Inappropriate antibiotic prescribing: understanding clinicians’ perceptions to enable changes in prescribing practices
Mah Laka A , Adriana Milazzo A and Tracy Merlin A B C
A B C
A School of Public Health, University of Adelaide, Adelaide, SA, Australia. Email: mah.laka@adelaide.edu.au; adriana.milazzo@adelaide.edu.au
B Adelaide Health Technology Assessment (AHTA), School of Public Health, University of Adelaide, Adelaide, SA, Australia.
C Corresponding author. Email: tracy.merlin@adelaide.edu.au
Australian Health Review 46(1) 21-27 https://doi.org/10.1071/AH21197
Submitted: 9 June 2021 Accepted: 6 July 2021 Published: 5 October 2021
Journal Compilation © CSIRO 2022 Open Access CC BY-NC-ND
Abstract
Objective The aim of this study was to identify perceived barriers to appropriate antibiotic prescribing across different healthcare settings.
Methods A cross-sectional survey of clinicians in Australian hospitals and primary care was undertaken between June and October 2019. The perceived barriers to appropriate antibiotic prescribing were considered as dependent variables, whereas age, sex, clinical experience, healthcare setting and the use of guidelines were considered independent variables. We used multivariate logistic regression to identify factors predictive of inappropriate antibiotic prescribing. Content analysis of free-text responses provided additional insights into the impediments to appropriate prescribing.
Results In all, 180 clinicians completed the survey. Overall, diagnostic uncertainty and limited access to guidelines and prescribing information were significant barriers to appropriate antibiotic prescribing. Factors associated with these barriers were clinical experience, care setting (hospitals vs primary care) and the use of guidelines. Experienced clinicians (>11 years) were less likely to consider that limited access to information negatively affected prescribing practices (experience 11–20 years, odds ratio (OR) 0.66, 95% confidence interval (CI) 0.31–0.84; experience >20 years, OR 0.51, 95% CI 0.24–0.91). Conversely, general practitioners considered diagnostic uncertainty (OR 1.31, 95% CI 1.09–1.63) and patient expectations (OR 1.41, 95% CI 1.12–1.84) were more likely to be perceived barriers to appropriate prescribing. The use of guidelines and clinical experience may counteract this.
Conclusion Years of experience, use of guidelines and type of setting were predictors of clinicians’ perceptions regarding antibiotic prescribing. Our data highlight the importance of individual and setting characteristics in understanding variations in prescribing practices and designing targeted interventions for appropriate antibiotic prescribing.
What is known about the topic? Inappropriate antibiotic prescribing is a significant health issue in Australia. Drivers of inappropriate prescribing are known, but how individual and setting characteristics contribute to variations in prescribing behaviour has not been fully understood.
What does this paper add? Diagnostic uncertainty and limited access to prescribing information, including guidelines, formulary restrictions and antibiotic resistance patterns, can limit appropriate antibiotic prescribing. Clinicians’ years of experience, the healthcare settings and clinician use of guidelines are important predictors of antibiotic prescribing behaviour.
What are the implications for practitioners? The findings of this study can inform the design of tailored interventions to promote rational antibiotic prescribing practices in general practice and hospital settings.
Introduction
Antibiotic resistance has emerged as a significant public health issue leading to an increasing health and economic burden. Many studies have attributed antibiotic resistance to excessive and inappropriate antibiotic use.1,2 Worldwide, antibiotic consumption increased by 65% from 2000 to 2015, with inappropriate antibiotic use established as one of the main factors contributing to this.3
In Australia, antibiotic consumption is high compared with other high-income countries.4 It is estimated that 22 million antibiotics are prescribed yearly, equating to one antibiotic per person each year.5 Overall, 46% of the Australian population in 2014 was dispensed antibiotics, of which it is estimated half were unnecessary.5 Most patients over 18 years of age who were seen in primary health care in Australia were prescribed antibiotics for conditions for which antibiotics are not generally recommended by prescription guidelines. This included 92.4% of patients with acute bronchitis, 92.9% patients with pneumonia, 90.2% patients with sinusitis and 62.3% patients with acute upper respiratory tract infections (URTIs).5
Antimicrobial stewardship (AMS) initiatives have been introduced to improve prescribing behaviour by increasing access to evidence-based antibiotic prescribing guidelines. However, adherence to these guidelines remains problematic. Studies have highlighted limited compliance, ranging from 30% to 70%.6,7 Behavioural and contextual determinants may influence the prescribing behaviour of healthcare practitioners; hence, it is important to consider these factors when designing interventions to promote rational antibiotic prescribing. This includes identifying the opportunities and challenges at an individual or organisational level for promoting sustainable AMS.
Previous studies have reported that several factors influence inappropriate antibiotic prescribing,1,4,8,9 but there is little information on how different individual- and setting-specific characteristics contribute to variability in prescribing patterns. The aims of this study were to identify perceived barriers to appropriate antibiotic prescribing and to assess the impact of individual and setting characteristics on clinicians’ perceptions around antibiotic prescribing. This knowledge may inform the design of stewardship interventions that encourage greater levels of compliance with appropriate prescribing.
Methods
Study design
An online survey of primary care and hospital clinicians was conducted across Australia. The questionnaire was developed following a detailed review of existing literature8,10–13 (provided as Supplementary material S1), and its design has been described elsewhere.14 In the survey preamble, inappropriate antibiotic prescribing was defined as when antibiotics are not required, are prescribed for a non-optimal duration and dose, or when the wrong type of antibiotic has been selected. The present study specifically examined factors related to inappropriate antibiotic prescribing, whereas the broader study14 was concerned with barriers and facilitators for computer decision support system adoption for antibiotic management. The survey question concerning barriers to appropriate antibiotic prescribing was presented in a multiple-response format; however, if participants did not agree with the provided options, an ‘Others (please specify)’ option was provided with a free-text box to write additional comments. Prior to its release, the survey was piloted on 10 clinicians, amended and then distributed.
This study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Human Research Ethics Committee of the University of Adelaide (Approval no. H-2019-094). Informed consent was obtained from all subjects involved in the study.
Study participants
On behalf of the study investigators, information about the study and the survey link were distributed to clinicians by the Royal Australasian College of Physicians, Royal Australasian College of General Practitioners and Royal Australasian College of Surgeons and local health networks across Australia through their newsletters, websites and social media accounts. The National Health Workforce Data Set on medical practitioners (2015–18) (https://hwd.health.gov.au/resources/data/summary-mdcl.html) was used to establish the sampling framework. We estimated a sample size of 350 clinicians from hospitals and primary health care based on a 5% margin of error, 95% confidence interval (CI) and power of 0.80.
Data collection
The survey questionnaire included a section on demographic characteristics (sex, age, clinical experience (years) and practice in hospital or primary care), as well as questions about antibiotic prescribing, including the availability and frequency of use of antibiotic prescribing guidelines and perceived barriers to appropriate antibiotic prescribing. The online survey was administered using SurveyMonkey.
Data analysis
Quantitative analysis
Initially, data were analysed descriptively to identify the characteristics of the survey participants and the perceived barriers to appropriate antibiotic prescribing. Because it was not mandatory to respond to all questions, the total number of participants varied depending on the number of responses for that question. For the multiple-response question, a separate dichotomous variable was created for each valid response. Each variable was assigned two possible values (1 if the response was selected; 0 if not selected). We considered demographic characteristics and the use of guidelines as independent variables, whereas perceived barriers to appropriate prescribing were analysed as the dependent variable. We used multivariate logistic regression to estimate associations between the dependent and independent variables. Results are reported as odds ratios (OR) with 95% CIs relative to a reference category. Data were analysed using Stata15 (StataCorp, College Station, TX, USA).
Content analysis
The questionnaire allowed for one free-text comment box for respondents to include additional information on their perceptions of barriers to appropriate antibiotic prescribing. These comments were analysed for contextual content in NVivo12 (QSR International, Melbourne, Vic., Australia). Most responses were brief; thus, manifest content analysis was an appropriate approach to understand the context of the data.15 The manifest content analysis aided interpretation through examination of the obvious elements in the data. Open coding identified preliminary categories that were then organised into relevant first- and second-order codes through multiple iterations. Themes were compared with results from the quantitative analysis to triangulate the data for an in-depth understanding of responses.
Ethics approval
This study was approved by the University of Adelaide Human Research Ethics Committee (Approval no. H-2019–094). Participation was voluntary and the data collected were non-identifiable. To offset expected low participation rates from clinicians, the respondents were provided the opportunity to take part in a draw to win either an iPad or have an equivalent value donation made in their name to a hospital research foundation.
Results
Respondent characteristics
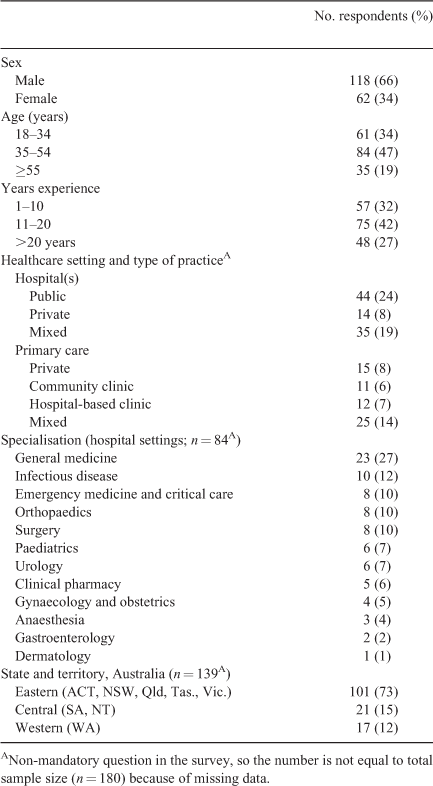
In all, 180 clinicians completed the survey; of these, 74 (41%) were from primary health care and 106 (59%) were from hospitals. Participants’ demographic characteristics are presented in Table 1.
|
Use of guidelines and antibiotic prescribing
Most respondents (68%) used specific guidelines when prescribing antibiotics. Clinicians in hospitals used guidelines more frequently than those in primary care (48% using guidelines daily vs 33%, respectively). Of respondents who used specific guidelines for antibiotic prescribing, 78% reported using national guidelines (Therapeutic Guidelines: Antibiotics; https://tgldcdp.tg.org.au/guideLine?guidelinePage=Antibiotic&frompage=etgcomplete), whereas 22% used local or intranet guidelines.
As indicated in Fig. 1, most respondents (75%) stated that delays in receiving diagnostic tests or cultures contributed to inappropriate antibiotic prescribing, particularly in primary care (OR 1.31, 95% CI 1.09–1.63). Similarly, participants who use guidelines less frequently (monthly or longer intervals) were 70% less likely than regular guidelines users to believe that delays in diagnostic tests can negatively affect their prescribing behaviour (Fig. 2).
More than half the respondents believed that their knowledge and perceptions (55%), and the absence of antibiotic prescribing guidelines (53%) limit clinicians’ ability to appropriately prescribe antibiotics (Fig. 1). General practitioners (GPs) were more likely to perceive that the lack of guidance was a barrier to appropriate antibiotic prescribing (OR 1.24, 95% CI 1.05–1.64). Participants who used guidelines for antibiotic prescribing were nearly one-third more likely to believe that access to guidelines can improve prescribing practices (OR 1.31, 95% CI 1.1–1.73). This was consistent with the observation that clinicians accessing guidelines less frequently (monthly), compared with daily, were more likely to believe that their personal perceptions can be a contributing factor to inappropriate prescribing (OR 1.24, 95% CI 1.04–1.77). Conversely, clinicians with more than 20 years clinical experience were 34% less likely to believe that they require guidance on appropriate antibiotic prescribing (OR 0.66, 95% CI 0.45–0.94). Presumably this was because they felt more comfortable relying on their own experience. Similarly, older clinicians were less likely to believe that their knowledge and perceptions contribute to inappropriate prescribing (age 35–54 years, OR 0.44, 95% CI 0.16–0.80; age >55 years, OR 0.27, 95% CI 0.09–0.42; Fig. 2).
The impact of patient expectations on inappropriate prescribing was considered important by 42% of respondents. Those working in primary care were 41% more likely than those in hospitals to report that patient expectations influence inappropriate antibiotic prescribing (OR 1.41, 95% CI 1.12–1.84). Clinicians with longer clinical experience (11–20 years, OR 0.54, 95% CI 0.21–0.73; >20 years, OR 0.39, 95% CI 0.19–0.56) and those using guidelines for prescribing antibiotics (OR 0.68, 95% CI 0.45–0.97) were less likely to be influenced by this pressure.
Content analysis
Free-text comments on perceived barriers to appropriate antibiotic prescribing were reported from primary care (n = 11) and hospital (n = 16) clinicians. Major themes included limited diagnostic certainty, interprofessional practices and adaptability of guidelines. Some of these themes provided elaboration on data measures included in the quantitative analysis, whereas others, such as interprofessional practices, emerged as an additional theme, as highlighted through triangulation of the data.
Limited diagnostic certainty
Respondents indicated that to avoid missing potential infection, antibiotics are sometimes prescribed as a precautionary measure:
At times, it is difficult to identify the exact source of infection for whatever reason, but as a professional you know the symptoms are definitely there, then surely, antibiotics are given more as a safety blanket. (P05, primary care)
Antibiotics are prescribed even when there is ‘uncertainty about infection being bacterial or viral’ (P117, primary care). Participants identified that the main driver of antibiotic prescribing decisions is to reduce the potential risk of any future complication.
Interprofessional practices
Different interprofessional factors, such as clinical hierarchy and collaboration between different departments, are important issues influencing antibiotic prescribing:
…in-patient teams that escalate to broad-spectrum antibiotics for any patient that is remotely unwell. This does have a trickle-down effect on [emergency department] doctors’ prescribing patterns. (P77, hospital)
Junior clinicians indicated that there is a significant effect of clinical hierarchy on prescribing patterns, because they expressed reluctance to challenge prescriptions made by their seniors even when they felt antibiotics were not required.
Adaptability of guidelines
Clinicians expressed scepticism regarding the utility of guidelines because of their poor specificity and adaptability for context-specific decision making. Lack of trust in guidelines was a perceived barrier because participants believed that guidelines do not capture the complexity of the clinical environment:
Guidelines not covering the context of the particular patient and their problems e.g. poorly controlled diabetes or immune suppression and major surgery, where a prolonged course of antibiotics is prescribed. (P09, hospital)
Discussion
This study provides insight into the behavioural drivers of inappropriate antibiotic prescribing and the impact of individual- and setting-specific characteristics on prescribing behaviour. Antibiotic prescribing practices are dependent on individuals’ perceptions and knowledge, but are also influenced by the type of care setting, clinical experience, availability of data for decision making and patient expectations. We identified several differences and similarities between clinicians regarding factors that influence antibiotic prescribing practices. Previous studies have investigated different drivers of inappropriate prescribing,1,8,9 but there was not enough information on how different individual and setting specific characteristics influence the prescribing behaviour. Our findings have significant implications for understanding variations in antibiotic prescribing behaviour. We believe our results can help guide the design of appropriate AMS interventions and help identify who should be targeted by these interventions.
We found that type of care setting, the use of guidelines and clinical experience are important predictors of self-described antibiotic prescribing behaviour. Primary care clinicians were more likely to perceive factors such as delays in diagnostic test or culture results, lack of antibiotic prescribing guidelines and patient expectations as perceived barriers to appropriate antibiotic prescribing. Many factors may contribute to diagnostic ambiguity, including the overlap between clinical features of different viral and bacterial infections, and the time constraints in a clinical consult to carry out a detailed assessment.12,16 Diagnostic uncertainty has been consistently identified by other studies conducted in primary care settings as a barrier to appropriate antibiotic use.17,18 In primary care, limited follow-up of patients and limited time to assess patients presenting with comorbid conditions may affect clinicians’ capacity to identify the most likely pathogen, thus contributing to unnecessary antibiotic prescribing.13
Primary care clinicians were also more likely to perceive that lack of timely access to prescription guidelines can negatively affect the appropriateness of antibiotic prescribing. This suggests that primary care clinicians may consider these guidelines to be beneficial for managing patients. Clinicians who accessed guidelines frequently agreed that easy access to guidelines at the point of care is important for reducing the risk of inappropriate prescribing. In the context of current clinical practice in Australia, in primary care approximately 33–73% of prescriptions assessed did not comply with antibiotic prescribing guidelines, compared with 23% in hospitals.19
In primary care, clinicians may be more inclined to prescribe antibiotics because of patients’ expectations, which, in many cases, may differ from guidelines.20 It has been reported that explicit requests for antibiotics were made in only 1% of total visits, but in 34% of cases clinicians perceived that patients expected to be prescribed antibiotics.21 Our findings are confirmed by many studies conducted in primary care that frequently report ‘demand’ and ‘expectations’ as drivers of prescriptions, with 10–30% of patients expecting antibiotics for acute respiratory infections, a common presentation.22,23
Our results further indicate that the level of clinical experience and the use of guidelines counteract the effect of patient expectations on prescribing practices. Given the uncertainty integral to antibiotic prescribing, research has suggested that less experienced clinicians may passively comply with patient demands due to fear of criticism, to garner the approval of patients and to manage their own reputation.24 Conversely, experienced clinicians in our study were less likely to be concerned about the absence of guidelines and lack of information, such as formulary restrictions and local resistance patterns, during clinical decision making; they tended to trust their own clinical reasoning and judgement when their opinion differed from the information presented in guidelines. A study conducted by Charani et al.9 found that decision making autonomy in a healthcare setting is directly related to the experience and knowledge of clinicians, with senior clinicians considering themselves exempted from following guidelines or policy. Conversely, less experienced clinicians, specifically those in training, are more likely to follow guidelines to ensure standard practice and avoid the risk of malpractice.9 Our findings also suggest that frequent consultation of guidelines can help reduce the negative effect of patient pressure on prescribing decisions, although this is less likely to be needed by experienced clinicians. Most experienced clinicians in our study engaged with guidelines less frequently than their younger or less experienced counterparts. This is consistent with other studies conducted in other countries showing that clinical experience affects clinicians’ adoption and adherence to clinical guidelines.25,26 AMS interventions targeted at more experienced clinicians to enable them to engage with guidelines would not only affect their own prescribing behaviour, but also potentially that of their junior colleagues.
Due to the complexity of the clinical environment, interventions for rational antibiotic prescribing need to consider the requirements of specific settings (primary care or hospitals), with an explicit focus on interprofessional networks and prescribing practices to ensure there is a cultural shift across all individuals involved in prescribing decisions. This study contributes to the field by evaluating different predictors that may help explain variability in appropriate antibiotic prescribing in Australia. Consideration of these individual- and setting-specific factors that determine prescribing behaviour is vital for designing targeted interventions to promote appropriate antibiotic prescribing. Table 2 outlines the key findings in this study and provides recommendations to address the barriers identified.

|
One of the limitations of this study was that we did not achieve the target sample size, although this may have been an ambitious target. We did achieve participation from 180 clinicians, but this may not be sufficient to generalise the findings to all Australian clinicians. We were unable to determine the true survey response rate because the denominator, or number from the targeted population who viewed the survey notices published across different platforms, could not be identified. It is recognised that the response rate among physicians and GPs is comparatively lower than among the general public.27 For example, an Australian longitudinal survey reported a response rate of 17.6% GPs and 22.3% specialists.28 The findings in the present study are also based on respondents’ perceptions and opinions, and these may not reflect their actual clinical practice. To mitigate this issue, we allowed open-ended responses regarding potential perceived barriers for appropriate antibiotic prescribing. The free-text responses were brief and provided limited contextual data. However, triangulation of quantitative and qualitative data established a better understanding of participants’ perceptions concerning the different barriers.
Conclusion
Our results provide a robust assessment of the range of factors associated with inappropriate antibiotic prescribing in Australia. The comparison of perceptions by different clinicians indicates that variation in antibiotic prescribing patterns can be attributed, in part, to clinical experience, care setting dynamics in hospitals and primary care and the disparate use of guidelines. Efforts should be directed at improving accessibility to information through evidence-based guidelines, understanding clinical culture and actively engaging clinicians across different age groups, as well as explicitly identifying strategies to address clinician concerns about patient expectations for antibiotic prescribing. AMS strategies should be tailored to specific users’ requirements and the nature of the setting in which these are implemented to ensure compliance with appropriate prescribing. These strategies can provide limited benefits if these contextual factors remain unacknowledged.
Competing interests
The authors declare no competing interests.
Acknowledgements
The authors thank the Royal Australasian College of Surgeons, Royal Australasian College of Physicians and Royal Australasian College of General Practitioners, the Rural Doctors Association of Australia and local health networks all over Australia for their help disseminating the survey. The authors greatly appreciate the contribution of all the healthcare professionals who responded. The authors also thank Kelly Hall (Adelaide Health Technology Assessment) for help with the statistical analysis of the survey data. This research did not receive any specific funding.
References
[1] Machowska A, Stålsby Lundborg C. Drivers of irrational use of antibiotics in Europe. Int J Environ Res Public Health 2019; 16 27| Drivers of irrational use of antibiotics in Europe.Crossref | GoogleScholarGoogle Scholar |
[2] Munita JM, Arias CA. Mechanisms of Antibiotic Resistance Microbiol Spectr 2016; 4 4.2.15
| Mechanisms of Antibiotic ResistanceCrossref | GoogleScholarGoogle Scholar |
[3] Klein EY, Van Boeckel TP, Martinez EM, Pant S, Gandra S, Levin SA, et al Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Natl Acad Sci USA 2018; 115 E3463–70.
| Global increase and geographic convergence in antibiotic consumption between 2000 and 2015.Crossref | GoogleScholarGoogle Scholar | 29581252PubMed |
[4] Hardy-Holbrook R, Aristidi S, Chandnani V, DeWindt D, Dinh K. Antibiotic resistance and prescribing in Australia: current attitudes and practice of GPs. Healthc Infect 2013; 18 147–51.
| Antibiotic resistance and prescribing in Australia: current attitudes and practice of GPs.Crossref | GoogleScholarGoogle Scholar |
[5] Australian Commission on Safety and Quality in Health Care (ACSQHC). AURA 2019: Third Australian report on antimicrobial use and resistance in human health. Sydney: ACSQHC; 2019.
[6] Morse J, Blackburn L, Hannam JA, Voss L, Anderson BJ. Compliance with perioperative prophylaxis guidelines and the use of novel outcome measures. Paediatr Anaesth 2018; 28 686–93.
| Compliance with perioperative prophylaxis guidelines and the use of novel outcome measures.Crossref | GoogleScholarGoogle Scholar | 29961951PubMed |
[7] Fischer F, Lange K, Klose K, Greiner W, Kraemer A. Barriers and Strategies in Guideline Implementation—A Scoping Review. Health Care 2016; 4 36
| Barriers and Strategies in Guideline Implementation—A Scoping Review.Crossref | GoogleScholarGoogle Scholar |
[8] Schwartz KL, Brown KA, Etches J, Langford BJ, Daneman N, Tu K, et al Predictors and variability of antibiotic prescribing amongst family physicians. J Antimicrob Chemother 2019; 74 2098–105.
| Predictors and variability of antibiotic prescribing amongst family physicians.Crossref | GoogleScholarGoogle Scholar | 31002333PubMed |
[9] Charani E, Castro-Sanchez E, Sevdalis N, Kyratsis Y, Drumright L, Shah N, et al Understanding the determinants of antimicrobial prescribing within hospitals: the role of “prescribing etiquette”. Clin Infect Dis 2013; 57 188–96.
| Understanding the determinants of antimicrobial prescribing within hospitals: the role of “prescribing etiquette”.Crossref | GoogleScholarGoogle Scholar | 23572483PubMed |
[10] Manne M, Deshpande A, Hu B, Patel A, Taksler GB, Misra-Hebert AD, et al Provider Variation in Antibiotic Prescribing and Outcomes of Respiratory Tract Infections. South Med J 2018; 111 235–42.
| Provider Variation in Antibiotic Prescribing and Outcomes of Respiratory Tract Infections.Crossref | GoogleScholarGoogle Scholar | 29719037PubMed |
[11] Nowakowska M, van Staa T, Mölter A, Ashcroft DM, Tsang JY, White A, et al Antibiotic choice in UK general practice: rates and drivers of potentially inappropriate antibiotic prescribing. J Antimicrob Chemother 2019; 74 3371–8.
| Antibiotic choice in UK general practice: rates and drivers of potentially inappropriate antibiotic prescribing.Crossref | GoogleScholarGoogle Scholar | 31430365PubMed |
[12] Teixeira Rodrigues A, Roque F, Falcão A, Figueiras A, Herdeiro MT. Understanding physician antibiotic prescribing behaviour: a systematic review of qualitative studies. Int J Antimicrob Agents 2013; 41 203–12.
| Understanding physician antibiotic prescribing behaviour: a systematic review of qualitative studies.Crossref | GoogleScholarGoogle Scholar | 23127482PubMed |
[13] Dempsey PP, Businger AC, Whaley LE, Gagne JJ, Linder JA. Primary care clinicians’ perceptions about antibiotic prescribing for acute bronchitis: a qualitative study. BMC Fam Pract 2014; 15 194
| Primary care clinicians’ perceptions about antibiotic prescribing for acute bronchitis: a qualitative study.Crossref | GoogleScholarGoogle Scholar | 25495918PubMed |
[14] Laka M, Milazzo A, Merlin T. Factors That Impact the Adoption of Clinical Decision Support Systems (CDSS) for Antibiotic Management. Int J Environ Res Public Health 2021; 18 1901
| Factors That Impact the Adoption of Clinical Decision Support Systems (CDSS) for Antibiotic Management.Crossref | GoogleScholarGoogle Scholar | 33669353PubMed |
[15] Neuendorf KA, Kumar A. Content analysis. Int Encyclop Politic Comm 2015; 8 1–10.
| Content analysis.Crossref | GoogleScholarGoogle Scholar |
[16] Md Rezal RS, Hassali MA, Alrasheedy AA, Saleem F, Md Yusof FA, Godman B. Physicians’ knowledge, perceptions and behaviour towards antibiotic prescribing: a systematic review of the literature. Expert Rev Anti Infect Ther 2015; 13 665–80.
| Physicians’ knowledge, perceptions and behaviour towards antibiotic prescribing: a systematic review of the literature.Crossref | GoogleScholarGoogle Scholar | 25813839PubMed |
[17] Jeffs L, McIsaac W, Zahradnik M, Senthinathan A, Dresser L, McIntyre M, et al Barriers and facilitators to the uptake of an antimicrobial stewardship program in primary care: A qualitative study. PloS one 2020; 15 e0223822
| Barriers and facilitators to the uptake of an antimicrobial stewardship program in primary care: A qualitative study.Crossref | GoogleScholarGoogle Scholar | 32134929PubMed |
[18] Lum EPM, Page K, Whitty JA, Doust J, Graves N. Antibiotic prescribing in primary healthcare: Dominant factors and trade-offs in decision-making. Infect Dis Health 2018; 23 74–86.
| Antibiotic prescribing in primary healthcare: Dominant factors and trade-offs in decision-making.Crossref | GoogleScholarGoogle Scholar |
[19] Australian Commission on Safety and Quality in Health Care. AURA 2017 – Second Australian report on antimicrobial use and resistance in human health. Sydney; 2017.
[20] Pinder R, Berry D, Sallis A, Chadborn T. Behaviour change and antibiotic prescribing in healthcare settings: Literature review and behavioural analysis. London: Department of Health & Public Health England; 2015. Available at: http://hdl.handle.net/10044/1/22194.
[21] Mangione-Smith R, McGlynn EA, Elliott MN, McDonald L, Franz CE, Kravitz RL. Parent expectations for antibiotics, physician-parent communication, and satisfaction. Arch Pediatr Adolesc Med 2001; 155 800–6.
| Parent expectations for antibiotics, physician-parent communication, and satisfaction.Crossref | GoogleScholarGoogle Scholar | 11434847PubMed |
[22] Rose J, Crosbie M, Stewart A. A qualitative literature review exploring the drivers influencing antibiotic over-prescribing by GPs in primary care and recommendations to reduce unnecessary prescribing. Perspect Public Health 2021; 141 19–27.
| A qualitative literature review exploring the drivers influencing antibiotic over-prescribing by GPs in primary care and recommendations to reduce unnecessary prescribing.Crossref | GoogleScholarGoogle Scholar | 31633458PubMed |
[23] Kianmehr H, Sabounchi NS, Seyedzadeh Sabounchi S, Cosler LE. Patient expectation trends on receiving antibiotic prescriptions for respiratory tract infections: A systematic review and meta-regression analysis. Int J Clin Pract 2019; 73 e13360
| Patient expectation trends on receiving antibiotic prescriptions for respiratory tract infections: A systematic review and meta-regression analysis.Crossref | GoogleScholarGoogle Scholar | 31066959PubMed |
[24] Papoutsi C, Mattick K, Pearson M, Brennan N, Briscoe S, Wong G. Social and professional influences on antimicrobial prescribing for doctors-in-training: a realist review. J Antimicrob Chemother 2017; 72 2418–30.
| Social and professional influences on antimicrobial prescribing for doctors-in-training: a realist review.Crossref | GoogleScholarGoogle Scholar | 28859445PubMed |
[25] Carlsen B, Glenton C, Pope C. Thou shalt versus thou shalt not: a meta-synthesis of GPs’ attitudes to clinical practice guidelines. Br J Gen Pract 2007; 57 971–8.
| Thou shalt versus thou shalt not: a meta-synthesis of GPs’ attitudes to clinical practice guidelines.Crossref | GoogleScholarGoogle Scholar | 18252073PubMed |
[26] Teixeira Rodrigues A, Roque F, Falcão A, Figueiras A, Herdeiro MT. Understanding physician antibiotic prescribing behaviour: a systematic review of qualitative studies. Int J Antimicrob Agents 2013; 41 203–12.
| Understanding physician antibiotic prescribing behaviour: a systematic review of qualitative studies.Crossref | GoogleScholarGoogle Scholar | 23127482PubMed |
[27] Cho YI, Johnson TP, VanGeest JB. Enhancing Surveys of Health Care Professionals: A Meta-Analysis of Techniques to Improve Response Eval Health Prof 2013; 36 382–407.
| Enhancing Surveys of Health Care Professionals: A Meta-Analysis of Techniques to Improve ResponseCrossref | GoogleScholarGoogle Scholar | 23975761PubMed |
[28] Joyce CM, Scott A, Jeon S-H, Humphreys J, Kalb G, Witt J, et al The” Medicine in Australia: Balancing Employment and Life (MABEL)” longitudinal survey-Protocol and baseline data for a prospective cohort study of Australian doctors’ workforce participation. BMC Health Serv Res 2010; 10 50
| The” Medicine in Australia: Balancing Employment and Life (MABEL)” longitudinal survey-Protocol and baseline data for a prospective cohort study of Australian doctors’ workforce participation.Crossref | GoogleScholarGoogle Scholar | 20181288PubMed |
[29] Simpkin AL, Schwartzstein RM. Tolerating uncertainty—the next medical revolution? N Engl J Med 2016; 375 1713–15.
| Tolerating uncertainty—the next medical revolution?Crossref | GoogleScholarGoogle Scholar | 27806221PubMed |
[30] van Esch TE, Brabers AE, Hek K, van Dijk L, Verheij RA, de Jong JD. Does shared decision-making reduce antibiotic prescribing in primary care? J Antimicrob Chemother 2018; 73 3199–205.
| Does shared decision-making reduce antibiotic prescribing in primary care?Crossref | GoogleScholarGoogle Scholar | 30165644PubMed |




