No evidence of protracted population decline across 17 years in an unmanaged population of the green and golden bell frog in north-eastern New South Wales
Ross L. Goldingay A B , Jonathan Parkyn A and David A. Newell AA School of Environment, Science and Engineering, Southern Cross University, PO Box 157, Lismore, NSW 2480, Australia.
B Corresponding author. Email: ross.goldingay@scu.edu.au
Australian Journal of Zoology 65(2) 87-96 https://doi.org/10.1071/ZO16087
Submitted: 15 December 2016 Accepted: 22 June 2017 Published: 25 July 2017
Abstract
Describing the population trends of threatened species over time is central to their management and conservation. The green and golden bell frog (Litoria aurea) is a formerly common species of south-eastern Australia that has declined to ~40 populations in New South Wales, and experienced a substantial contraction of its geographic range. We aimed to determine whether an unmanaged population at the northern end of its range had declined across a 17-year period. We estimated population size at the beginning and end of this period, using several population models to fully characterise this population. Different modelling approaches gave different population estimates. Based on a similar number of survey occasions the adult male segment of the population was estimated using the Popan model at 112.0 (±13.5, s.e.; 95% CI: 85.5–138.8) in 1998/99 and 95.2 (±17.6; 60.8–129.7) in 2015/16. With the inclusion of maturing subadults following the practice of earlier studies, the population was estimated at 163.6 (±25.9; 112.8–214.5) males in 2015/16. These estimates represent an index of a larger population because the largest wetland was subsampled. Our data provide no evidence of a declining population. Our study highlights the need to understand the implications of using different population models and two age-classes to estimate population parameters.
Introduction
The susceptibility to extinction of amphibians is now well recognised. Global extinctions number 38 species with another 489 species listed as critically endangered, including 120 species considered ‘possibly extinct’ but which are yet to be confirmed (IUCN 2016). Australia, in particular, has lost up to six species, with many more still under severe threat (Hero et al. 2006; Skerratt et al. 2016). This has led to a large research effort to document the threats and devise effective conservation strategies. The amphibian chytrid fungus has been implicated in the Australian declines and has been a central focus of research (Berger et al. 1998; Schloegel et al. 2006; Kriger and Hero 2007; Hunter et al. 2010; Scheele et al. 2014; Skerratt et al. 2016). Concurrent with this has been recognition for greater knowledge of the population ecology of species and for population monitoring to document trends in population abundance (Lewis and Goldingay 2005; Richards and Alford 2005; Phillott et al. 2013). Currently there are few long-term datasets to demonstrate the stability or otherwise of Australian frog populations (see Richards and Alford 2005; Newell et al. 2013; Pickett et al. 2014; Scheele et al. 2014; Gillespie et al. 2015; Quick et al. 2015; Scheele et al. 2017).
The green and golden bell frog (Litoria aurea) is an Australian species that experienced a widespread decline during the 1970s and 1980s (White and Pyke 1996). Historically, its range extended ~1200 km along coastal eastern Australia. Its range also encompassed three tableland populations above 700 m elevation. Of these, only a single small population on the southern tablelands of New South Wales remains (White and Pyke 1999; Osborne et al. 2008; Hamer et al. 2010). Approximately 40 populations remain across New South Wales (White and Pyke 2008), which formerly contained 80% of the species’ historical geographic range. Habitat clearing, introduced gambusia fish and the amphibian chytrid fungus have been implicated as the causes of this species’ decline (Pyke and White 2001; Goldingay 2008; Mahony et al. 2013; Stockwell et al. 2015). The green and golden bell frog is listed as a vulnerable species under the Federal Environment Protection and Biodiversity Conservation Act 1999 and as an endangered species under the New South Wales Biodiversity Conservation Act 2016.
The green and golden bell frog may provide a model for understanding and managing declines of Australian frogs. Between 1995 and 2007, 23% of 31 populations in New South Wales disappeared and a further 13% probably also disappeared (White and Pyke 2008). No populations in New South Wales are considered to be secure. One at Sydney Olympic Park is actively managed (Darcovich and O’Meara 2008; O’Meara and Darcovich 2008; O’Meara and Darcovich 2015) and is estimated to contain ~300 individuals (Pickett et al. 2014). Another at Kooragang Island in Newcastle is also actively managed and may contain >1000 individuals (Hamer and Mahony 2007). The species is highly susceptible to the amphibian chytrid fungus (Penman et al. 2008; Stockwell et al. 2008, 2010), which may account for recent losses (Stockwell et al. 2015). Persistence at locations with infection warrants investigation of which factors allow persistence and suggests a need for increased surveillance of disease distribution (Stockwell et al. 2015).
Decline of some green and golden bell frog populations and persistence of others highlights the need for a better understanding of the species’ population ecology. We investigated the population ecology of a population at the very northern end of the geographic range. The population is the larger of two nearby populations (Goldingay and Newell 2005a). The loss of these northern populations would present a worsening outlook for the green and golden bell frog, representing a 110-km range contraction south on top of a 140-km southward contraction since the 1980s (see Lewis and Goldingay 1999). We conducted capture–mark–recapture studies in two periods: in 1998/99 and in 2015/16. The aim of our study was to estimate and compare population size for these two periods. We cannot know how the population fluctuated within the intervening 17-year period. However, comparing these two periods will reveal whether the population size in the later period was larger, smaller or equivalent. A severe decline in population size might precede local extinction. We use the pattern of decline observed by White and Pyke (1996) in a green and golden bell frog population in Sydney as a model. They studied the Eastlakes population over 28 years (1967–95) and reported survey data at 2-year intervals. A decline occurred between 1977 and 1982, and was then followed by a 12-year period of very low abundance (~15% of predecline abundance) before the species disappeared (see also White and Pyke 2008). Other studies involving detailed multiyear surveys have observed annual variation in abundance (~50% among years) but not a protracted decline, nor very low abundance (Goldingay and Lewis 1999; Pickett et al. 2014). Therefore, we hypothesise that if a protracted decline had occurred in our study population that the population size we estimate in 2015/16 would be substantially lower (<20%) than that estimated for 1998/99. Furthermore, there would be limited evidence of breeding success for the current or previous breeding season. The 17-year intervening period should be sufficient to detect a protracted decline should one have occurred.
Long-term monitoring of populations of threatened species is fundamental to understanding their conservation requirements. The New South Wales government has a program to manage threatened species (OEH 2013). At the core of this program are attempts to manage the threats to these species and to measure this against long-term monitoring of populations (OEH 2014). This monitoring needs to be cost-effective but provide robust data so that population recovery or stability can be ascertained. Our study describes an approach adopted to provide such monitoring data for one green and golden bell frog population and considers several factors that may influence population estimates.
Methods
Study area
This study was conducted at Station Creek in Yuraygir National Park, in north-east New South Wales. The frog habitat we sampled consisted of a large coastal lagoon, a swamp periodically connected to the lagoon and four ephemeral ponds located within adjacent sand dunes. The coastal lagoon (Fig. 1) measured 40–70 m wide by 700 m long (5.4 ha); the swamp was 30 m by 60 m and the ephemeral ponds ~25 m by 25 m. The lagoon and swamp were fringed by tall sawsedge (Gahnia clarkei), jointed twig rush (Baumea articulata) and cumbungi (Typha orientalis).
Frog surveys
We conducted eight non-consecutive nights of survey between August 1998 and February 1999, and 12 nights of survey divided among four primary periods in November and December 2015, and February and March 2016. Surveys were conducted independent of rainfall events in 1998/99 whereas in 2015/16, three of four survey periods followed days in which >20 mm of rain had fallen. Rainfall at Wooli, 12 km away, for a 1-year period (March–February) that encompassed our surveys totalled 1413 mm in 1998/99 and 1392 mm in 2015/16, representing 91% and 89% of the long-term mean, respectively (Bureau of Meteorology).
Surveys were conducted by 2–3 people in both 1998/99 and 2015/16. The ephemeral ponds were searched when they contained water in 1999 but they did not fill with water in 2015/16. Surveys sampled the same area, representing 50% of the lagoon, in each period. Repeat traverses were conducted of this area each night until few additional individuals were detected. In some months many frogs were captured floating on top of aquatic plants and algae (Fig. 2). Because we did not sample the entire lagoon our population estimates represent indices of the broader population and should not be viewed as absolute estimates.

|
Frogs were placed into separate plastic bags at capture and their location recorded by a GPS. They were later scanned with a portable tag reader (Trovan Ltd, Douglas, UK). Untagged individuals were subcutaneously implanted with a passive integrated transponder (PIT) tag and the entry point sealed with Vetbond adhesive. Each frog was sexed, and its weight and snout–vent length (SVL) measured. Sex was determined according to the presence or absence of pigmented nuptial pads, as well as the weight and size of the frog. Each frog was later released at its initial capture location.
Data analysis
We constructed capture histories for each male tagged frog for both the 1998/99 and 2015/16 periods. Too few female frogs were captured for them to be included in the modelling. The data collected in 1998/99 have been presented previously (Goldingay and Newell 2005a) but were not subjected to population modelling. We obtained rainfall data from the Bureau of Meteorology for Wooli Beach to include as a covariate in our analyses.
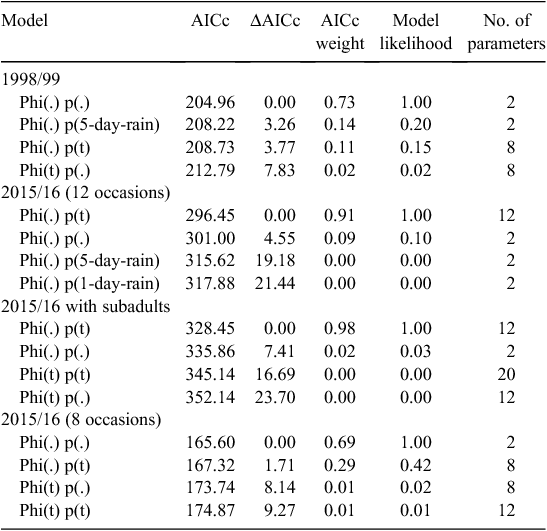
We used Program Mark 7.0 (White and Burnham 1999) to estimate the probabilities of capture and survival for this frog population. Survival is defined as ‘apparent survival’ because it includes actual survival with an unknown component of dispersal. We constructed models in which survival and capture parameters varied over time, were constant or were influenced by rainfall. Models were ranked on the basis of the Akaike Information Criterion corrected for small sample size (AICc). The top-ranked model showed the best fit to the data. The plausibility of other models was determined from their difference in AICc to the top model (i.e. ΔAICc). Models in which ΔAICc was <2 were considered equally plausible; models with higher values of ΔAICc were considered less plausible (Burnham and Anderson 2004). In some models the parameter estimates converged on values of 1 or 0. We subsequently fixed these values and ranked the model with the reduced number of parameters.
To facilitate comparison across studies and to explore differences in model outputs, we used three different model designs: the Popan formulation of the Jolly–Seber model (Schwarz and Arnason 1996), the Cormack–Jolly–Seber (CJS) model and the Robust Design (RD) model. Previous population modelling of the green and golden bell frog has used the CJS model (Hamer and Mahony 2007) and the Robust Design (Pickett et al. 2014). The Popan model has the advantage over the CJS model in that it provides a direct estimate of population size, and estimates recruitment between survey occasions. The Popan model considers a super-population from which individuals enter the capture sample (Schwarz and Arnason 1996). The estimate of the super-population can be used to represent all frogs within the survey area across the whole survey period. In an analogous way Hamer and Mahony (2007) estimated the total population by dividing the total number of individuals captured in a breeding period by the CJS model estimate of capture probability. To estimate population size from the CJS model in our study where capture probability varied with time, we used the average of the capture probability. The RD model incorporates secondary sampling periods nested within primary sampling periods to recognise that populations may be closed over short periods but open over long periods (Pollock 1982). The structure of the surveys in 1998/99 precluded the use of this model. The 2015/16 data conformed to the RD survey structure. The primary period in December was excluded from this analysis because this survey was abandoned on the second night due to an electrical storm and a substantial decline in frog encounters.
Reduction of 2015/16 data to eight occasions
The data for 1998/99 were derived from eight survey occasions. Comparing these to the data for 2015/16 derived from 12 occasions may lead to a bias. Therefore, in order to explore the influence of using fewer occasions the data for 2015/16 were reduced to eight occasions. This was done by removing the last night of sampling across the four primary sampling periods from the detection history.
Inclusion of subadults in the data
Earlier population studies on the green and golden bell frog included male and female individuals of a SVL greater than 45 mm (Pickett et al. 2014) or males >42.8 mm (Hamer and Mahony 2007). These values are based on the minimum size at sexual maturity (males with nuptial pads). This is likely to overestimate the number of adult individuals because not all individuals of that size will be sexually mature. Minimum male SV lengths were 51 mm at Port Kembla (Goldingay and Newell 2005b) and 56 mm at Yuraygir (Goldingay and Newell 2005a) based on the presence of darkened nuptial pads. Pyke and White (2001) stated that the adult stage starts in bell frogs when nuptial pads develop at ~50 mm SVL. At Yuraygir in 2015/16, the smallest male with darkened nuptial pads was 53 mm. Smaller frogs did not have dark pigmentation to their pads, suggesting that they had not reached sexual maturity.
Hamer and Mahony (2007) identified two age cohorts among male and three among female bell frogs. This is relevant because different age classes are likely to have age-specific apparent survival and capture probabilities. Juveniles are likely to have lower rates of survival (see Pike et al. 2008 for reptiles) because they are more vulnerable to predators than adults. They are also more prone to dispersal so their probability of apparent survival is predicted to be lower. Therefore, modelling of data that combines clearly adult and potentially subadult bell frogs is likely to produce lower estimates of capture and apparent survival than if only adult individuals are modelled. Furthermore, including more individuals in the dataset will inflate the total number estimated.
We investigated the influence of including a set of smaller frogs. We chose 43 mm SVL to be our minimum size based on Hamer and Mahony (2007). We captured 104 frogs that measured 43–52 mm SVL (average length 45 mm). Some of these frogs could be sexed as males but none had darkened nuptial pads. We randomly selected a subset of these frogs to include with our clearly adult male individuals. Hamer and Mahony (2007) identified two size and age classes among their male frogs. The smaller age class comprised 28% of the total number of male frogs. Therefore, we selected 23 smaller individuals to comprise 28% of the total when added to the adult individuals. This larger set of individuals was modelled as described above to compare the outputs with those for the adults only. This additional modelling was conducted only for the 2015/16 data because no frogs of this smaller size were captured in 1998/99 and only two were observed.
Goodness-of-fit was tested using the program Release within Mark for the Popan and CJS models. This indicated that there was no significant lack of fit for the 1998/99 data (χ2 = 4.08, d.f. = 13, P = 0.99), the 2015/16 data without subadults (χ2 = 13.53, d.f. = 23, P = 0.94) or with subadults (χ2 = 15.67, d.f. = 26, P = 0.94), and when the 2015/16 data were reduced to eight occasions (χ2 = 4.52, d.f. = 12, P = 0.97).
Results
In 1998/99, we captured 64 adult males, six adult females and no subadults across eight nights. Adult males were captured on 105 occasions. In 2015/16, we captured 56 adult males, 20 adult females and 104 subadult individuals across 12 nights. Adult males were captured on 111 occasions. The subadults were captured on 126 occasions.
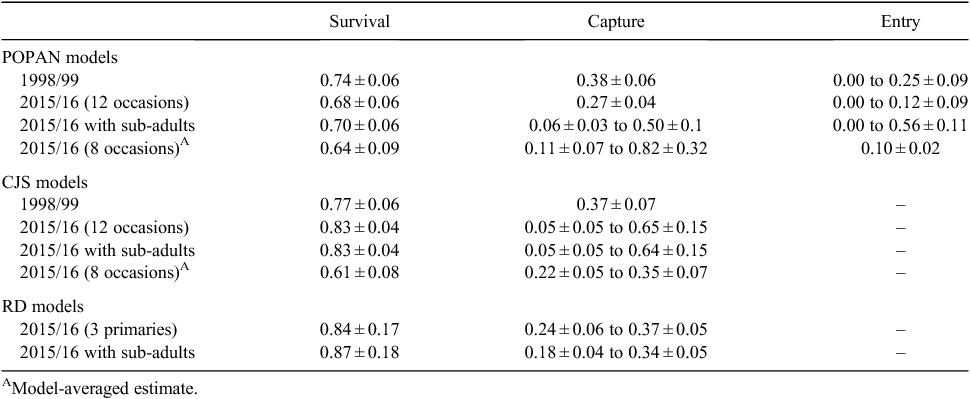
Popan model
The best-fitting model for the 1998/99 data was one where survival (0.74) and capture (0.38) were estimated as constant over time whilst the probability of entry varied with time (Tables 1, 2). This model had a model weight of 0.83. The best-fitting model for the 2015/16 (12 occasions) data was one where survival (0.68) and capture (0.27) were estimated as constant over time whilst the probability of entry varied with time (Tables 1, 2). This model had a model weight of 1.0. The best-fitting model for the 2015/16 data with subadults was one where survival was estimated as constant (0.70), capture varied over time (0.06–0.50), and the probability of entry varied over time (Tables 1, 2). This model had a model weight of 1.0. The best-fitting model for the 2015/16 (8 occasions) data was one where survival was estimated as constant (0.64), capture varied over time (0.11–0.82), and the probability of entry was estimated as constant (Tables 1, 2). This model had a model weight of 0.73.
Cormack–Jolly–Seber model
The best-fitting model for the 1998/99 data was one where survival (0.77) and capture probability (0.37) were estimated as constant over time (Tables 2, 3). This model had 5.2 times as much support as the next model, which included rain in the 5-day period before each capture occasion. The best-fitting model for the 2015/16 (12 occasions) data was one where survival (0.83) was estimated as constant over time whilst capture probability varied with time (0.05–0.65) (Tables 2, 3). This model had 10.1 times as much support as the next model with capture probability constant. The best-fitting model for these 2015/16 data with subadults was one where survival (0.83) was estimated as constant over time whilst capture probability varied with time (0.05–0.64) (Tables 2, 3). This model had a model weight of 0.98. Analysis of the 2015/16 (8 occasions) data identified two top models that were equally plausible with ΔAICc between them of 1.71 (Tables 2, 3). In the top model the probability of survival (0.60) and capture (0.3) were estimated as constant whereas in the second model the probability of survival was estimated as constant but capture varied with time. The top model had 2.4 times as much support as the next model. Model averaging was used to estimate parameters.
|
Robust design
The top model was one in which survival was estimated to be constant, capture and recapture were equal and differed across two of the three primary periods (Table 4). The probability that frogs temporarily emigrated (gʹʹ) was estimated at 0.34 whilst the probability that those outside the sample area remained outside it (gʹ) was close to 1 so was fixed to 1. Survival was estimated at 0.84 ± 0.17 per month. Capture probability was estimated at 0.37 ± 0.05 in Primary Period 1 and 0.24 ± 0.06 in Primary Periods 2 and 3.
When the subadults were added, the top model was similar to that with adults only. Survival was constant, while capture and recapture were equal, and differed across two of the three primary periods (Table 4). Temporary emigration was estimated at 0.39 while the probability of frogs remaining outside the sample area was close to 1 so was fixed to 1. Survival was estimated at 0.87 ± 0.18 per month. Capture probability was estimated at 0.34 ± 0.05 in Primary Period 1 and 0.18 ± 0.04 in Primary Periods 2 and 3.
Population estimates
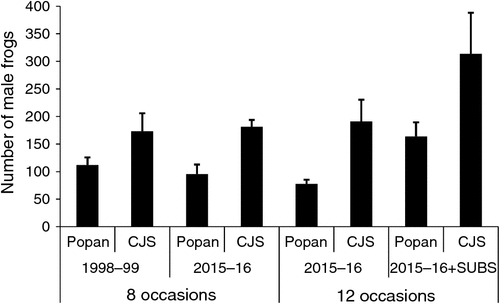
We generated population estimates from Popan and CJS models for the 1998/99 data and for three configurations of the 2015/16 data (Fig. 3). The CJS model estimates were 1.5–2.5 times higher than any of the Popan estimates. The 2015/16 Popan estimate for 12 occasions (95% CI: 62.5–92.5) showed a decline on the 1998/99 estimate (60.8–129.7). However, the 2015/16 Popan estimate for eight occasions (85.5–138.5) was similar to the 1998/99 estimate based on eight occasions. When 2015/16 data were modelled with the inclusion of a subset of subadults and compared to the 12 occasion estimate, the estimate was 1.6 and 2.1 times higher for the CJS (165.3–705.4) and Popan (112.8–214.5) models, respectively. The population estimates derived from the RD model for the primary periods in 2015/16 were: 40.2 ± 3.6 (95% CI: 36.2–51.9) (November), 35.5 ± 8.0 (25.9–60.2) (February), 26.6 ± 6.4 (19.2–46.9) (March). The population estimates derived from the RD model for the primary periods in 2015/16 with subadults were: 48.6 ± 4.8 (42.7–63.2) (November), 56.0 ± 13.9 (38.4–96.7) (February), 58.3 ± 14.4 (40.0–100.2) (March).
|
Discussion
Does the Yuraygir population show evidence of a long-term decline?
The primary aim of our study was to compare population estimates for the green and golden bell frog population at Station Creek in Yuraygir National Park at the beginning and end of a 17-year period. Because this species has shown a vulnerability to local extinction (White and Pyke 2008) we hypothesised that this may manifest itself in the form of a protracted decline in abundance before local extinction, as observed by White and Pyke (1996) for the Eastlakes population. Our population modelling revealed little difference in estimated abundance at the beginning and end of this period. We interpret this to indicate that there is no evidence of a protracted decline. We do not know how the population varied in the intervening period. One predicts there would be annual variation reflecting variation in climate (e.g. Brown and Shine 2016). On the basis of the available evidence it seems implausible that the Station Creek population could be in a long-term decline and be able to increase its abundance for a few years, before reverting to a declining trend. Multiyear population modelling of other Australian frogs indicate either: dramatic decline (Gillespie et al. 2015), a population increase (Newell et al. 2013; Quick et al. 2015) or an approximate steady-state (Phillott et al. 2013; Pickett et al. 2014).
Our population estimates represent an index of overall population size. We sampled a swamp, ephemeral ponds and 50% of a 700-m-long lagoon over 5–6 months in each period. Could subsampling the lagoon misrepresent the broader population in the two periods? That is, could habitat quality vary between year periods such that a large proportion of the population occupied our sample area in one year and a small proportion in the other year, but still produce equivalent estimates of abundance? Habitat quality did differ across years in that the ephemeral ponds did not fill in 2015/16. However, most of the frogs captured in the ephemeral ponds in 1998/99 were first tagged in the lagoon or swamp (Goldingay and Newell 2005a), indicating the mobility of the frogs and that sampling these sites was not sampling a different portion of the population. This is consistent with Hamer et al. (2008), who recorded 47% of tagged male frogs moving between waterbodies: moving a mean distance of 220 m (maximum 750 m) to and from ephemeral ponds and 140 m (maximum 1100 m) between permanent ponds. A survey of calling males across the broader lagoon in our study area in 2001 suggested that frog abundance in different portions of the lagoon was equivalent (Goldingay and Newell 2005a). If the broader population changed its pattern of habitat use in the lagoon across our two study periods due to changes in habitat quality one might expect a difference in capture probability because lower-quality habitat should produce lower persistence and therefore lower catchability but this was not the case (Popan 1998/99: 0.36 ± 0.06; 2015/16 (8 occasions): 0.32 ± 0.06, when estimated as constant across occasions). It seems implausible that bell frogs in our study area would shift their use from one end of the lagoon to the other in a differing pattern across the sample years. Therefore, we conclude that our sampling has provided a consistent index over time.
Does the population have the signature of a stable population? Our two population estimates, 17 years apart, were equivalent. In 2015/16 we captured 56 adult male frogs and 104 subadult frogs. In 1998 we captured 64 adult male frogs but no subadults. This difference may simply reflect different patterns of rain early in 2015 compared with early in 1998 but it also reflects a high level of successful reproduction in 2015, which should provide the basis of a stable population. This is a significant finding because this population is not managed in any way, unlike bell frog populations at Sydney Olympic Park (O’Meara and Darcovich 2008; Bower et al. 2013) and more recently at Kooragang Island, Newcastle (Klop-Toker et al. 2016).
Factors influencing population estimates
Our investigation has revealed several factors that may bias population modelling and preclude comparison among studies: (1) differences in survey design over time, (2) differences among population models, and (3) differences arising from data inclusions or exclusions.
Differences in survey design between our two study periods arose due to the ad hoc timing of surveys in 1998/99. We had a structured approach in 2015/16 where we followed a robust design, with secondary sampling periods embedded within primary periods. We had eight survey occasions in 1998/99 and 12 occasions in 2015/16. We analysed the 2015/16 data based on 12 occasions and also reduced the data to eight occasions to enable direct comparison. Reducing the number of survey occasions did not produce lower population estimates. For the Popan model it produced a higher estimate with a larger confidence interval. Differences in survey design among studies of bell frogs preclude comparison of parameter estimates. Pickett et al. (2014) employed three survey occasions per year for one area and 2–6 secondary periods within two primary periods in two other areas. Hamer and Mahony (2007) employed two secondary periods with seven primary periods. Heard et al. (2012) employed 8 and 10 occasions to survey the growling grass frog (Litoria raniformis) across two years. The influence of survey design should be investigated using simulations (e.g. Lanier et al. 2016).
We explored differences arising from different population models. Two previous studies have employed population modelling to estimate population size of the green and golden bell frog. Hamer and Mahony (2007) relied on the CJS model, whereas Pickett et al. (2014) used the RD model for two wetland complexes and the CJS model for a third. We found that the CJS model estimated population size at least 1.5 times higher (with higher standard errors) than the estimate of the Popan model. The CJS estimate for the 2015/16 data without subadults was 2.5 times higher than the equivalent Popan estimate. The CJS and Popan models estimated the population as the total number in the study area across the whole study period. In contrast, the RD model provides individual estimates for the primary sampling periods. The Popan model can also provide estimates for survey occasions. The Popan estimate for March (25.4 ± 5.4 individuals) was equivalent to the RD estimate (26.6 ± 6.4 individuals). The difference in how these models estimate abundance highlights the need for caution in how they are applied and compared.
Although the critical requirement for population monitoring is that a standardised approach be used over time (Richards et al. 1994; Lewis and Goldingay 2005; Richards and Alford 2005; Gillespie et al. 2015), it is worth asking, what is the most biologically relevant approach to characterise a bell frog population? Due to the high mobility of bell frog individuals (Pyke and White 2001; Goldingay and Newell 2005a; Hamer et al. 2008; Hamer and Mahony 2010), it may be most relevant to estimate the adult male population across a breeding season as we have attempted to do in this study. This follows the approach of Hamer and Mahony (2007) in which population size was estimated based on the total number of males captured in a breeding season. Such estimates may not be directly comparable to those based on primary periods such as by Pickett et al. (2014). Our estimates for the March 2016 primary period from the RD, CJS and Popan models were at least 50% lower than the estimate for the whole breeding season.
The differences among models suggest that comparisons cannot be made across studies that use different models. Hamer and Mahony (2007) estimated the male bell frog population on Kooragang Island to be 1995 ± 315 (s.e.) in one year using the CJS model. Pickett et al. (2014) estimated the bell frog population (males and females) with the RD model in primary periods for two separate precincts at Sydney Olympic Park. Population estimates in one precinct ranged between 103 and 249 over a 6-year period (95% CI up to ~±50), and in another precinct it ranged from 87 to 171 over a 3-year period (95% CI up to ~±20). Obviously, male-only estimates cannot be compared with male+female estimates.
The other factor we explored that may influence the population estimate was whether subadult individuals were included in the modelling. Both Hamer and Mahony (2007) and Pickett et al. (2014) included individuals in their modelling below a recognised size threshold for adult frogs. Pyke and White (2001) indicated a threshold of 50 mm SVL whereas Goldingay and Newell (2005a, 2005b) identified a value of 51–56 mm. The concern with this is that these individuals may exhibit behaviour and survival different to that of adult frogs, which violates key assumptions of the population modelling if they are not modelled separately, that each frog has an equal probability of being captured and an equal probability of survival (Hamer and Mahony 2007; Lampo et al. 2012). Subadult frogs are likely to have different probabilities of apparent survival and are not part of the breeding population. Indeed, Hamer and Mahony (2007) stated that they were unable to use age-structure in their modelling due to low recapture rates in frogs <6 months of age. For conservation purposes, it will be of greatest interest to characterise different populations based on the sizes of adult populations. This cannot be done if population estimates include subadult individuals. We investigated the influence of including subadult individuals in our modelling. The number of subadults represented the same proportion of the total number of individuals as that reported by Hamer and Mahony (2007). We found the inclusion of subadults led to estimates 1.6–2.1 times higher than when based on adults alone.
Apparent survival
We estimated apparent survival probabilities of 0.60–0.87 per month. Hamer and Mahony (2007) estimated apparent survival at 0.76 per 2-week period (i.e. 0.58 per month). Our values, based on recapture over the breeding season, cannot be converted to annual values because it is unlikely survival would be equivalent in and outside the breeding period. Pickett et al. (2014) were able to provide annual estimates that ranged from 0.06 to 0.44, but this included males and females, and subadults. Heard et al. (2012) estimated active season (October to March) survival probabilities in the growling grass frog of 0.04–0.14. They suggested that these apparent survival values may be underestimated due to emigration. That is almost certainly the case with our estimates at Station Creek. The RD model estimated the probability of temporary emigration between primary periods at 0.34. We sampled ~50% of our study area, which has probably led to tagged individuals being unavailable for recapture. Our earlier study detected individuals moving 300–500 m between waterbodies at Station Creek after rain (Goldingay and Newell 2005a). Christy (2001) recorded movements between captures of up to 630 m while Hamer et al. (2008) recorded movements up to 1100 m. The extent that bell frogs may have moved away from the edge of the lagoon where our surveys were focussed or to the unsurveyed perimeter of the lagoon, or even away from the lagoon, is unknown. Estimates of apparent survival have probably been influenced by emigration in the study of Pickett et al. (2014), wich also employed subsampling of wetland habitat.
Conclusions
Continuing threats to populations of the green and golden bell frog (White and Pyke 2008; Mahony et al. 2013) highlight the need for long-term monitoring. Our estimates of population abundance were equivalent in two periods separated by 17 years. From this we infer that this population is not showing long-term decline. We recommend monitoring at intervals of 3–5 years for continued understanding of population trends (e.g. Quick et al. 2015). Whilst annual monitoring might be preferred by some researchers we recognise that this is unrealistic and not cost-effective. Our initial study in 1998/99 and subsequent study in 2015/16 were self-funded. Funds were not available for detailed surveys between those years. More generally, if active management of a green and golden bell frog population is taking place, or there is suspicion that a population is subject to decline, then annual monitoring would be appropriate. This is a form of adaptive monitoring (Lindenmayer and Likens 2009).
Consideration of the factors that may bias population estimates are of central interest because estimates may be used to rank conservation priorities. We show the problems that might arise when attempting to compare population estimates among studies when different estimators are used and when data are compiled in different ways. Population indices must be based on equivalent data over time for long-term monitoring to be reliable. Recognition of the factors that may bias estimates is important. Although we focus on population size it is population trend that is of greatest importance. Populations such as our study population at Station Creek that are able to persist over long time frames without active management are of utmost conservation value to the long-term survival of a species.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
We thank Darren McHugh, Sergio Jacomy, Jenine Dempster and Sally McMahon for assistance with field surveys. The comments of two anonymous referees greatly improved our manuscript.
References
Berger, L., Speare, R., Daszak, P., Green, D. E., Cunningham, A. A., Goggin, C. L., Slocombe, R., Ragan, M. A., Hyatt, A. D., McDonald, K. R., Hines, H. B., Lips, K. R., Marantelli, G., and Parkes, H. (1998). Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proceedings of the National Academy of Sciences of the United States of America 95, 9031–9036.| Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXkvFaltbc%3D&md5=14ce13b2554e46c7df865a156184e323CAS |
Bower, D. S., Stockwell, M. P., Pollard, C. J., Pickett, E. J., Garnham, J. I., Clulow, J., and Mahony, M. J. (2013). Life stage specific variation in the occupancy of ponds by Litoria aurea, a threatened amphibian. Austral Ecology 38, 543–547.
| Life stage specific variation in the occupancy of ponds by Litoria aurea, a threatened amphibian.Crossref | GoogleScholarGoogle Scholar |
Brown, G. P., and Shine, R. (2016). Frogs in the spotlight: a 16-year survey of native frogs and invasive toads on a floodplain in tropical Australia. Ecology and Evolution 6, 4445–4457.
| Frogs in the spotlight: a 16-year survey of native frogs and invasive toads on a floodplain in tropical Australia.Crossref | GoogleScholarGoogle Scholar |
Burnham, K. P., and Anderson, D. R. (2004). Multimodel inference: understanding AIC and BIC in model selection. Sociological Methods & Research 33, 261–304.
| Multimodel inference: understanding AIC and BIC in model selection.Crossref | GoogleScholarGoogle Scholar |
Christy, M. T. (2001). The ecology and conservation biology of the green and golden bell frog Litoria aurea (Lesson 1829) (Anura: Hylidae). Ph.D. Thesis, University of Sydney.
Darcovich, K., and O’Meara, J. (2008). An Olympic legacy: green and golden bell frog conservation at Sydney Olympic Park 1993–2006. Australian Zoologist 34, 236–248.
| An Olympic legacy: green and golden bell frog conservation at Sydney Olympic Park 1993–2006.Crossref | GoogleScholarGoogle Scholar |
Gillespie, G. R., Hunter, D., Berger, L., and Marantelli, G. (2015). Rapid decline and extinction of a montane frog population in southern Australia follows detection of the amphibian pathogen Batrachochytrium dendrobatidis. Animal Conservation 18, 295–302.
| Rapid decline and extinction of a montane frog population in southern Australia follows detection of the amphibian pathogen Batrachochytrium dendrobatidis.Crossref | GoogleScholarGoogle Scholar |
Goldingay, R. L. (2008). Conservation of the green and golden bell frog: what contribution has ecological research made since 1996? Australian Zoologist 34, 334–349.
| Conservation of the green and golden bell frog: what contribution has ecological research made since 1996?Crossref | GoogleScholarGoogle Scholar |
Goldingay, R., and Lewis, B. (1999). Development of a conservation strategy for the green and golden bell frog in the Illawarra Region of NSW. Australian Zoologist 31, 376–387.
| Development of a conservation strategy for the green and golden bell frog in the Illawarra Region of NSW.Crossref | GoogleScholarGoogle Scholar |
Goldingay, R. L., and Newell, D. A. (2005a). Aspects of the population ecology of the green and golden bell frog at the northern end of its range. Australian Zoologist 33, 49–59.
| Aspects of the population ecology of the green and golden bell frog at the northern end of its range.Crossref | GoogleScholarGoogle Scholar |
Goldingay, R. L., and Newell, D. A. (2005b). Population estimation of the green and golden bell frog at Port Kembla. Australian Zoologist 33, 210–216.
| Population estimation of the green and golden bell frog at Port Kembla.Crossref | GoogleScholarGoogle Scholar |
Hamer, A. J., and Mahony, M. J. (2007). Life history of an endangered amphibian challenges the declining species paradigm. Australian Journal of Zoology 55, 79–88.
| Life history of an endangered amphibian challenges the declining species paradigm.Crossref | GoogleScholarGoogle Scholar |
Hamer, A. J., and Mahony, M. J. (2010). Rapid turnover in site occupancy of a pond-breeding frog demonstrates the need for landscape-level management. Wetlands 30, 287–299.
| Rapid turnover in site occupancy of a pond-breeding frog demonstrates the need for landscape-level management.Crossref | GoogleScholarGoogle Scholar |
Hamer, A. J., Lane, S. J., and Mahony, M. J. (2008). Movement patterns of adult green and golden bell frogs Litoria aurea and the implications for conservation management. Journal of Herpetology 42, 397–407.
| Movement patterns of adult green and golden bell frogs Litoria aurea and the implications for conservation management.Crossref | GoogleScholarGoogle Scholar |
Hamer, A. J., Lane, S. J., and Mahony, M. J. (2010). Using probabilistic models to investigate the disappearance of a widespread frog-species complex in high-altitude regions of south-eastern Australia. Animal Conservation 13, 275–285.
| Using probabilistic models to investigate the disappearance of a widespread frog-species complex in high-altitude regions of south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Heard, G. W., Scroggie, M. P., and Malone, B. S. (2012). The life history and decline of the threatened Australian frog, Litoria raniformis. Austral Ecology 37, 276–284.
Hero, J.-M., Morrison, C., Gillespie, G., Roberts, J. D., Newell, D., Meyer, E., McDonald, K., Lemckert, F., Mahony, M., Osborne, W., Hines, H., Richards, S., Hoskin, C., Clarke, J., Doak, N., and Shoo, L. (2006). Overview of the conservation status of Australian frogs. Pacific Conservation Biology 12, 313–320.
| Overview of the conservation status of Australian frogs.Crossref | GoogleScholarGoogle Scholar |
Hunter, D. A., Speare, R., Marantelli, G., Mendez, D., Pietsch, R., and Osborne, W. (2010). Presence of the amphibian chytrid fungus Batrachochytrium dendrobatidis in threatened corroboree frog populations in the Australian Alps. Diseases of Aquatic Organisms 92, 209–216.
| Presence of the amphibian chytrid fungus Batrachochytrium dendrobatidis in threatened corroboree frog populations in the Australian Alps.Crossref | GoogleScholarGoogle Scholar |
IUCN (2016). The IUCN Red List of Threatened Species. Available at: www.iucnredlist.org [accessed 7 November 2016].
Klop-Toker, K., Valdez, J., Stockwell, M., Fardell, L., Clulow, S., Clulow, J., and Mahoney, M. (2016). We made your bed, why won’t you lie in it? Food availability and disease may affect reproductive output of reintroduced frogs. PLoS One 11, e0159143.
| We made your bed, why won’t you lie in it? Food availability and disease may affect reproductive output of reintroduced frogs.Crossref | GoogleScholarGoogle Scholar |
Kriger, K. M., and Hero, J. M. (2007). Large-scale seasonal variation in the prevalence and severity of chytridiomycosis. Journal of Zoology 271, 352–359.
Lampo, M., Celsa, S. J., Rodríguez‐Contreras, A., Rojas‐Runjaic, F., and García, C. Z. (2012). High turnover rates in remnant populations of the harlequin frog Atelopus cruciger (Bufonidae): low risk of extinction? Biotropica 44, 420–426.
| High turnover rates in remnant populations of the harlequin frog Atelopus cruciger (Bufonidae): low risk of extinction?Crossref | GoogleScholarGoogle Scholar |
Lanier, W. E., Bailey, L. L., and Muths, E. (2016). Integrating biology, field logistics, and simulations to optimize parameter estimation for imperilled species. Ecological Modelling 335, 16–23.
| Integrating biology, field logistics, and simulations to optimize parameter estimation for imperilled species.Crossref | GoogleScholarGoogle Scholar |
Lewis, B., and Goldingay, R. (1999). Preliminary assessment of the status of the green and golden bell frog in north-eastern New South Wales. In ‘Declines and Disappearances of Australian Frogs’. (Ed. A. Campbell.) pp. 94–98. (Environment Australia: Canberra.)
Lewis, B. D., and Goldingay, R. L. (2005). Population monitoring of the vulnerable wallum sedge frog (Litoria olongburensis) in north-eastern New South Wales. Australian Journal of Zoology 53, 185–194.
| Population monitoring of the vulnerable wallum sedge frog (Litoria olongburensis) in north-eastern New South Wales.Crossref | GoogleScholarGoogle Scholar |
Lindenmayer, D. B., and Likens, G. E. (2009). Adaptive monitoring: a new paradigm for long-term research and monitoring. Trends in Ecology & Evolution 24, 482–486.
| Adaptive monitoring: a new paradigm for long-term research and monitoring.Crossref | GoogleScholarGoogle Scholar |
Mahony, M. J., Hamer, A. J., Pickett, E. J., McKenzie, D. J., Stockwell, M. P., Garnham, J. I., Keely, C. C., Deboo, M. L., O’Meara, J., and Pollard, C. J. (2013). Identifying conservation and research priorities in the face of uncertainty: a review of the threatened bell frog complex in eastern Australia. Herpetological Conservation and Biology 8, 519–538.
Newell, D. A., Goldingay, R. L., and Brooks, L. O. (2013). Population recovery following decline in an endangered stream-breeding frog (Mixophyes fleayi) from subtropical Australia. PLoS One 8, e58559.
| Population recovery following decline in an endangered stream-breeding frog (Mixophyes fleayi) from subtropical Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXks1Wns7c%3D&md5=d7a2ea4be88dce6dd5c39900e059da5eCAS |
O’Meara, J., and Darcovich, K. (2008). Gambusia control through the manipulation of water levels in Narawang Wetland, Sydney Olympic Park 2003–2005. Australian Zoologist 34, 285–290.
| Gambusia control through the manipulation of water levels in Narawang Wetland, Sydney Olympic Park 2003–2005.Crossref | GoogleScholarGoogle Scholar |
O’Meara, J., and Darcovich, K. (2015). Twelve years on: ecological restoration and rehabilitation at Sydney Olympic Park. Ecological Management & Restoration 16, 14–28.
| Twelve years on: ecological restoration and rehabilitation at Sydney Olympic Park.Crossref | GoogleScholarGoogle Scholar |
OEH (2013). Saving our species technical report. (Office of Environment & Heritage: Sydney.)
OEH (2014). Monitoring, evaluation and reporting framework: approach to conservation projects for site-managed species. (Office of Environment & Heritage: Sydney.)
Osborne, W., Patmore, S. R., Hunter, D., and Pietsch, R. (2008). Preliminary observations on a highly-restricted tableland population of green and golden bell frogs on the Upper Molonglo River, NSW. Australian Zoologist 34, 271–284.
| Preliminary observations on a highly-restricted tableland population of green and golden bell frogs on the Upper Molonglo River, NSW.Crossref | GoogleScholarGoogle Scholar |
Penman, T. D., Muir, G. W., Magerey, E. R., and Burns, E. L. (2008). Impact of a chytrid-related mortality event on a population of the green and golden bell frog Litoria aurea. Australian Zoologist 34, 314–318.
| Impact of a chytrid-related mortality event on a population of the green and golden bell frog Litoria aurea.Crossref | GoogleScholarGoogle Scholar |
Phillott, A. D., Grogan, L. F., Cashins, S. D., McDonald, K. R., Berger, L., and Skerratt, L. F. (2013). Chytridiomycosis and seasonal mortality of tropical stream-associated frogs 15 years after introduction of Batrachochytrium dendrobatidis. Conservation Biology 27, 1058–1068.
| Chytridiomycosis and seasonal mortality of tropical stream-associated frogs 15 years after introduction of Batrachochytrium dendrobatidis.Crossref | GoogleScholarGoogle Scholar |
Pickett, E. J., Stockwell, M. P., Bower, D. S., Pollard, C. J., Garnham, J. I., Clulow, J., and Mahony, M. J. (2014). Six-year demographic study reveals threat of stochastic extinction for remnant populations of a threatened amphibian. Austral Ecology 39, 244–253.
| Six-year demographic study reveals threat of stochastic extinction for remnant populations of a threatened amphibian.Crossref | GoogleScholarGoogle Scholar |
Pike, D. A., Pizzatto, L., Pike, B. A., and Shine, R. (2008). Estimating survival rates of uncatchable animals: the myth of high juvenile mortality in reptiles. Ecology 89, 607–611.
| Estimating survival rates of uncatchable animals: the myth of high juvenile mortality in reptiles.Crossref | GoogleScholarGoogle Scholar |
Pollock, K. H. (1982). A capture–recapture design robust to unequal probability of capture. Journal of Wildlife Management 46, 752–757.
| A capture–recapture design robust to unequal probability of capture.Crossref | GoogleScholarGoogle Scholar |
Pyke, G. H., and White, A. W. (2001). A review of the biology of the green and golden bell frog Litoria aurea. Australian Zoologist 31, 563–598.
| A review of the biology of the green and golden bell frog Litoria aurea.Crossref | GoogleScholarGoogle Scholar |
Quick, G., Goldingay, R. L., Parkyn, J., and Newell, D. A. (2015). Population stability in the endangered Fleay’s barred frog (Mixophyes fleayi) and a program for long-term monitoring. Australian Journal of Zoology 63, 214–219.
| Population stability in the endangered Fleay’s barred frog (Mixophyes fleayi) and a program for long-term monitoring.Crossref | GoogleScholarGoogle Scholar |
Richards, S. J., and Alford, R. A. (2005). Structure and dynamics of a rainforest frog (Litoria genimaculata) population in northern Queensland. Australian Journal of Zoology 53, 229–236.
| Structure and dynamics of a rainforest frog (Litoria genimaculata) population in northern Queensland.Crossref | GoogleScholarGoogle Scholar |
Richards, S. J., McDonald, K. R., and Alford, R. A. (1994). Declines in populations of Australia’s endemic tropical rainforest frogs. Pacific Conservation Biology 1, 66–77.
| Declines in populations of Australia’s endemic tropical rainforest frogs.Crossref | GoogleScholarGoogle Scholar |
Scheele, B. C., Guarino, F., Osborne, W., Hunter, D. A., Skerratt, L. F., and Driscoll, D. A. (2014). Decline and re-expansion of an amphibian with high prevalence of chytrid fungus. Biological Conservation 170, 86–91.
| Decline and re-expansion of an amphibian with high prevalence of chytrid fungus.Crossref | GoogleScholarGoogle Scholar |
Scheele, B. C., Skerratt, L. F., Grogan, L. F., Hunter, D. A., Clemann, N., McFadden, M., Newell, D., Hoskin, C. J., Gillespie, G. R., Heard, G. W., Brannelly, L., Roberts, A. A., and Berger, L. (2017). After the epidemic: ongoing declines, stabilizations and recoveries in amphibians afflicted by chytridiomycosis. Biological Conservation 206, 37–46.
| After the epidemic: ongoing declines, stabilizations and recoveries in amphibians afflicted by chytridiomycosis.Crossref | GoogleScholarGoogle Scholar |
Schloegel, L. M., Hero, J. M., Berger, L., Speare, R., McDonald, K., and Daszak, P. (2006). The decline of the sharp-snouted day frog (Taudactylus acutirostris): the first documented case of extinction by infection in a free-ranging wildlife species? EcoHealth 3, 35–40.
| The decline of the sharp-snouted day frog (Taudactylus acutirostris): the first documented case of extinction by infection in a free-ranging wildlife species?Crossref | GoogleScholarGoogle Scholar |
Schwarz, C. J., and Arnason, A. N. (1996). A general methodology for the analysis of capture–recapture experiments in open populations. Biometrics 52, 860–873.
| A general methodology for the analysis of capture–recapture experiments in open populations.Crossref | GoogleScholarGoogle Scholar |
Skerratt, L. F., Berger, L., Clemann, N., Hunter, D. A., Marantelli, G., Newell, D. A., Philips, A., McFadden, M., Hines, H. B., Scheele, B. C., Brannelly, L. A., Speare, R., Versteegen, S., Cashins, S. D., and West, M. (2016). Priorities for management of chytridiomycosis in Australia: saving frogs from extinction. Wildlife Research 43, 105–120.
| Priorities for management of chytridiomycosis in Australia: saving frogs from extinction.Crossref | GoogleScholarGoogle Scholar |
Stockwell, M. P., Clulow, S., Clulow, J., and Mahony, M. (2008). The impact of the amphibian chytrid fungus Batrachochytrium dendrobatidis on a green and golden bell frog Litoria aurea reintroduction program at the Hunter Wetlands Centre Australia in the Hunter Region of New South Wales. Australian Zoologist 34, 379–386.
| The impact of the amphibian chytrid fungus Batrachochytrium dendrobatidis on a green and golden bell frog Litoria aurea reintroduction program at the Hunter Wetlands Centre Australia in the Hunter Region of New South Wales.Crossref | GoogleScholarGoogle Scholar |
Stockwell, M. P., Clulow, J., and Mahony, M. J. (2010). Host species determines whether infection load increases beyond disease-causing thresholds following exposure to the amphibian chytrid fungus. Animal Conservation 13, 62–71.
| Host species determines whether infection load increases beyond disease-causing thresholds following exposure to the amphibian chytrid fungus.Crossref | GoogleScholarGoogle Scholar |
Stockwell, M. P., Bower, D. S., Bainbridge, L., Clulow, J., and Mahony, M. J. (2015). Island provides a pathogen refuge within climatically suitable area. Biodiversity and Conservation 24, 2583–2592.
| Island provides a pathogen refuge within climatically suitable area.Crossref | GoogleScholarGoogle Scholar |
White, A. W., and Pyke, G. H. (1996). Distribution and conservation status of the green and golden bell frog Litoria aurea in New South Wales. Australian Zoologist 30, 177–189.
| Distribution and conservation status of the green and golden bell frog Litoria aurea in New South Wales.Crossref | GoogleScholarGoogle Scholar |
White, A. W., and Pyke, G. H. (1999). Past distribution of the bell frogs Litoria aurea and Litoria castanea in the Bathurst–Orange district of New South Wales. Herpetofauna 29, 1–9.
White, A. W., and Pyke, G. H. (2008). Green and golden bell frogs in New South Wales: current status and future prospects. Australian Zoologist 34, 319–333.
| Green and golden bell frogs in New South Wales: current status and future prospects.Crossref | GoogleScholarGoogle Scholar |
White, G. C., and Burnham, K. P. (1999). Program MARK: survival estimation from populations of marked animals. Bird Study 46, S120–S139.
| Program MARK: survival estimation from populations of marked animals.Crossref | GoogleScholarGoogle Scholar |