table 1 reports summary statistics on the study participants. Their median age was 60.35 with an interquartile range of 54.17–67.17 years old. The recall to assessment rate for the sample was 3.7%.
|
Table 2 summarises the results of the diagnostic-workup sequence frequency analysis derived from client records of the screening clinic. The four most frequent diagnostic work-up sequences in descending order were:
Assessment mammography and ultrasound following screening (assessment mammography uses digital breast tomosynthesis)
Assessment mammography only following screening
Assessment mammography, ultrasound, physical examination and core biopsy following screening
Assessment mammography, physical examination and core biopsy following screening.

|
A full listing of all sequences is provided in Supplementary Table S3.
Table 3 presents total costs in 2016 incurred for all diagnostics (including routine screening which did not result in the detection of abnormalities) and resulting cost shares in the screening clinic that we used as the basis for national projections. 13% of total screening and assessment costs were incurred from assessment of false-positive mammograms, more than the share of costs of investigating true-positive mammograms (10%).

|
Table 4 presents total annual costs in 2016 incurred for all national BreastScreen Australia-related diagnostics (including routine screening which did not result in the detection of abnormalities) based on the extrapolated and age adjusted frequency data.

|
Annual screening and assessment costs totalled $73.88 million with 15.4% due to assessments caused by false-positive mammograms, more than the share of costs of investigating true-positive mammograms (13%). The average assessment cost for each false-positive mammogram was $277.80 compared to $861.99 for each true-positive mammogram.
As would be expected, excluding patients outside the invited screening age range resulted in a lower false positive rate of 2.9% and lower cost share of false-positive mammogram investigations of 13.4% as did excluding only those below the screening range (Table 5). Results were broadly unchanged if only patients above the screening range were excluded (e.g. cost share of 15.3% for false-positive investigations).

|
Discussion
This is the first study to our knowledge to estimate total annual screening and assessment costs of a national breast-screening programme for women aged ≥40 years, partitioned into the costs of investigating false-positive and true-positive mammograms and their respective shares. Total costs of investigating false-positive mammograms are approximately $11.37 million or 15.4% of the annual screening and assessment costs of $73.88 million compared to a share of 13% for investigating true-positive mammograms. Our study is the first to base cost estimates on detailed analysis of complete annual data on diagnostic frequency patterns of women, combined with cost estimates from the same screening clinic and extrapolated nationally. This approach may be widely applicable.
The average investigation cost per false-positive mammogram was $277.80 (US$190.65), much lower than estimates from the US and Sweden which (converted to 2020 USD) range from US$623 to US$1600.9–11 The estimated national false-positive rate of 3.8% is much lower than the 11% estimated for the US.7
Saxby et al. (2020)14 using Australian data reported an average investigation cost of $196 per false-positive mammogram and $676 per true-positive mammogram (in 2020 AUD). They did not report cost shares or total screening program costs. Our estimates include diagnostic open biopsy costs, and when these are removed to facilitate comparability the differences between our estimates are small ($198.70 per false-positive diagnosis and $787 per true-positive diagnosis compared to $196 and $676 respectively for Saxby et al.14).
Based on 2000–14 data, Australia’s false-positive mammography rate is 3.3% for ages 50–69 and 4.8% for ages 40–49,15 which is not directly comparable with our 3.8% estimate though our estimate nonetheless lies within the range of these two figures.
Strengths and weaknesses
While there have been numerous studies on the psychological impact of false-positive mammograms, the healthcare costs of investigating false-positive mammograms is understudied. Our study used a novel and transparent approach which may be widely applicable.
The economy of using data from one screening clinic to construct a frequency analysis of screening and diagnosis tests forming the basis for cost-modelling is a strength. A corresponding weakness is the reliance on data from one clinic. However, while it is true that policies and practices of screening clinics through Australia vary and they work to different service delivery and budgetary models, all clinics operating as part of the national breast-screening program follow standardised practice and policies, and undergo nationally-aligned accreditation and quality assurance processes.
While data from multiple screening clinics could produce a more representative frequency analysis, a cross-check of our estimates against estimates from other Australian studies did not find major disparities in terms of average diagnosis costs or false-positive rates, increasing our confidence in our estimates, including that approximately 15% of diagnostic costs are from false-positive screens. Our sensitivity analysis indicates that excluding patients outside screening age reduces this slightly, as would be expected, but the range of variation produced from our sensitivity analysis is narrow (13.4–15.4%).
While not a weakness per se, the scope of our study excludes private assessment costs (including the costs of false-positive investigations) outside the national screening program which may account for 26% of total screening (see caveats to this estimate in Section 2, Supplementary Material).
Implications for practice and policy
A key contribution of this study is the transparent and non-onerous (in terms of data requirements) approach described to estimate the costs associated with false-positive and true-positive screens. We encourage future researchers to adopt and refine similar approaches using whatever screening clinic data is available so that more national estimates of false-positive and true-positive costs can be developed and compared. We hope that such estimates can facilitate better informed decision-making about the costs and benefits of screening.
However, in undertaking any benchmarking using these methods, we caution against the implication that if false-positive costs associated with one screening program are high relative to screening in other jurisdictions that this necessarily means that such costs can or should be further reduced. The approach that we have outlined suggests that the costs associated with a given false-positive recall rate will depend on the relative costs and frequencies of the different kinds of diagnostic-workup sequences to investigate these false-positive screens so there may be multidimensional means of managing these costs (for instance through refining diagnostic algorithms). Trying to reduce false-positive costs simply by reducing false-positive risks may have second-order effects by increasing false-negative risks, reducing the cancer-detection rate, resulting in delayed diagnosis and treatment of cancers that were missed. Importantly, we quantify costs of false-positive and true-positive screens in the context of a national program with ongoing monitoring of these outcomes which recognises that ‘an effective breast cancer screening program will limit any unnecessary investigations by minimising the proportion of women recalled for further assessment without affecting the achievement of high breast cancer detection rates’.13
Conclusion
To our knowledge we have produced the first estimates of the share of annual national screening and assessment costs of Australia’s national breast-cancer screening from assessment of false-positive mammograms. Our 15.4% estimate is higher than that of the share of assessment costs from true-positive mammograms (13%). Excluding patients outside screening age range did not significantly change these results.
We believe that these estimates and the transparent and non-onerous methodology underlying them may be of interest to future researchers who wish to better monitor these costs and benchmark against estimates from other screening programs (whether for breast or other forms of cancer).
Future studies should extend the analysis to cover patients after they have exited screening and assessment to include treatment following positive diagnosis (including estimates of overdiagnosis costs) as well as to cover patients through the full screening, assessment, and treatment journey outside the national screening program.
Supplementary material
Supplementary material is available online.
Data availability
The data that support this study were obtained from BreastScreen Maroondah by permission/licence. Data will be shared upon reasonable request to the corresponding author with permission from BreastScreen Maroondah.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
Funding for this research was provided by National Health and Medical Research Council (NHMRC)/Centres of Research Excellence GNT1104136.
Acknowledgements
We gratefully acknowledge the contribution of Karinna Saxby and Dennis Petrie who shared the costing assumptions used in their paper Saxby et al.14
References
[1] Roder D, Houssami N, Farshid G, et al. Population screening and intensity of screening are associated with reduced breast cancer mortality: evidence of efficacy of mammography screening in Australia. Breast Cancer Res Treat 2008; 108 409–416.| Population screening and intensity of screening are associated with reduced breast cancer mortality: evidence of efficacy of mammography screening in Australia.Crossref | GoogleScholarGoogle Scholar |
[2] Department of Health and Ageing. BreastScreen Australia Evaluation. Screening monograph no. 4/2009. Canberra: Department of Health and Ageing; 2009.
[3] Lerman C, Track B, Rimer BK, Boyce A, Jepson C, Engstrom PF. Psychological and behavioral implications of abnormal mammograms. Ann Intern Med 1991; 114 657–661.
| Psychological and behavioral implications of abnormal mammograms.Crossref | GoogleScholarGoogle Scholar |
[4] Gram I, Lund E, Slenker S. Quality of life following a false positive mammogram. Br J Cancer 1990; 62 1018–1022.
| Quality of life following a false positive mammogram.Crossref | GoogleScholarGoogle Scholar |
[5] Swanson V, McIntosh IB, Power KG, Dobson H. The psychological effects of breast screening in terms of patients’ perceived health anxieties. Br J Clin Pract 1996; 50 129–135.
[6] Hofvind S, Ponti A, Patnick J, et al. False-positive results in mammographic screening for breast cancer in Europe: a literature review and survey of service screening programmes. J Med Screen 2012; 19 57–66.
| False-positive results in mammographic screening for breast cancer in Europe: a literature review and survey of service screening programmes.Crossref | GoogleScholarGoogle Scholar |
[7] Lehman CD, Arao RF, Sprague BL, et al. National Performance Benchmarks for Modern Screening Digital Mammography: Update from the Breast Cancer Surveillance Consortium. Radiology 2017; 283 49–58.
| National Performance Benchmarks for Modern Screening Digital Mammography: Update from the Breast Cancer Surveillance Consortium.Crossref | GoogleScholarGoogle Scholar |
[8] Ho TH, Bissell MCS, Kerlikowske K, et al. Cumulative Probability of False-Positive Results After 10 Years of Screening With Digital Breast Tomosynthesis vs Digital Mammography. JAMA Netw Open 2022; 5 e222440
| Cumulative Probability of False-Positive Results After 10 Years of Screening With Digital Breast Tomosynthesis vs Digital Mammography.Crossref | GoogleScholarGoogle Scholar |
[9] Lidbrink E, Elfving J, Frisell J, Jonsson E. Neglected aspects of false positive findings of mammography in breast cancer screening: analysis of false positive cases from the Stockholm trial. BMJ 1996; 312 273–276.
| Neglected aspects of false positive findings of mammography in breast cancer screening: analysis of false positive cases from the Stockholm trial.Crossref | GoogleScholarGoogle Scholar |
[10] Chubak J, Boudreau DM, Fishman PA, Elmore JG. Cost of breast-related care in the year following false positive screening mammograms. Med Care 2010; 48 815–820.
| Cost of breast-related care in the year following false positive screening mammograms.Crossref | GoogleScholarGoogle Scholar |
[11] Ong MS, Mandl KD. National expenditure for false-positive mammograms and breast cancer overdiagnoses estimated at $4 billion a year. Health Aff 2015; 34 576–583.
| National expenditure for false-positive mammograms and breast cancer overdiagnoses estimated at $4 billion a year.Crossref | GoogleScholarGoogle Scholar |
[12] Australian Bureau of Statistics. 3101.0 National, state and territory population, September 2020, Table 4. 2022. Available at https://www.abs.gov.au/statistics/people/population/national-state-and-territory-population/latest-release#data-download [accessed 11 May 2021].
[13] BreastScreen Australia. National Accreditation Standards, Information for clinical staff. 2019. https://www.health.gov.au/sites/default/files/documents/2019/09/breastscreen-australia-national-accreditation-standards-nas-breastscreen-australia-national-accreditation-standards.pdf [accessed 14 June 2021].
[14] Saxby K, Nickson C, Mann GB, et al. The financial impact of a breast cancer detected within and outside of screening: lessons from the Australian Lifepool cohort. Aust N Z J Public Health 2020; 44 219–226.
| The financial impact of a breast cancer detected within and outside of screening: lessons from the Australian Lifepool cohort.Crossref | GoogleScholarGoogle Scholar |
[15] Australian Institute of Health and Welfare. Analysis of breast cancer outcomes and screening behaviour for BreastScreen Australia. Cancer series no. 113. Cat. no. CAN 118. Canberra: AIHW; 2018.


