A survey of reimbursement practices of private health insurance companies for pharmaceuticals not covered under the Pharmaceutical Benefits Scheme 2008
Senthil M. Lingaratnam A E , Sue W. Kirsa A , James D. Mellor A , John Jackson B , Wallace Crellin C , Michael Fitzsimons D and John R. Zalcberg CA Pharmacy Department, Peter MacCallum Cancer Centre, St Andrew’s Place, Melbourne, VIC 3002, Australia. Email: sue.kirsa@petermac.org; dan.mellor@petermac.org
B APHS Pharmacy, 6 Dividend Street, Mansfield, QLD 4122, Australia. Email: john.jackson@aphs.com.au
C Peter MacCallum Cancer Centre, St Andrew’s Place, Melbourne, VIC 3002, Australia. Email: whc@melbpc.org.au; john.zalcberg@petermac.org
D Medicines Australia, Level 1, 16 Napier Close, Deakin, ACT 2600, Australia. Email: michael.fitzsimons@medicinesaustralia.com.au
E Corresponding author. Email: senthil.lingaratnam@petermac.org
Australian Health Review 35(2) 204-210 https://doi.org/10.1071/AH10894
Submitted: 28 February 2010 Accepted: 8 September 2010 Published: 25 May 2011
Journal Compilation © AHHA 2011
Abstract
Objective. To describe the current practices and policy of Australian private health insurance (PHI) companies with respect to cover for pharmaceuticals not subsidised under the Pharmaceutical Benefits Scheme (PBS).
Design, setting and participants. A 2008 review of web-published policy statements for top-level hospital and comprehensive general treatment insurance, and survey of reimbursement practices by way of questionnaire, of 31 Australian-registered, open-membership PHI companies.
Main outcome measures(s). Description of the level of pharmaceutical cover and important considerations identified by PHI companies for funding non-PBS pharmaceuticals through benefit entitlements or ex-gratia payments.
Results. Nine of thirty-one PHI companies (29%) provided responses accounting for ~60% market share of PHI. The majority of smaller PHI firms either declined participation or did not respond. The maximum limits offered for non-PBS pharmaceuticals, under comprehensive general treatment insurance, varied significantly and typically did not adequately cover high-cost pharmaceuticals. Some companies occasionally offered ex-gratia payments (or discretionary payments in excess of the policyholder’s entitlement benefits) for high cost-pharmaceuticals. Factors considered important in their decision to approve or reject ex-gratia requests were provided. All results were de-identified.
Conclusions. There is little consistency across PHI companies in the manner in which they handle requests for high-cost pharmaceuticals in excess of the defined benefit limits. Such information and processes are not transparent to consumers.
What is known about the topic? Pharmaceuticals that are not accessible via the Pharmaceutical Benefits Scheme (PBS) may be subsidised through private health insurance. The level of cover through general treatment insurance and hospital insurance varies according to the insurer or policy type and hospital–insurer agreement respectively.
What does this paper add? An increasing proportion of lower cost, high volume pharmaceuticals that are available to consumers without any form of Commonwealth subsidy, under current arrangements, also do not attract any form of PHI cover. There is also little consistency across PHI companies in the manner in which they handle requests for high-cost pharmaceuticals in excess of the defined benefit limits and that such information and processes are not transparent to consumers.
What are the implications for practitioners? PHI could be better engaged to play a more significant role in helping maintain consumer access to essential medicines.
Introduction
For most Australians, access to pharmaceuticals occurs via the tax-funded Pharmaceutical Benefits Scheme (PBS). However, there are two significant non-government funding arrangements for pharmaceuticals; payment by individual consumers and by private health insurance (PHI).
In response to a burgeoning pharmaceutical bill and in keeping with most advanced western economies, the Australian government has introduced reforms to limit its expenditure on pharmaceuticals.1,2 Although these measures are aimed at ‘creating more headroom’ for the new generation pharmaceuticals to be listed on the PBS,2 recent evidence suggests that patient affordability and access to essential medicines is diminishing.3 Furthermore, non-concessional individuals now pay the full amount for a greater number of PBS-listed pharmaceuticals that cost less than the annually increasing PBS patient contribution threshold for Commonwealth subsidy.3–5 In this current climate, consumers may assume their private health insurance will provide an adequate level of assistance to help shoulder the private sector’s share of health costs (e.g. costs for pharmaceuticals that are not Commonwealth subsidised) as has been asserted by the industry in a health economics report submitted to the Australian government in support of government subsidy of PHI.6 This study was aimed at understanding the policies of PHI companies towards reimbursement of non-PBS pharmaceuticals. This paper reports on two aspects of the study:
-
a review of published PHI policy statements regarding the level of cover for pharmaceuticals; and
-
findings of survey responses from the PHI funds in relation to high-cost pharmaceutical claims made by their members or by healthcare providers on behalf of their members.
Method
A steering committee comprising representatives from industry and government groups was established in order to inform the study of legislative, political and economic factors influencing the current practice of PHI fund reimbursement policies for pharmaceutical expenses. The steering committee was made up of lead representatives from the Peter MacCallum Cancer Centre including a consumer representative, Medicines Australia, Australian Private Hospital Association, the Pharmacy Guild of Australia (Victoria Branch) and the Private Hospital Pharmacy Committee of Speciality Practice, Society of Hospital Pharmacists Australia. Among those organisations that declined invitations to join the steering committee were the Australian Health Insurance Association (AHIA), Department of Health and Ageing, and the Private Health Insurance Ombudsman’s Office that instead offered assistance on specific queries.
This study was carried out in two stages:
-
Stage 1 was a review of Standard Information Statements (SIS) published by PHI companies at www.privatehealth.gov.au. This was undertaken after ethics approval was received for this study. A comparison was undertaken of the pharmaceutical cover under top-level hospital and comprehensive general treatment insurance policies offered by each of the Australian registered, open-membership health insurers. Open-membership health insurers are those insurers that do not restrict membership to a specific industry or group. SIS are up-to-date summaries of key product features offered by PHI companies, provided in a standardised format to allow consumers to clarify details and more easily compare products. PHI company websites were also reviewed and, where available, further information pertaining to pharmaceutical entitlements was collated.
-
Stage 2 involved analysis of survey responses from all Australian registered open-membership PHI companies. A survey tool was designed in consultation with the steering committee. This may be obtained upon request from the corresponding author. The questionnaire was aimed at:
-
-
Exploring the company’s standard policy and practice in relation to reimbursement of pharmaceutical costs covered under top-level hospital and comprehensive general treatment (GT) insurance and
-
Exploring the factors considered by the company when considering reimbursement requests for high-cost pharmaceuticals that exceed the typical benefit entitlements for policyholders.
-
-
In July 2008, a covering letter and self-addressed-stamped envelope were posted out to the medical consultant at the head offices of all Australian open-registered PHI companies. In order to ensure that the questionnaires were received by relevant authorities, a reminder letter and copy of survey, addressed to the CEO, were sent four weeks later to PHI companies that had not acknowledged receipt of the documents. All survey recipients were informed that findings would be de-identified before reporting and were also given the option of anonymous return of their responses.
Results
In July 2008 there were 31 Australian-registered, open-membership PHI companies eligible for evaluation in this study.
Stage 1. Review of published policy statements
SIS from www.privatehealth.gov.au for top-level hospital and comprehensive GT insurance policies were reviewed for the 31 eligible PHI companies. Reportable data pertaining to non-PBS pharmaceuticals across company websites and SIS were only available for the non-hospital admitted setting i.e. pharmaceutical entitlements considered under GT insurance. There was no specific information detailing restrictions on pharmaceuticals consumed during hospital admission, other than to mention excesses or out-of-pocket expenses incurred for hospital admission, specific to the level of cover under each policy.
As summarised in Fig. 1 the maximum reimbursements varied from $20 to $350 per prescription after the patient paid an initial amount per prescription equivalent to the non-concession PBS patient contribution fee. The cumulative maximum annual limits varied from $150 to $750 for the policyholder. As these levels of reimbursement provide minimal protection against high-cost non-PBS pharmaceuticals, stage 2 of the study was crucial to ascertaining PHI company responses to requests for ex-gratia reimbursement for high-cost pharmaceuticals.
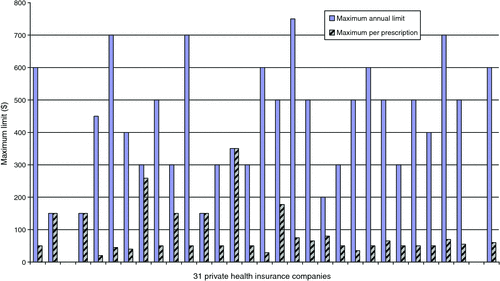
|
Many PHI companies limit the types of pharmaceuticals for which GT insurance is available; however, details of exclusions were not available from SIS. This information was collated from company websites and is summarised in Fig. 2. Company websites also stipulated that GT insurance did not cover any portion of PBS pharmaceuticals including the patient contribution fee, as is currently legislated under the National Health Act 1953. Many companies acknowledged that policyholders would be charged a minimum set charge equivalent to the non-concessional patient contribution fee for any non-PBS pharmaceutical covered under their GT policy.
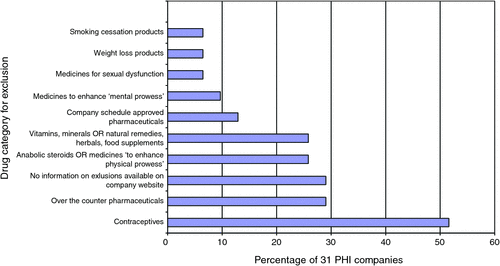
|
Stage 2. Survey findings
Under current legislation, a private health fund may operate multiple PHI companies. Of the 22 private health funds, equivalent to 31 PHI companies, that were invited to participate, nine responses from the funds (accounting for 15 of the 31 PHI companies) were received. Of the nine responses returned, three included completed questionnaires – this amounted to a response rate of 29% (9 out of 31 PHI companies). These nine PHI companies accounted for ~60% of PHI market share according to statistics published in the Private Health Insurance Ombudsman 2008 Annual Report. None of these respondents exercised their option to remain anonymous. The remaining six responses were notifications from PHI funds registering their non-participation in the study. Thirteen funds did not respond to repeated invitations to participate in the study.
All respondents identified non-PBS pharmaceuticals as eligible for entitlement benefit or ex-gratia consideration if they satisfied criteria, as summarised in Box 1. Further information relating to entitlement benefits under GT insurance is also summarised in Box 1. Any pharmaceutical costing in excess of the policy limit would not be eligible for entitlement benefit. Instead, any further consideration by the fund to finance higher-cost pharmaceuticals not covered under the member’s policy would be done so on a discretionary basis. Such ex-gratia requests were usually forwarded to hospital contracts the claims office or a medical consultant within the PHI company.
| Box 1. Definition of non-PBS pharmaceuticals for PHI benefit entitlement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Two insurers provided responses to the questions relating to hospital-insurer agreements. One indicated that 49 of 50 hospital agreements included non-PBS drug costs in the accommodation and theatre benefits already paid to the hospital, whereas only 1 of 50 hospital agreements had non-PBS benefits paid on application by the hospital. In the latter instance expenditure for non-PBS pharmaceuticals were equally shared by the hospital, insurer and policyholder. Another insurer reported that all of its hospital agreements had non-PBS benefits paid on application by the hospital but did not provide further information on cost-sharing. Most of the hospital-insurer agreements that oblige the hospitals to meet pharmaceutical costs for in-patient treatment from the benefits paid by the insurer also limit the capacity of hospitals to recover costs from patients. With limited arrangements for supplementary or ex-gratia payments by the insurance funds, some high-cost in-patient pharmaceuticals, such as non-PBS oncology drugs, can cost the hospital more than the level of income from the fund caring for the patient. One example is azacitidine, which was shown to improve progression free survival in patients with myelodysplastic syndromes and reduce their blood transfusion dependence, thereby reducing the need for recurrent hospital admissions. Although azacitidine was TGA-approved in November 2009, it is yet to be listed for Commonwealth subsidy via the PBS or Section 100. Currently, this agent is available to newly diagnosed patients at considerable financial costs to the patient or treating hospital. Another example is rituximab in combination with chemotherapy for the treatment of chronic lymphocytic leukemia (CLL). Although multiple randomised controlled trials have shown clinical benefit over standard chemotherapy alone, particularly in patients with better performance status, rituximab for the treatment of CLL is not a PBS-listed indication.
Of the various respondents, one reported that they had no ex-gratia policy, citing that ‘members are only covered for the features/benefits of the product they purchased’. The remaining respondents identified that such requests would be decided on a case-by-case basis.
As summarised in Box 2, the respondents that considered ex-gratia payments on a case-by-case basis indicated that non-admitted patients were less likely to receive favourable outcomes for their ex-gratia requests compared to patients that were hospital in-patients. One of the respondents additionally noted that when identifying cases to receive ex-gratia payment, a degree of caution had to be exercised in case the PHI Ombudsman stipulated that all patients with similar clinical scenarios should receive the same decision outcome to ensure equitable outcomes to all members. As such, the perceived effect of any ex-gratia request on the fund’s benefits outlays needed to be factored into decision making (i.e. ‘how many other patients may request similar payments. An example that was cited was the ex-gratia requests for use of trastuzumab for locally advanced breast cancer prior to PBS listing’).
| Box 2. Factors considered by PHI funds when considering ex-gratia requests Entries with an asterisk (*) are the most important factors considered by PHI funds | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
Respondents ranked in-patient use, TGA approval of the drug and the member’s policy type (and whether the member was a resident or an overseas policyholder) within the 5 most highly ranked factors considered in approving ex-gratia requests. One fund additionally cited that only privately contracted hospitals were considered for ex-gratia approvals, and that another important factor for consideration was the outcome of submissions to the PBAC for that pharmaceutical to be listed on the PBS. One fund cited length of membership and the member’s previous claims history as two other factors that ranked highly in consideration for approval of ex-gratia requests.
Respondents differed in opinion as to whether the following factors were important in considering approval for ex-gratia payments: client’s life expectancy, whether the hospital was public or private and the duration the patient had been suffering with a particular illness.
Respondents agreed that the following factors were not considered in decisions regarding ex-gratia requests: client’s age, client’s state of residence, client’s smoking status, whether the prescriber was familiar or had recognised standing among peers and the FDA approval status of the pharmaceutical being requested.
Discussion
Owing to the voluntary nature of PHI company participation in this survey, and the request for commercially sensitive data, limited responses were received. Despite a low response rate of completed surveys (3 of 22 funds or 9 of 31 insurers), responses were representative of ~60% of the Australian population with PHI coverage, and as such these findings are considered to be of interest to the wider public. We acknowledge that large-scale structural changes, such as the broadening of PHI coverage under new legislation enacted in 2007, demutualisation of some PHI funds and more recent changes to the Medicare rebate and Medicare levy surcharge threshold may ultimately result in changes to the findings that have been described in this report.
Notably, length of membership, previous claims history, client’s life expectancy and the duration of illness were considered by some insurers as influential in their decision making, although this goes against government-enforced community rating principles (i.e. the principles associated with equitable access to healthcare) that premiums paid for health insurance and benefit entitlements or ex-gratia payments made by PHI companies should not be dependant upon a person’s actual or perceived health status. Related practices have been reported previously where insurers have offered different attractions in their plans to tease out lower risk from higher risk members and accordingly charge different premiums.7
These practices are thought to have contributed to the introduction of ‘a bewildering array of [health insurance] products and tables’ in the PHI market,8 thus making informed consumer choice a difficult undertaking. More recently, the Commonwealth Government instituted measures, under the Private Health Insurance Act 2007 to facilitate consumer understanding by requiring that insurers publish SIS for all hospital and general treatment insurance products on a Commonwealth Government website: www.privatehealth.gov.au. The information relevant to non-PBS pharmaceuticals contained within SIS is only a summary and direct contact with the insurer is required to clarify more specific information. Some health insurers hold their own schedule for reimbursable non-PBS pharmaceuticals; however, the lists of pharmaceuticals are not readily available to the public. Further, there is some variability between funds as to which pharmaceuticals are excluded from entitlement benefits as shown in Fig. 2.
PHI funds have determined that patients must pay an amount towards each private prescription equivalent to the statutory PBS non-concessional patient co-payment. This amount is automatically increased on 1 January each year in line with inflation, which results in a reduction in the level of PHI benefit. As this threshold increases according to Government stipulation, and PBS reference prices fall under the reference pricing strategies encompassed within the PBS reform package, increasingly more PBS-listed pharmaceuticals will fall under the threshold for any form of government subsidy. This trend is based on the assumption that the new reference pricing strategies encompassed within the PBS Reforms package promotes reductions of ex-manufacturer prices below the Commonwealth subsidy thresholds, which are continually rising in line with healthcare inflation. Thus increasingly, the lower cost, high volume pharmaceuticals are available to the consumer without any form of subsidy from the Commonwealth. Section 92B of the National Health Act 1953 (the Act) establishes that an insurer must not enter ‘into a contract of insurance that comprises or contains a refund agreement’ – that is, PHI cannot cover any cost of pharmaceuticals that have a Commonwealth benefit payable via the PBS.
It is understood that high-cost pharmaceuticals, if they are deemed to be cost-effective, fall within the domain of the Commonwealth for funding.9,10 Relevant pharmacoeconomic assessments are considered by the Pharmaceutical Benefits Advisory Committee (PBAC) before their recommendation to list a pharmaceutical onto the PBS. Manufacturers of pharmaceuticals not on the PBS have either had their PBS submissions rejected or have not made these available to the PBAC for listing onto the PBS. Since 2007, the PBAC has disclosed aspects of their deliberations on pharmaceuticals considered for PBS-listing but these publicly available documents are not routinely utilised by PHI companies. Also, there is often insufficient reporting of the appraisal of economic evaluations. In particular, the non-disclosure of cost-effectiveness thresholds presented in PBAC submissions make it difficult for PHI companies or any other potential funders such as private or public hospitals to consider this information. One large fund cited in its survey response that one of the top 5 factors that governed a decision to fund an ex-gratia payment was the outcome of a PBAC evaluation of the pharmaceutical. Yet the details of such evaluation cannot be readily considered in subsequent decisions made by these companies.
Private health insurers were polarised in their positions on ex-gratia considerations for high-cost pharmaceuticals. Because ex-gratia payments are part or full payments in excess of actuarially fair entitlements, some insurers (who wish to remain anonymous) inform that offering a greater allowance of healthcare funding to a smaller proportion of their membership adversely affects the wider proportion of their membership by compromising the financial footing of the fund and promoting premium rate rises. Personal communication with the office of the PHI Ombudsman and PHIAC confirms that these are legitimate concerns that a fund must factor in its prudential management of its members’ interests. Other independent commentators contend that PHI is aimed at minimising the burden on public expenditure of healthcare; that the highest level of health insurance should comprehensively insure against the highest level of health risk. However, survey responses from participating health insurers have revealed that most treatments involving high-cost pharmaceuticals, especially oral therapies for non-admitted patients (i.e. the more cost-effective treatment options where the costs associated with hospitalisation may be avoided) are not adequately covered under the most comprehensive general treatment or hospital insurance policies.
Conclusion
Accessibility to pharmaceuticals remains an issue in healthcare with the cost to consumers for pharmaceuticals continuing to increase. PHI companies may pay some or all of the costs of pharmaceuticals that are not reimbursed by the Commonwealth Government. How PHI companies handle requests for high-cost pharmaceuticals in excess of the defined benefit limits is neither consistent across the major funds nor transparent to consumers. Our findings suggest that if PHI is to offer more equitable access to high-cost non-PBS pharmaceuticals, companies will need to be better informed of the clinical benefit and cost-effectiveness of these pharmaceuticals and must be assured that the risks associated with each application are equitably borne by all stakeholders; health insurers, hospitals, pharmaceutical industry, consumers and government alike.
Competing interests
Roche Products (Australia) Pty Ltd provided an unrestricted research grant to the Peter MacCallum Cancer Centre. At no time did Roche Products (Australia) Pty Ltd influence any part of the study. Roche had no direct involvement in the conception, scoping, execution or publication of any part of this work. The authors declare that no other conflicts of interest exist.
Acknowledgements
We thank Ken Gray from the Pharmacy Guild of Australia (Victoria Branch) for his support in the steering committee, and Roche Products (Australia) Pty Ltd (Dee Why, NSW) for providing an unrestricted research grant to carry out this study. The unrestricted research grant was paid to the Peter MacCallum Cancer Centre and this was utilised to fund a part-time research officer to complete the project.
References
[1] National Health Amendment (Pharmaceuticals Benefits Scheme) Act 2007.[2] Media Release. Strengthening your PBS. Minister for Health and Ageing, Tony Abbott MHR. 16 November 2006.
[3] Hynd A, Roughead EE, Preen DB, Glover J, Semmens J. The impact of co-payment increases on dispensings of government-subsidised medicines in Australia. Pharmacoepidemiol Drug Saf 2008; 17 1091–9.
| The impact of co-payment increases on dispensings of government-subsidised medicines in Australia.Crossref | GoogleScholarGoogle Scholar | 18942671PubMed |
[4] Australian Institute of Health and Welfare. Health Expenditure Australia 2006–2007. Health and Welfare expenditure series no. 35. Canberra: AIHW; 2008.
[5] Sub-Committee DU. Australian Statistics on Medicines 2007. Canberra: Department of Health and Ageing, Drug Utilisation Sub Committee; 2009.
[6] Easing the pressure: the intergenerational report and Private Health Insurance. Submission to the Productivity Commission, prepared for Medibank Private. Econtech Pty Ltd; 2004. Available at http://www.econtech.com.au/Health.aspx [verified 2 May 2011].
[7] Vaithianathan R. An economic analysis of the Private Health Insurance Incentives Act (1998), Discussion Paper No. 427. Canberra: Centre for Economic Policy Research, The Australian National University; 2001.
[8] Banks G. The Industry Commission’s Report on Private Health Insurance in Australia. Chairman’s keynote address to the Conference: ‘The White Paper on PMI – Looking to the Future’, Dublin, Ireland, 28 May 1998. Productivity Commission. Available at http://www.pc.gov.au/speeches/cs19980528 [verified 21 April 2011].
[9] Duckett SJ. Drug policy down under: Australia’s Pharmaceutical Benefits Scheme. Health Care Financ Rev 2004; 25 55–67.
| 15229996PubMed |
[10] Sansom L. The subsidy of pharmaceuticals in Australia: process and challenges. Aust Health Rev 2004; 28 194–205.
| The subsidy of pharmaceuticals in Australia: process and challenges.Crossref | GoogleScholarGoogle Scholar | 15527399PubMed |


