Charophytes of Australia’s Northern Territory – II. Tribe Nitelleae
Michelle T. Casanova A B C E * and Kenneth G. Karol
A B C E * and Kenneth G. Karol  D
D
A Royal Botanic Gardens, Melbourne, Birdwood Avenue, South Yarra, Vic. 3141, Australia.
B The Future Regions Research Centre, Institute of Innovation, Science and Sustainability, Federation University Australia, Mount Helen, PO Box 663, Ballarat, Vic. 3350, Australia.
C The Natural History Museum, Cromwell Road, London, UK.
D The Lewis B. and Dorothy Cullman Program for Molecular Systematics, New York Botanical Garden, Bronx, NY, USA.
E Present address: 273 Casanova Road, Westmere, Vic. 3351, Australia.
Australian Systematic Botany 36(4) 322-353 https://doi.org/10.1071/SB22029
Submitted: 27 October 2022 Accepted: 21 July 2023 Published: 18 August 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
This study of Northern Territory charophytes deals with the tribe Nitelleae in family Characeae. We recognise 16 species of Nitella for the Territory. The list includes seven previously described species (Nitella belangeri, N. biformis, N. congesta, N. heterophylla, N. micklei, N. myriotricha and N. tumulosa, of which N. belangeri and N. tumulosa are newly recorded for the Australian flora), and nine newly described species (N. acanthospora, N. boreali-australis, N. crocodylus, N. limosa, N. martinii, N. nitida, N. oollooensis, N. silicea and N. townsendii). Of the five previously reported Nitella species in the Northern Territory (N. hyalina, N. myriotricha, N. penicillata, N. pseudoflabellata and N. subtilissima), only N. myriotricha is recorded in this study, because the other records were based on erroneous identifications or localities. All Nitella species described here can be distinguished on the basis of their morphology and reproductive arrangement. Keys, illustrations and descriptions of all the species are provided.
Keywords: charophyte, morphology, Nitella, Northern Territory, oospore, sp. nov., taxonomy, wetlands.
Introduction
Charophytes are water-plants, green macroalgae in family Characeae; they occur in wetlands (lagoons, waterholes, claypans) and riparian areas (rivers, streams, springs) in northern Australia and world-wide, including the Antarctic continent (Schubert et al. 2018). Their growth habit can be diffuse, as in species of Nitella C.Agardh, compact, as in many species of Chara L., slimy when there is mucus on the reproductive whorls, or stony when calcium carbonate is deposited. They can grow in deep or shallow water, when sufficient light is available (Casanova 2007), in turbid water (Casanova and Porter 2013), and sometimes exist emergent at the edges of rivers, in a thin film of water (Casanova and Nairn 2015). They have been called ‘ecosystem engineers and permanent pioneers’ as a consequence of their multiple and species-specific roles in wetland ecosystems (Schubert et al. 2018). Charophytes often grow in mixed species communities, although single species ‘beds’ or ‘meadows’ can develop under stable, low-nutrient conditions (Casanova 2009).
Although members of Nitelleae were recorded or described for northern Australia in early studies (Nitella conglobata A.Braun nom. Illeg. [=N. lhotzkyi (A.Braun) A.Braun], N. hyalina (DC.) C.Agardh and N. sonderi A.Braun (Mueller 1889)), the most significant study on northern Australian charophytes was completed by G. O. Allen (Groves and Allen 1935). That study was exclusively on Queensland charophytes, and 22 species of Nitella were recognised. Zaneveld (1940) provided the first comprehensive study on tropical Asian species, some of which also occur in Australia, and his results contributed to clarification of species concepts in the Nitelleae. The next significant work on northern Australian Characeae was initiated when Ray Specht visited Arnhem Land and Groote Eylandt during the American–Australian expedition in 1948 (Wood 1958). He sent his Characeae material to R. D. Wood in USA, who identified the Nitella specimens as N. sonderi (following a determination by I. L. Ophel, see Casanova 2007) and N. pseudoflabellata A.Braun, following the works of Braun (in Nordstedt 1883) and Zaneveld (1940). Wood (1962) and Wood and Imahori (1965) later recorded the first listed specimen as N. pseudoflabellata subsp. pseudoflabellata var. imperialis f. sonderi (A.Braun) R.D.Wood. Wood and Imahori (1965) also included Nitella congesta (R.Br.) A.Braun for the Northern Territory, on the basis of an erroneous interpretation of locality data in Nordstedt (1883). However, N. congesta was not included for the Northern Territory in Wood (1971).
Wood attempted a more thorough compilation after collecting in the vicinity of Darwin with Nancy Eddy (Wood 1971). In that treatment, he listed five Nitella species for the Northern Territory (N. hyalina, N. myriotricha A.Braun ex Kütz., N. penicillata A.Braun, N. pseudoflabellata and N. subtilissima A.Braun). No specimen was cited for N. subtilissima in Wood (1971), nor in Wood and Imahori (1965). Two of the other species were cited in error, as the specimen of N. hyalina was collected in Cawarral, Queensland (cited as ‘Carawal’ in Wood 1971), and N. penicillata was collected from the Orara River in New South Wales. Wood (1971) allocated specimens to various subspecies, varieties or forms of those taxa.
Since the 1970s, more comprehensive collections have been made, particularly by Peter Dostine, Peter Latz, Julia Schult and Simon Townsend, and on Bush Blitz expeditions, greatly increasing the number of specimens and allowing a more comprehensive delineation of known and new species for the Northern Territory.
Northern Territory charophytes were included in works by García and Chivas (2006) and (to genus) in Cowie et al. (2000). Since then, a study on the ecology of the Daly River (Schult et al. 2007) included a report of charophytes collected there, and elsewhere in the Northern Territory, documenting 13 species, some of which were undescribed.
The taxonomy of charophytes has been, in the past, complicated by subdivision of species into subspecies, varieties and forms by various authors. The genus Nitella was subdivided (Wood 1962) into subgenera (Nitella subg. Hyella R.D.Wood, N. subg. Nitella and N. subg. Tieffallenia R.D.Wood), each of which was divided into several sections containing one to several species. The species were further divided into subspecies, varieties and forms. This approach has been found to be not only difficult to use but also erroneous in the amalgamation of previously described species, and the phylogenetic structure implied by the hierarchy; see Casanova and Karol (2014). Because the classification proposed by previous workers, in both organisation and arrangement, has been shown to be unnatural, in this treatment, we present an alphabetical arrangement of species. This species concept is based on phylogenetic and morphological evidence (Sakayama et al. 2002, 2005, 2006; Karol 2004; Casanova 2005, 2009, 2013; Casanova and Karol 2014).
This is the second of two related papers concerning the Characeae of the Northern Territory. Tribe Nitelleae comprises members of the genera Nitella C.Agardh, Sphaerochara Mädler and Tolypella (A.Braun) A.Braun; however, Sphaerochara and Tolypella have not been collected in the Northern Territory. Members of tribe Chareae that occur in the Northern Territory (Chara L., Lamprothamnium J.Groves and Lychnothamnus (Rupr.) Leonh.) are dealt with in a previous paper (Casanova and Karol 2023).
Materials and methods
Approximately 300 fresh, pressed and spirit-preserved specimens collected in the Northern Territory, deposited in Australian and overseas herbaria and several private collections, were examined for the present study, including type material. In general, each specimen was allocated a letter–number combination (e.g. p###, r###, t###, v###), so that all data obtained from individual specimens including measurements, photographs, chromosome counts and scanning electron micrographs could be related back to the specimen. Initial examination involved measurements (size, number) directly from the specimen (e.g. length of branchlets, number of furcations) and with the use of a microscope (e.g. axis and branchlet diameter, number and morphology of dactyls and dactyl end-cells, arrangement of gametangia). Oospore features were examined and measured with the aid of light microscopy and scanning electron microscopy (SEM).
Where possible and appropriate, oospores were removed from the herbarium specimens or obtained from live material. Approximately 50% of the specimens examined had oospores. They were prepared for SEM examination by cleaning, if required, with a detergent solution, using a modification of the methods in Crawford et al. (2001). Sometimes the enveloping cells were removed by hand, with fine needles. As a general guide, if there were more than eight oospores on a specimen, up to three oospores would be used. If there were five to eight oospores present, then no more than two were removed. If there were three or four oospores present, then one was removed. If fewer than three oospores were present, then an oospore was removed only in exceptional circumstances. For type specimens, very old material, or specimens with a single oospore available for examination, the oospores were handled with great care, and with minimal manipulation. Oospores were removed from the SEM stubs after microscopy and stored in alcohol and deposited with the specimen for possible future examination.
Results
This paper deals only with the Nitelleae, and only the genus Nitella has been found to occur in the Northern Territory. The genera Sphaerochara and Tolypella are not expected to occur in northern Australia because they are generally restricted to >26° latitude (although there are exceptions in the northern hemisphere: see Ramdani et al. 2001; Cirujano Bracamonte et al. 2013; Pérez et al. 2014).
Nitella
Nitella C.Agardh, Syst. Alg. xxvii (1824)
Monoecious or dioecious. Plant axis and branchlets ecorticate. Stipulodes absent; branchlets in 1 whorl, or more than 1 whorl, per node, ecorticate; primary segments usually distinctly forked into 2–8 secondary segments, or further furcate. Terminal end segments (dactyls) 1–8 cells long. Gametangia arranged at the branchlet nodes, rarely at the axial nodes; antheridia terminal and oosporangia lateral. Helical cells of the oosporangium terminated by a coronula of 10 cells, in 2 rows of 5. Oospores usually flattened spheres or ovals, with 1–3 basal-cell impressions.
Type: Nitella opaca (C.Agardh ex Bruzelius) C.Agardh.
Oospores are a reliable morphological means of identifying species of Nitella. A comparison of specimen oospores with the pictures provided in the illustrations can provide definitive identification. In the absence of oospores (or a microscope), the following key can assist in distinguishing among species. A good-quality hand-lens is necessary to see features of the dactyls and whorls in most species.
| 1. | Plants monoecious...2 Plants dioecious...5 |
| 2. | Branchlets once-furcate; dactyls consisting of a single cell...N. belangeri Branchlets 2 or 3× furcate; dactyls consisting of 2 cells...3 |
| 3. | Axes narrow, 0.4–0.5 mm in diameter; plants less than 15 cm high; dactyls long...4 Axes ~1 mm in diameter; plants up to 25 cm high; dactyls very short (brachydactylous)...N. tumulosa |
| 4. | Plants unevenly 2(–3)× furcate; sterile branchlets ~5 mm long; end-cells long-conical...N. townsendii Plants evenly 3(–4)× furcate; sterile branchlets up to ~10 mm long; end-cells shortly conical...N. boreali-australis |
| 5. | Branchlets in a single whorl of 6–8 at a node...8 Branchlets in more than 1 whorl, with more than 10 (up to ~40) branchlets arising at the nodes...6 |
| 6. | Branchlets in 2 whorls, up to ~10 in the shorter (secondary) whorl...7 Branchlets in 3 whorls, or so many branchlets as to be difficult to count...N. congesta |
| 7. | Plants up to 10 cm high; branchlets up to 5 mm long; primary branchlet whorl 1–2× furcate...N. biformis Plants up to 40 cm high; branchlets up to 10 mm long; primary branchlet whorl 2–3× furcate...N. heterophylla |
| 8. | Branchlets 2 or 3× furcate; end-cells conical and acute...10 Branchlets 3 or more× furcate; end-cells long and obtuse...9 |
| 9. | Branchlet segments at all fucations 4–7; fertile whorls dispersed, with long primary segments...N. limosa Branchlet segments at all furcations 2–5; fertile whorls compact, with short primary segments...N. myriotricha |
| 10. | Plants less than 10 cm high; in arid- and semi-arid-zone waters...11 Plants greater than 10 cm high; in wet–dry tropics...12 |
| 11. | Dactyls notably wider than the branchlet segments (inflated), tapering towards the end-cell...N. micklei Dactyls similar to branchlet segments; end-cell an obtuse bristle on the branchlet tips...N. acanthospora |
| 12. | Primary branchlet segment >60% of branchlet length (the remaining segments make up little brushes on the tips) ...13 Primary branchlet segment up to 50% of the total branchlet length...15 |
| 13. | Plants with mucus; 6 branchlets in a whorl...N. crocodylus Plants without mucus; 6–8 branchlets in a whorl...14 |
| 14. | Branchlets without a central (percurrent) secondary segment...N. nitida Branchlets with a central (percurrent) secondary segment...N. oollooensis |
| 15. | Axes wiry and tangled, gametangia at the branchlet tips...N. martinii Axes soft and flabellate...N. silicea |
Nitella acanthospora Casanova & Karol, sp. nov.
Type: Nardoo Lake, 35 km south-east of The Granites, Tanami Desert, 16 Aug. 2001, P.K.Latz 17988 (holo: DNA!).
Dioecious. Plants lightly calcified, with tiny, elongate homeoclemous fertile whorls (Fig. 1a). Axes up to 200 μm wide; internodes upwards of 5 mm long, longer than the branchlet whorls. Fertile branchlets 6 or 7 in a whorl; whorls up to 7 mm in diameter, decreasing in diameter progressively up the axis, 1 or 2× furcate; primary segments up to 1 mm long; secondary segments 3 or 4, of which 1–3 are dactyls, up to 1 mm long, usually 1 of the segments longer and again furcate into 3 or 4 dactyls (Fig. 1b). Sterile branchlets longer and more evenly furcate, up to 5 mm long, 2 or 3× furcate; primary segments 2–4 mm long; secondary segments up to 1 mm long, furcate into 1–5 segments, of which 1–3 are dactyls, the remainder up to 1 mm long and again furcate into 2–4 dactyls (Fig. 1d). Dactyls up to 0.5 mm long, bicellulate; end-cell somewhat obtuse (Fig. 1e), deciduous on older whorls. Heads (contracted reproductive whorls distinguished from the vegetative parts) not really formed, although the fertile branchlet whorls have consecutively shorter branchlets and are separated by long internodes. Gametangia on separate plants; oosporangia single, geminate or clustered at all furcations; antheridia single, central to the first furcation. Oosporangia up to 200–300 μm long, 250 μm wide, with 7 or 8 helical stripes, the coronula cells slightly longer in the upper row (Fig. 1c). Oospores 180–240 μm long, 150–180 μm wide with 7 striae (Fig. 1k), ornamentation densely spinose on the fossa and sometimes continuing up the flanges (Fig. 1i), with 6–16 very small spines across the fossa (Fig. 1h–j), sometimes in dentate rows (Fig. 1f). Antheridia up to 300–400 μm in diameter. Chromosome numbers not known.
Nitella acanthospora from the holotype specimen P.K.Latz 17988 (DNA). (a) Habit of whole plant, scale: 10 mm. (b) Fertile whorl, scale: 1 mm. (c) Oosporangium, scale: 500 µm. (d) Sterile branchlet, scale: 1 mm. (e) Dactyls, scale: 0.1 mm, (f) Light micrograph (LM) of oospore wall showing dentate ornamentation in rows perpendicular to the striae, scale: 5 µm. (g) LM of oospore wall showing detail of dentate ornamentation, scale: 5 µm. (h) LM of oospore wall showing density of dentate ornamentation, scale: 5 µm. (i) Scanning electron microscope (SEM) image of oospore wall, scale: 2 µm. (j) SEM image of dentate ornamentation, scale: 1 µm. (k) SEM image of whole oospore in side view, scale: 50 µm.

The oospores of this species are distinctly and densely spinose, with spines sometimes continuing up the flanges on the oospore. Nitella acanthospora was compared with other species with elongate projections on the oospore wall. Of these, Nitella sonderi has generally sparse papillae that do not continue onto the flanges; N. penicillata has similar oospores, but the vegetative morphology is very different; N. mathuatae Allen is monoecious, with spines much less pronounced, and the vegetative morphology is very different; N. haageniae Raam is monoecious and the papillae are flattened terminally and much broader, and N. knightiae J.Groves & E.L.Stephens has unicellulate dactyls and projections that are much more conical and surrounded by iteratively smaller conical projections. There is variation in oospore size and number of spines across the fossa among populations of N. acanthospora, and further material is needed to clarify the limits of species characters.
This species is distinguished from other arid-zone species on the basis of its homeoclemous whorls and the somewhat obtuse end-cells of the dactyls. The oospores are also distinctively different from those of other small Nitella species, with which it might be confused (see images for N. heterophylla (A.Braun) A.Braun, and N. biformis A.Braun).
Occurring in the arid zone of the Northern Territory and New South Wales, in tanks and bores.
From the Greek ‘akanthos’ (spiny) and ‘sporus’ (a seed or spore) in reference to the spiny oospores.
NORTHERN TERRITORY: 11 km S of Newhaven Homestead, common in clear, shallow gypsum lake, 29 Apr. 2001, P.K.Latz & D.E.Albrecht 17713 (DNA); 7 km S of Sangsters Bore, Tanami Desert, saline water, 14 Aug. 2001, P.K.Latz 17985 (DNA) [a mix of two species]. NEW SOUTH WALES: Dead Horse Tank, 11 Dec. 1998, J.L.Porter 169 (MEL).
Nitella belangeri (A.Braun) A.Braun, Monatsber. Königl. Preuss. Akad. Wiss. Berlin 1858: 353, 355 (1859)
Nitella acuminata var. belangeri A.Braun. Hookers J. Bot. Kew Gard. Misc. 1: 292 (1849), as ‘Bellangeri’
Nitella acuminata β belangeri (A.Braun) Wallman, Actes Soc. Linn. Bordeaux sér. 3, 21: 30 (1856), as ‘Bellangeri’
Nitella acuminata f. belangeri (A.Braun) R.D.Wood, Nova Hedwigia 22: 41 (1971).
Type: in pools near Gengu, on the coast of Coromandel, 1826–28, Bélanger s.n. (holo: B n.v. [destroyed, fide R.D.Wood & K.Imahori, Revis. Charac. 1: 409 (1965)]; Bombay, Concan, 1847, J.L.Stockes s.n. (neo: BM 000904580!, here designated).
Monoecious. Plants not calcified, 10–15 cm high (Fig. 2a), tangled and compact in shallow water. Axes up to 450 µm wide; internodes up to 6 mm long, as long as the sterile branchlets. Fertile branchlets homeoclemous (i.e. in a single whorl), 5 or 6 in a whorl, 1× furcate; primary segments up to 2 mm long; secondary segments 2 or 3, up to 4 mm long (usually dactyls) (Fig. 2d); sterile branchlets homeoclemous, 6 in a whorl, 1× furcate; primary segments up to 5 cm long; secondary segments 1–3, up to 2.5 cm long (Fig. 2b). Fertile and sterile dactyls similar, single-celled; between 1 and 5 mm long, tapering gradually to an acuminate tip (Fig. 2c, d). Heads not formed, the fertile branchlets shorter and closer together than the sterile branchlets, without mucus. Gametangia conjoined at the fertile branchlet nodes (Fig. 2e). Oosporangia solitary or geminate (Fig. 2e); coronula up to 30 µm high, with equal-sized cells (Fig. 2f). Oospores up to 280 µm long and 215 µm wide, with 7 flanged striae (Fig. 2g), the flanges up to 30 µm high; ornamentation of scattered, flaccid dentate scales up to 2 µm high (Fig. 2h); basal-cell impressions 65 µm wide at the widest part, the larger cell pentagonal, the smaller cell rectangular (Fig. 2i). Antheridia up to 300 µm in diameter (Fig. 2e). Chromosome numbers not known.
Nitella belangeri from the specimen M.T.Casanova r737 (MEL). (a) Habit of whole plant, scale: 5 cm. (b) Sterile branchlet whorl, scale: 5 mm. (c) Fertile branchlet whorl, scale: 1 mm. (d) Three fertile branchlets, scale: 1 mm. (e) Dactyls and gametangia, scale: 0.5 mm. (f) Oogonium, scale: 100 µm. (g) Scanning electron microscope (SEM) image of oospore, scale: 100 µm. (h) SEM image of detail of oospore wall, scale: 10 µm. (i) SEM image of view of basal-cell impressions, scale: 50 µm.

The lectotype material of Nitella acuminata A.Braun (Commerson, s.d. (LD!)), and other material from Indian Ocean Islands (Réunion, Mauritius, Madagascar (BM!, PC!)) was compared with specimens of N. acuminata from India, Indonesia, Philippines and Australia (particularly those mentioned by Braun in Nordstedt 1883 and by Zaneveld 1940). The Australian material has identical oospores and similar morphology to collections of the same taxon from India, whereas the material from Indian Ocean Islands (and other specimens included in N. acuminata from North America) has substantially different oospores (porate, or flocculate and with densely arranged flaccid conical elements). Eastern Asian specimens seen are similar to the Indian material.
In the absence of the holotype, the specimen ‘Concan, 1847, J.L.Stockes s.n.’ (BM 000904580) is designated as neotype of Nitella acuminata var. belangeri A.Braun. The specimen was collected from East India, conforms to the type description and was examined by Braun (in Nordstedt 1883, p. 38) and by Zaneveld (1940, p. 60), who both assigned the name N. acuminata var. belangeri to it. The variety is here recognised at species rank, pending further investigation.
Braun’s (1849, p. 292) first mention of this taxon was a Latin description of Nitella acuminata var. belangeri, with a separate diagnosis in English validating the species name N. acuminata given in notes on the following page. Zaneveld (1940, p. 60) stated that the holotype specimen (Coromandelia, in pools near Gengu, 1826–28, Bélanger s.n.) was annotated by Braun, first as ‘Nitella Bellangeri A. BR. 1838’, then as ‘Nitella (acuminata) Belangeri 1858’. Nitella acuminata was cited with a Latin description by Wallman (1856), with a reference to Braun’s manuscript name Chara flexilis var. acuminata for a specimen from Réunion (Île Bourbon, legit Commerson (LD)). A slightly modified Latin description for Nitella acuminata var. belangeri (as Nitella acuminata β belangeri (A.Braun) Wallm.) was provided in that publication, with the Bélanger collection cited. Braun (1859) later referred to it at species level without further description.
This is the only Nitella species in Australia with single-celled dactyls and a single whorl of branchlets at the axis nodes. The other species with single-celled dactyls in Australia, Nitella stuartii A.Braun, is distinguished by having more than one whorl of branchlets at the nodes, and it occurs in eastern Australia, north to central Queensland (Casanova and Karol 2008). Nitella belangeri is distinguished from other species related to N. acuminata chiefly via the ornamentation on the oospore wall, and by the arrangement of gametangia.
Nitella belangeri appears to be restricted to the north in Australia. It grows in shallow wetlands and temporary streams at the end of the wet season in northern Australia and eastern Asia, being fertile from June to September.
Named for the collector of the original material, Charles Paulus Bélanger (1805–1881), a French explorer and naturalist working in Pondicherry between 1825 and 1829.
WESTERN AUSTRALIA: Moochalabra Dam, 8 Aug. 1995, S.W.Jacobs s.n. (MEL, NSW); Caliwingina Spring, M.Curran 2 (MEL). NORTHERN TERRITORY: George Brown Darwin Botanic Garden, M.T.Casanova r737 (DNA, MEL, NY); floodplain billabong near Gunlom Campground, Kakadu National Park, J.Schult 11-vi-2012 (MEL) [oospores only]. QUEENSLAND: Davies Creek, Mareeba, H.S.McKee 9372 (CANB, MEL, NY); Edge Hill, 31 May 1936, H.Flecker 1707 (BM, NY). INDONESIA: Java, Malang, Roemah, Kampok, 14 May 1936, JH 75 (L). PHILIPPINES: Rangii, on swamp brook and open llocus norto, 40 cm ASL, 22 Feb. 1917, M.Ramos 27630 (NY).
Nitella biformis A.Braun, Hookers J. Bot. Kew Gard. Misc. 1: 197 (1849)
Nitella conglobata var. biformis (A.Braun) A.Braun in C.F.O.Nordstedt, Abh. Königl. Akad. Wiss. Berlin 1882: 77 (1883); Nitella subtilissima f. biformis (A.Braun) R.D.Wood, Taxon 11: 21 (1962).
Type: WESTERN AUSTRALIA: Swan River, J.Drummond 8 (lecto: BM! [oosporangial specimen]); isolecto: BM! [antheridial specimen], fide R.D.Wood & K.Imahori, Revis. Charac. 1: 652 (1965)).
Nitella congesta f. subtilissimoides R.D.Wood, Taxon 11: 22 (1962); Nitella lhotzkyi f. subtilissimoides (R.D.Wood) R.D.Wood, Revis. Charac. 1: 668 (1965); Nitella subtilissimoides R.D.Wood, Revis. Charac. 1: 779 (1965), nom. inval.
Type: WESTERN AUSTRALIA: in shallow water near margin of Lake Parkeyerring, near Wagin, 28 Dec. 1955, N.Burbidge 4951 (holo: AD 20411!; iso: NY!).
[Nitella lhotzkyi auct. non A.Braun: R.D.Wood, Nova Hedwigia 22: 76 (1971)]
Dioecious. Plants up to 10 cm high, male plants with more open and diffuse sterile whorls than those in female plants; upper whorls with mucus (Fig. 3a). Axes 300–330 µm wide. Internodes up to 2 cm long, 1–3× the length of the branchlets. Fertile branchlets heteroclemous, 7 or 8 primary branchlets in a whorl; the primary whorl branchlets 1 or 2× furcate; 1 or 2 simple (not furcate) branchlets in the secondary whorl on male plants (i.e. scarcely heteroclemous, Fig. 3c); the secondary branchlets 1× furcate on the female plant; sterile branchlets heteroclemous with 7 or 8, 1 or 2× furcate primary branchlets in a whorl with 2–5 simple or 1×furcate secondary branchlets (Fig. 3b); primary segments up to 0.8 mm long, secondary segments 4–5, up to 0.5 mm long, at least 2 of them again furcate into 3–5 tertiary segments (=dactyls) up to 400 µm long. Fertile dactyls up to 400 µm long, bicellulate; the end-cell conical and acute (Fig. 3c). Sterile dactyls up to 0.8 mm long, bicellulate; the end-cell conical and acute (Fig. 3d). Heads not formed, the whorls all similar. Gametangia on separate plants, at axial nodes (oosporangia) and first and second branchlet nodes (oosporangia and antheridia). Oosporangia up to 350 µm long × 350 µm wide with 8 helical stripes, coronula cells similar in both rows (Fig. 3e). Oospores up to 320 µm long and 300 µm wide with 6 or 7 flanged striae (flanges can break off) and a distinctive ‘crest’ or ‘beak’ at the apex (Fig. 3g), the oospore wall construction is fibrous (Fig. 3h), with apparently a single basal-cell impression present (Fig. 3i). Antheridia up to 250 µm in diameter (Fig. 3c). Chromosome numbers n = 9 (specimen M.T.Casanova p795, Fig. 3f).
Nitella biformis. (a–c, f) From specimen M.T.Casanova p795 (MEL). (d, e, g–i) From the lectotype specimen J.Drummond 8 (BM). (a) Habit of whole plant, scale: 1 cm. (b) Sterile branchlet whorl, scale: 1 mm. (c) Fertile antheridial branchlet whorl, scale: 1 mm. (d) Dactyls, scale: 100 µm. (e) Oogonium, scale: 100 µm. (f) Light micrograph of chromosomes, 1000× magnification. (g) Scanning electron microscope (SEM) image of oospore, scale: 100 µm. (h) SEM image of detail of oospore wall, scale: 20 µm. (i) SEM image of basal-cell impression, scale: 50 µm.

Braun (1849) initially named this species, and in a later work amalgamated it with Nitella lhotzkyi and renamed the amalgamated taxon N. conglobata A.Braun, nom. illeg. Wood (1962) synonymised N. biformis with N. subtilissima (as N. subtilissima f. biformis (A.Braun) R.D.Wood) on the basis that it was unlikely that Drummond could have collected four new species sequentially in a single site (Swan River) (Wood 1971, p. 71). Wood (1962) described another specimen of this species as N. congesta f. subtilissimoides R.D.Wood, later transferring it to N. lhotzkyi f. subtilissimoides (R.D.Wood) R.D.Wood (Wood 1971). The morphology of these plants and their oospores are very similar, so they are here amalgamated into one species, for which the name N. biformis has priority.
A species with short branchlets and fine axes, with large antheridia and very few secondary branchlets, forming dense, mossy cushions in shallow water, occasionally as individual shoots among other species. It is scarcely heteroclemous and the smooth-looking oospores are distinctive for this dioecious entity in the group of heteroclemous Nitella species. Oospores have essentially fibrous wall construction, but are smaller than those of N. heterophylla and N. congesta.
Known from Western Australia, semi-arid South Australia and the Northern Territory, although this inconspicuous species is probably more widespread.
Assumed to be named for the two kinds of branchlets in the whorls (i.e. heterophyllous branchlet whorls).
WESTERN AUSTRALIA: Fortescue Marsh-west, Pilbara Biological Study site PSW003, seed-bank culture, 16 Oct. 2005, M.T.Casanova p770 (MEL, PERTH); in shallow, brackish, temporary lake growing on sandy mud, 3 miles [~4.8 km] SE of Morawa, 22 July 1953, R.Melville & J.Calaby 4271 (BM); Murchison River, Oldfield s.n. (BM). NORTHERN TERRITORY: 7 km SW of Newhaven Homestead, common in a small seasonal lake, sandy bed, 2 Oct. 2000, P.K.Latz 16805 (DNA); 22 km S of Palmer Valley Homestead, 6 Mar. 1995, P.K.Latz 14064 (DNA). SOUTH AUSTRALIA: Kurdimichi Outstation, seed-bank culture, 11 Nov. 2005, M.T.Casanova p795 (MEL).
Nitella boreali-australis Casanova & Karol, sp. nov.
Type: Mitchell Creek, Northern Territory, 24 May 2010, P.Dostine 12DW23-2 (holo: MEL! [spirit]).
Nitella pseudoflabellata f. bancroftii R.D.Wood, Nova Hedwigia 22: 69 (1971), nom. inval., nom. prov.
[Nitella mucosa auct. non. (Nordst.) J.Groves: J.Groves in J.Groves & G.O.Allen, Proc. Roy. Soc Queensland 46: 44 (1935)].
Monoecious. Plants fine and flexible, mucus-covered, >10 cm high. Axes up to 500 µm wide; internodes up to 10 mm long, shorter than the branchlet whorls (Fig. 4a). Fertile branchlets 7 or 8 in a whorl, smaller and less furcate than the sterile whorls; primary segments up to 5 mm long, secondary segments 5 or 6, 0.5–1.5 mm long, divided into 4–6 tertiary segments of which one can be a dactyl, up to 0.5 mm long, further divided into 4 or 5 dactyls (Fig. 4c); sterile branchlets 7 or 8 in a whorl, 10–20 mm long with longer segments than fertile branchlets; primary segments up to 10 mm long, secondary segments 4–7, 3–4 mm long, divided into 3–6 tertiary segments (=dactyls), up to 2 mm long, sometimes 1 or 2 again divided into 3–5 dactyls (Fig. 4b). Fertile dactyls 3–5, up to 1 mm long, but usually 0.5–0.7 mm long, bicellulate; the end-cell conical and acute, confluent with the end of the penultimate cell (Fig. 4d). Where a dactyl is formed at the second furcation, the dactyls can appear 3 cells long (i.e. a suppressed furcation). Sterile dactyls are similar to fertile dactyls but up to 2 mm long. Mucus-covered heads present, really consisting of sequentially smaller whorls distally. Male and female gametangia on the same plant; antheridia single and central in the lowest 2 furcations (Fig. 4c); oosporangia single, lateral at all but the lowest furcation (Fig. 4c). Oosporangia up to 400 µm long, 300 µm wide with 8 or 9 helical stripes, coronula up to 30 µm high, the upper cells slightly longer than the lower cells. Oospores 210–230 µm long, 190–210 µm wide with 8 or 9 flanged striae ~7 µm high (Fig. 4e); the ornamentation fibrous-papillate and porate; the wall appears to be constructed of flocculate fibres, built into papillae occasionally, with rimmed pores (variolae) also occasional (Fig. 4f). Antheridia up to 200 µm in diameter. Chromosome numbers not known.
Nitella boreali-australis from specimen P.Dostine 12DW23-2 (DNA). (a) Habit of whole plant, scale: 10 mm. (b) Sterile whorl, scale: 5 mm. (c) Fertile branchlet, scale: 1 mm. (d) Dactyls, scale: 100 µm. (e) Scanning electron microscope (SEM) image of oospore, scale: 100 µm. (f) SEM image of detail of oospore wall, scale: 20 µm.

Three specimens of Nitella were collected by T. L. Bancroft in Queensland, two growing at ‘Stannary Hills’ in 1909 (possibly at the same locality, but the collection details are not specific) and one in Murphy’s Creek in 1910. James Groves (in Groves 1935) identified the two specimens from Stannary Hills as Nitella mucosa (Nordst.) J.Groves and N. orientalis T.F.Allen, and the specimen from Murphy’s Creek as N. phauloteles J.Groves. Wood and Imahori (1965) referred only to the specimen from Murphy’s Creek and provided an illustration (Wood and Imahori 1964, icon 275). Wood (1971) listed a specimen ‘Stannary Hills: Bancroft in 1909’ under N. pseudoflabellata var. imperialis T.F.Allen, allocating it the provisional designation of ‘form…(8) bancroftii’, but did not distinguish between the two Bancroft specimens collected in this locality. Wood (1971) also amalgamated N. pseudoflabellata with N. mucosa, and N. orientalis with N. phauloteles (all of which are separate species). Although the epithet bancroftii was provisionally attached to one of these specimens, neither is used here as type material, and owing to the potential for confusion, the name ‘bancroftii’ is not adopted for the taxon at species rank.
A very soft, flabellate and mucus-covered monoecious species, distinguished from Nitella limosa Casanova & Karol by the two-celled dactyls. On the basis of oospore similarity, N. boreali-australis is probably closely related to N. imperialis (T.F.Allen) Sakayama from Japan, but that species has less porate oospores, gametangia missing from the first and last furcations, longer internodes and shorter branchlets.
On the basis of the localities in Queensland and the Northern Territory, it is assumed that this species will be found across the wet tropics in northern Australia.
The name boreali-australis literally means ‘northern-southern’, but the intent is to refer to northern Australia.
NORTHERN TERRITORY: Groote Eylandt, 18 May 1948, R.L.Specht A28 (BM, NY); Groote Eylandt, 18 May 1948, R.L.Specht A29a (NY); Port Darwin, 4 Apr. 1896, T.B.Blow A103 (BM). QUEENSLAND: Stannary Hills, 1909, T.L.Bancroft (BM); Lake Eacham, 11 Sep. 1993, T.J.Entwisle 2310 (MEL); Mareeba, 28 Apr. 1962, H.S.McKee 9373 (NY).
Nitella congesta (R.Br.) A.Braun, Hookers J. Bot. Kew Gard. Misc. 1: 198 (1849)
Chara congesta R.Br., Prodr. 1: 346 (1810).
Type: Bay I [Lucky Bay], South Coast, Novae Hollandiae oram septentrionalem et australem, Western Australia, Jan. 1802, R.Brown s.n. [Iter Austral. 276] (lecto: BM000904640!; isolecto: BM000610394!, BM 000610395 fide M.T.Casanova in D.J.Mabberley & D.T.Moore, Robert Brown Handb. 108 (2022)).
Dioecious. Plants up to 40 cm tall, robust with very strong stems, with dense, often spherical whorls of branchlets (string-of-beads morphology), with mucus, especially in the upper parts (Fig. 5a). Axes up to 1 mm in diameter, usually wiry and strong; internodes up to 10 cm long. Fertile branchlets at the apices; heteroclemous (at least 3 whorls at each node, but frequently so compacted that it is impossible to distinguish separate whorls); 3 or 4× furcate (Fig. 5e). Approximately 8 branchlets in the primary whorl (i.e. the longer branchlets), with primary segments up to 10 mm long, at least half the branchlet length; secondary segments 5–8, 1 of them central, up to 1/3 of them dactyls, tertiary segments 3–7, sometimes dactyls, one of them central (in female plants) and again furcate, quaternary segments 1–5, sometimes one of them central and again furcate into 3 quinary segments = dactyls. There can be 20–40 accessory whorl branchlets, up to 3× furcate, but fewer in the upper fertile whorls; sterile branchlets are similar to fertile branchlets but the primary whorl branchlets can be longer and emergent from the congested sphere of branchlets. In older whorls, sometimes only the primary branchlet segments remain. Dactyls are 2 cells long, the end-cell long-conical and acute (Fig. 5f). Heads are not formed, but the upper fertile whorls are well separated and often sequentially smaller in size and number of accessory branchlets (Fig. 5a). Gametangia on separate plants at all fertile branchlet nodes (Fig. 5h, i), including sometimes oosporangia at the base of the whorl on the axial nodes. Oosporangia are solitary or aggregate (up to 4 at a node) 400–500 µm long and 350–450 µm wide, with 8–10 helical stripes; coronula small with equal-sized cells (Fig. 5h). Oospores pale to dark brown, appearing ‘felted’ (i.e. intermingled fibres) under light microscopy, 350–450 µm long, 300–400 µm wide with 8 or 9 ridged striae and a crest of the joined ridges at the apex of the oospore (Fig. 5b). The oospore wall is superficially smooth, but densely fibrous and felted underneath (Fig. 5c, d), the basal-cell impression is of 2 cells of equal size. Antheridia are solitary, up to 400 µm long (Fig. 5i). Chromosome numbers n = ~9 (Tasmanian specimen, H. & A.Wapstra HAW071 (HO, MEL)).
Nitella congesta. (a–d) From the isolectotype specimen R.Brown 276 (BM). (e–h) From specimen M.T.Casanova r022 (MEL), (i) from specimen H. & A.Wapstra HAW059 (HO). (a) Habit of whole plant, scale: 5 cm. (b) Scanning electron microscope (SEM) image of oospore with 8 or 9 striae, scale: 200 µm. (c) SEM image of detail of oospore wall, scale: 20 µm. (d) SEM image of detail of the construction of the oospore wall, scale: 5 µm. (e) Sterile branchlet whorl, scale: 5 mm. (f) Sterile dactyls, scale: 0.5 mm. (g) Oogonium, scale: 100 µm. (h) Fertile oogonial branchlet, scale: 1 mm. (i) Fertile antheridial branchlet, scale: 1 mm.

There is some variation among populations particularly in oospore size and development of flanges, along with variation in the number of branchlets. Sometimes only female plants can be found, suggesting the capacity for parthenogenesis, as in Chara canescens Loisel. (Casanova and Nicol 2009). Specimens appear to be perennial and old specimens can be blackish with degraded whorls of branchlets (mere bristles) at the nodes.
Nitella congesta is usually easy to identify on the basis of its densely packed whorls of branchlets and pale brown, felted-looking oospores.
Specimens have been recorded from Western Australia, South Australia, Victoria and Tasmania, as well as the Northern Territory.
WESTERN AUSTRALIA: Bremer Gorge, 1 Sep. 1965, M.Wittwer 476 (PERTH); Lake Richmond, 11 Feb. 1978, K.F.Kenneally 6530 (PERTH); Fitzgerald River, 11 Jan. 1980, B.G.Muir s.n. (PERTH); Lake Joondalup, 19 Jan. 1989, Schmidt s.n. (PERTH); Bannister River, 29 Sep. 2002, M.T.Casanova 0209291 (MEL, NY); Palm Spring near Millstream, 17 Sep. 2003, M.N.Lyons PBS3021 (PERTH); Lake Leschenaultia, 5 Oct. 2010, M.T.Casanova r810, r811 (MEL, NY, PERTH).
NORTHERN TERRITORY: Willoughby Bore, 15 Feb. 1971, G.C.Taylor 7 (DNA, MEL); Gorge behind old Serpentine Chalet, ~110 km W of Alice Springs, 7 Sep. 1985, G.Leach 716 (DNA). SOUTH AUSTRALIA: Dead Man’s Lagoon near Naracoorte, 16 July 1991, M.T.Casanova 910716-1A (MEL); Gahnia Lagoon, 24 Feb. 1997, M.A.Brock s.n. (NE); Henry Creek, 13 May 2004, M.Hammer s.n. (MEL); Lake Edward, 13 June 2004, M.T.Casanova p611 (MEL); Lake Leake, 13 June 2004, T.M.Dugdale & K.Dixon p614 (MEL); Mt Monster quarry, 27 Oct. 2007, M.T.Casanova r022 (MEL); Freshwater Lake, Little Dip Conservation Park, near Robe, 31 Oct. 2010, M.T.Casanova r849 (MEL, NY). VICTORIA: Lake Mombeong, 14 June 2004, R.L.A.Casanova t017 (MEL). TASMANIA: Sandford Lagoon, 1905, G.S.Perrin s.n. (HO); Blackmans Lagoon, 16 Apr.1997, M.Cameron 5447 (HO); Meredith R, Swansea, 10 July 2008, H. & A.Wapstra 016 (HO, MEL, NY); Latrobe near Dooleys Hill, 17 Nov. 2008, H. & A.Wapstra 071 (HO, MEL).
Nitella crocodylus Casanova & Karol, sp. nov.
Type: Manton Dam, 5 Sep. 2010, M.T.Casanova r760 (holo: DNA!; iso: BM!, MEL!, NY!).
[Nitella pseudoflabellata f. sonderi auct. non (A.Braun) R.D.Wood: R.D.Wood in R.D.Wood & K.Imahori, Revis. Charac. 1: 589–590 (1965)].
[Nitella pseudoflabellata var. imperialis auct. non T.F.Allen: R.D.Wood, Nova Hedwigia 22: 69 (1971)].
Dioecious. Plants up to 30 cm high, with some long branchlets along the axis; plant apex with mucus-covered heads (Fig. 6a, c). Axes 400–500 µm wide; internodes up to 12 cm long in the lower parts, 10–20 mm in the upper parts, approximately as long as the sterile branchlets. Fertile branchlets 6 in a whorl; variable in length, some very short and in contracted whorls, others with very long (6–9 mm) primary branchlet segments; mostly 2× furcate, primary segments 1–9 mm long, secondary segments 4–6, up to 0.5–2 mm long or more if not bearing gametangia, 3–6 tertiary segments (dactyls) up to 0.6 mm long. Sterile branchlets 6 in a whorl, 2× furcate, primary segments 5–15 mm long, secondary segments 3–5, up to 6 mm long but often shorter, tertiary segments (dactyls) 2 or 3, up to 3 mm long (Fig. 6b). Fertile and sterile dactyls similar, bicellulate, up to 1 mm long, tapering to a conical, acute end-cell (Fig. 6d). Fertile branchlets often contracted into axial and apical heads (Fig. 6c). Post-fertile branchlets apparently extend the primary branchlet segment, appearing as little brushes of segments and dactyls at the tips (Fig. 6e), mucus present around young fertile branchlets. Gametangia on separate plants at all fertile branchlet nodes. Oosporangia solitary, coronula up to 20 µm high, upper cells approximately the same as lower (Fig. 7f). Oospores dark brown, 270–300 µm long and 240–270 µm wide (Fig. 6h) with 6 or 7 striae with porous flanges 5–19 μm high and ornamentation in 2 layers; the lower layer perforate with sinuate holes (Fig. 6g), the upper layer incompletely reticulate, consisting of ropey lines (Fig. 6i). Basal-cell impressions almost rectangular (Fig. 6j). Antheridia up to 250 µm in diameter. Chromosome numbers not known.
Nitella crocodylus from isotype specimen Casanova r760 (MEL). (a) Habit of oosporangial plant, scale: 10 mm. (b) Sterile branchlet tip and dactyls, scale: 1 mm. (c) Habit of male plant, scale: 10 mm. (d) Female fertile branchlet whorl, scale: 1 mm. (e) Male branchlet whorl, scale: 5 mm. (f) Fertile female branchlet tip, scale: 1 mm (g) Scanning electron microscope (SEM) image of detail of the oospore ornamentation, a raised reticulum on a sinuously porate ground, scale: 2 µm. (h) SEM image of oospore in side view, scale: 100 µm. (i) Detail of oospore wall, scale: 20 µm. (j) View of the basal-cell impressions, scale: 50 µm.

Nitella heterophylla. (a–c) From specimen M.T.Casanova r460 (MEL). (d–i) From specimen M.T.Casanova p770 (PERTH). (a) Whole plant, scale: 1 cm. (b) Whorl of branchlets, scale: 1 mm. (c) Fertilised oogonium, scale: 200 µm. (d) Fertile female shoot, scale: 1 mm. (e) Fertile male shoot, scale: 1 mm. (f) Chromosomes in metaphase, 1000× magnification. (g) Scanning electron microscope (SEM) image of oospore in side view, scale: 100 µm. (h) SEM image of detail of oospore wall, scale: 20 µm. (i) SEM image of basal view of oospore, showing end-cell impressions on the oospore wall, scale: 100 µm.

This species is characterised by abundant mucus and branchlets with very long primary segments (most likely post-reproductive whorls), as well as other, more regularly furcate branchlets. It is distinguished from Nitella oollooensis by the presence in N. oollooensis of central secondary and tertiary branchlet-segment lengths. The oospores are also distinctive.
Nitella heterophylla (A.Braun) A.Braun, Hookers J. Bot. Kew Gard. Misc. 1: 198 (1849)
Chara heterophylla A.Braun, Linnaea 17: 113 (1843).
Nitella lhotzkyi f. heterophylla (A.Braun) R.D.Wood, Taxon 11: 22 (1962).
Type: in fluvio ‘Adams River’ (Grantham), Western Australia, 7-iii-1841, L.Preiss 1876 (holo: B n.v. [apparently destroyed]; iso: LD! [fragment], LE! [fragment]).
Dioecious. Plants up to 40 cm high (Fig. 7a); heterophyllous whorls well separated on the axis; branchlets with little clusters of dactyls at their tips, like pom-poms; frequently covered in mucus and attached debris (Fig. 7b). Axes up to 800 µm wide; internodes up to 70 mm long, much longer than the branchlet whorls. Fertile branchlets 1× furcate (Fig. 7d, e), in 2 whorls; primary branchlets 7 or 8 in a whorl, similar to the sterile branchlets but somewhat smaller, contracted and covered in mucus; primary segments up to 0.5 mm long, secondary segments (=dactyls), 2–8, up to 1 mm long; secondary branchlets simple or 1× furcate, up to 1 mm long, shorter than primary branchlets; sometimes also bearing reproductive structures; sterile branchlets in 2 whorls, 2 or 3× furcate (Fig. 7b), 7 or 8 primary branchlets in a whorl, up to 8 mm long, primary segments up to 4 mm long, secondary segments 3–8, up to 2 mm long, tertiary segments 3–7, up to 1 mm long, some very short and divided into 3 or 4 quaternary segments, all dactyls up to 1.2 mm long; secondary branchlets ~6, simple or 1× furcate, sometimes a secondary segment becoming a central (percurrent) ray, up to 2 mm long. Dactyls usually 5–7, up to 1.2 mm long; bicellulate, the end-cell shortly conical and acute. Sterile dactyls similar to fertile; 3–8, up to 2 mm long. Heads somewhat spike-like, the upper whorls narrow and contracted and often covered in thick mucus. Gametangia at the fertile branchlet nodes. Oosporangia up to 500 µm long × 420 µm wide with ~7 helical stripes (Fig. 7c); coronula up to 35 µm high. Oospores 380–420 µm long × 250–300 µm wide with 7 or 8 large flanges (Fig. 7g), the ornamentation fibrous and the fibres somewhat ropey (Fig. 7h), visible as fibres under the light microscope. The impressions of the basal cells are angular, maximum diameter ~40 µm (Fig. 7i). Antheridia up to 350 µm in diameter. Chromosome numbers n = 9 (Fig. 7f).
Nitella heterophylla was established as a species on the basis of its heteroclemous whorls of branchlets, the number of dactyls, the axis diameter and the size of the oospores. It was amalgamated with N. lhotzkyi by Wood (1962), but it has large fibrous oospores whereas N. lhotzkyi has smaller papillate oospores. It was initially distinguished in the Pilbara Biological Survey (Lyons 2015) because of its dioecy and long primary branchlet segments in the primary whorls, along with the distinctive oospore. Investigation of the isotype material (fragments in LD and LE) helped in the application of the correct name for this taxon.
Nitella heterophylla is dioecious with heteroclemous whorls and distinctive oospores. The oospores of N. heterophylla are similar to those of both N. hyalina (which is monoecious) and N. congesta (dioecious, but with ~40 accessory branchlets in the sterile whorls). Additionally, N. congesta has more furcations and uniformly shorter primary branchlet segments.
Arid and semi-arid zones in New South Wales, the Northern Territory and Western Australia, but probably more widespread.
WESTERN AUSTRALIA: in 0–6″ [~0–15.2 cm] of water, black muck covered with red silt, in a pond immediately NE of railroad crossing, just N of Watheroo, 1 Oct. 1960, R.D.Wood 60-10-1-9 (L, NY); Coondiner Pool, 5 Sep. 2003, M.N.Lyons 3000 (PERTH); Gnalka Gnoona, 4 Sep. 2003, M.N.Lyons 3046 (PERTH); Watrara Creek Pool, 8 Sep. 2003, M.N.Lyons 3046a (MEL, NY, PERTH); Carawine Gorge T1, 12 Sep. 2003, M.N.Lyons & S.D.Lyons 3035-b (PERTH); Gnalka Gnoona CT2, 4 Sep. 2003, M.N.Lyons & S.D.Lyons 3047-a (PERTH); cultured from seed bank in a glasshouse in Westmere, ex Rudal River, 4 Oct. 2005, M.T.Casanova p701 (MEL); cultured from seed bank in a glasshouse in Westmere, ex Coondiner Pool, 4 Oct. 2005, M.T.Casanova p760 (MEL, NY); cultured from seed bank in a glasshouse in Westmere, ex Ethel Creek Claypan, 12 Oct. 2005, M.T.Casanova p768 (MEL, NY); cultured from seed bank in a glasshouse in Westmere, ex Fortescue Marsh East, 16 Oct. 2005, M.T.Casanova p771 (MEL, NY); Bobswim Pool T2, 12 May 2005, M.N.Lyons & D.A.Mickle 3149 (PERTH); pool on Lower Fortescue T1, 22 Aug. 2005, M.N.Lyons & D.A.Mickle 3156 (PERTH); Rocky Island Pool T2, 9 Sep. 2005, M.N.Lyons & D.A.Mickle 3185 (PERTH); Koodjeepindawarranna Pool, 17 Aug. 2006, M.T.Casanova p943 (MEL); Watrara Creek Pool T1, 3 Sep. 2006, M.N.Lyons & D.A.Mickle 3255 (PERTH); Desert Queen Baths Adj, 2 Sep. 2006, M.N.Lyons & D.A.Mickle 3251-a (PERTH); Pewar West T1/T2, 21 May 2006, D.A.Mickle & N.Y.Huang 39-3118 (PERTH); Coondiner Pool T1, 15 Aug. 2006, M.T.Casanova PBS2 (MEL); Hamersley Gorge T1, 18 Aug. 2006, M.T.Casanova PBS20 (MEL); Gnalka Gnoona T1, 19 Aug. 2006, M.T.Casanova PBS36 (MEL); Koodjeepindawarranna Pool Adj, 19 Aug. 2006, M.T.Casanova PBS39 (MEL); Bobswim Pool T1, 20 Aug. 2006, M.T.Casanova PBS46 (MEL); Palm Spring on Cave Creek T2, 21 Aug. 2006, M.T.Casanova PBS53 (MEL); Palm Spring near Millstream T2, 22 Aug. 2006, M.T.Casanova PBS72 (MEL); Weeli Wolli T1, 16 Aug. 2006, M.T.Casanova PBS8 (MEL); Koodjeepindawarranna Pool, 31 Aug. 2006, I.J.Powling s.n (MEL); WPT155, 5 Sep. 2006, I.J.Powling s.n. (MEL). NORTHERN TERRITORY: Dingo Waterhole, Lander River, 5 Dec. 1986, P.K.Latz 81234 (DNA); 75 km NW of Lake Surprise, submerged in freshwater, 19 Aug. 1991, P.K.Latz 12209 (DNA). NEW SOUTH WALES: Dumaresq Dam, shallow water, near car park, 1 Dec. 2009, M.T.Casanova r460 (MEL, NY).
Nitella limosa Casanova & Karol, sp. nov.
Type: Girraween Lagoon near Darwin, 3 Sep. 2010, M.T.Casanova & J.Schult r738 (holo: DNA!; iso: MEL!, NY!).
Dioecious. Plants 15–40 cm high; elongate, diffuse and flexible with mucus-covered fertile parts (Fig. 8a). Axes up to 450 µm wide; internodes up to 60 mm long, but usually less than 40 mm, longer than the sterile branchlets when growing in deep water. Fertile branchlets 6 in a whorl; 3 to rarely 4× furcate; primary segments up to 5 mm long; secondary segments 4 or 5, to 0.5–1 mm long; tertiary segments 3–5, 0.2–0.5 mm long; quaternary segments (=dactyls) 2–4, 2 cells long, both cells elongate, each up to 500 μm long, the end-cell apex obtuse (Fig. 8c); sterile branchlets 6 in a whorl; 3× furcate; primary segments up to 2 cm long; secondary segments 6–8, up to 4 mm long; tertiary segments 4–6, up to 3 mm long, again furcate into 3–5 dactyls (Fig. 8b), up to 5 mm long, made up of 2 or 3 cells; end-cells elongate and obtuse. Fertile branchlets somewhat contracted, but the fertile branchlet primary segments remain elongate, and the remaining segments are contracted into tufts: mucus present and sometimes abundant. Gametangia occurring singly at the 2nd and 3rd fertile branchlet nodes. Oosporangia solitary at nodes, up to 450 µm long with ~7 helical stripes; coronula up to 30 µm high, cells equal sized (Fig. 8c). Oospores up to 350–400 µm long × 280–320 µm wide with 9 or 10 flanged striae (Fig. 8d); the flanges up to 20 µm high; ornamentation reticulate, a network of 10–15 shallow holes across the fossa, the edges of the holes sometimes well defined (~1 µm wide), ornamentation continues up the flanges for at least 10 µm (Fig. 8e). Basal-cell impressions 50 µm wide at the widest part, the larger cell pentagonal, the smaller cell rectangular (Fig. 8f). Antheridia up to 300 µm in diameter. Chromosome numbers not known.
Nitella limosa from holotype specimen M.T.Casanova & J.Schult r738 (DNA). (a) Habit of whole plant, scale: 1 cm. (b) Sterile whorl, scale: 10 mm. (c) Female fertile branchlet, scale: 500 µm. (d) Scanning Electron Microscope (SEM) image of oospore, scale: 200 µm. (e) SEM image of detail of oospore wall, scale: 20 µm. (f) SEM image of impression of end-cells on oospore wall, scale: 100 µm.
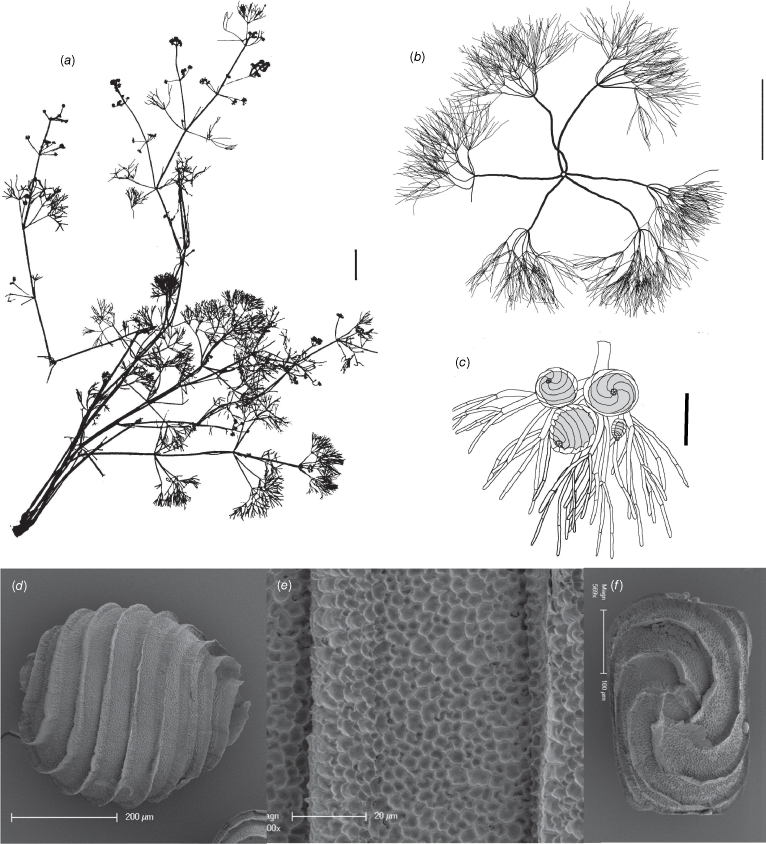
This species was originally determined as Nitella myriotricha (Wood 1971) because of the pluricellulate branchlets, dioecy and abundant mucus, but examination of the oospores using SEM showed a much larger and more reticulate oospore. This species has 3× furcate branchlets, and the distal fertile segments on very long primary segments, so that the appearance of fertile parts in the water is of glistening orange stars.
From the Latin limosus (slimy); although this word has negative connotations in the English language, it is a field character that is very distinctive.
NORTHERN TERRITORY: Yamburram Range, 15 May 1994, N.Walsh 3773 (MEL); Lyons Lagoon near Darwin, 3 Sep. 2010, M.T.Casanova & J.Schult r748 (DNA, MEL, NY); Woodford Lagoon near Darwin, 3 Sep. 2010, M.T.Casanova & J.Schult r743 (DNA, MEL, NY); Groote Eylandt, Little Lagoon, in Gulf of Carpentaria, in freshwater swamp, 28 May 1948, R.Specht A27 (BM, MEL, NY 02022347); pool on Stuart? Creek, E of Humpty Doo Road, 50 miles [~80.5 km] S of Darwin, 20 Apr. 1961, R.D.Wood, N.Eddy & A.Wilson 61-4-20-20 (BM, NY 02020653, NY 02022339, NY 02285722).
Nitella martinii Casanova & Karol, sp. nov.
Type: Roadside Borrow Pit on the Arnhem Hwy, 8 Sep. 2010, M.T.Casanova r785. (holo: DNA!, iso: BM!, MEL!, NY!).
Dioecious. Plants up to at least 30 cm high; branchlets up to 3× furcate; all branchlet segments long, spreading and diffuse, without mucus (Fig. 9a). Axes up to 400 µm wide; internodes up to ~60 mm long in most parts, some exceptionally long (15 cm). Fertile branchlets 6 in a whorl, ranging from shorter and less furcate than the sterile whorls to approximately the same (Fig. 9c); primary segments up to 6 mm long; secondary segments 4 or 5, 3–7 mm long, divided at the tips to 2 or 3 brachydactylous tertiary segments, up to 0.5 mm long; sterile branchlets 6 or 7 in a whorl; up to 25 mm long, with longer segments than fertile branchlets, 3(–4)×furcate (Fig. 9b); primary segments up to 12 mm long, secondary segments ~5, of which one is central, 7–13 mm long; the shorter ones divided into 2 or 3 tertiary segments, 5–13 mm long, some of these are dactyls and 1 or 2 of them again divided into 2 or 3 dactyls. Fertile dactyls 2 or 3, bicellulate, the end-cell shortly conical and acute, somewhat bristle-tipped, some very long (5–7 mm) others very short and brachydactylous (Fig. 9f). In some cases, the final branchlet segments in fertile whorls appear to be stipes for gametangia. Sterile dactyls similar to fertile but longer. Some shoots appear to be specialised vegetative reproductive organs (turions), with contracted, starch-filled whorls of branchlets (the starch in the primary branchlet segments, and all other segments reduced). Heads not formed. Gametangia on separate plants, antheridia single and geminate at final 2 furcations, often appearing to be terminal to the entire branchlet (Fig. 9d), oosporangia single, geminate and clustered lateral to most of the fertile branchlet nodes (Fig. 9c). Oosporangia up to 500 µm long, 350 µm wide with 6 or 7 helical stripes; coronula up to 30 µm high, the upper cells as long as the lower cells (Fig. 9e). Oospores 400–450 µm long × 350–400 µm wide with ~8 flanges ~20 µm high united in a crest at the apex of the oospore (Fig. 9g); the ornamentation appears lowly verrucate, and dry oospores are pale, spongy brown (Fig. 9h). Basal-cell impression apparently single, rectangular (Fig. 9i). Antheridia up to 300 µm in diameter. Chromosome numbers not known.
Nitella martinii from holotype specimen M.T.Casanova r785 (DNA). (a) Habit of whole plant, scale: 10 cm. (b) Sterile branchlet whorl, scale: 10 mm. (c) Fertile female branchlet axis, scale: 10 mm. (d) Male fertile branchlet, scale: 1 mm. (e) Oogonia at branchlet tips, scale: 250 µm. (f) Branchlet tips, scale: 500 µm. (g) Scanning Electron Microscope (SEM) image of oospore in side view, scale: 100 µm. (h) SEM image of detail of oospore wall, scale: 20 µm. (i) SEM image of view of basal-cell impressions, scale: 100 µm.

Only one gathering has been made, but this species is so distinctive that it warrants description.
In the only gathering, Nitella martinii formed wiry masses of clear green shoots in shallow water. The diffuse and spreading habit could be confused with Chara lucida (A.Braun) Casanova & Karol, with which it was growing, but the stems were more wiry. The absence of mucus and dispersed fertile branchlets are distinctive.
Only one locality is known, which is likely an ephemeral one (roadside borrow-pit) on the Arnhem Highway. It is likely to be more widely distributed in temporary wetlands in the Northern Territory.
Nitella micklei Casanova in M.T.Casanova & J.L.Porter, Muelleria 31: 55 (2013)
Type: WESTERN AUSTRALIA. Mulga Downs Outcamp Claypan, 17 Aug. 2006, M.T.Casanova PBS17: r114 (holo: MEL!).
Dioecious. Plants sometimes calcified, homeoclemous, up to 90 mm tall, internodes up to 35 mm long (Fig. 10a). Axes stout, up to 650 µm in diameter. Fertile branchlets 5 or 6 in a whorl, up to 2 mm long, 1–22×furcate (Fig. 10b, d, e). Primary segments up to 1.5 mm long, 2 or 3 secondary segments to 1.2 mm long; rarely 2 or 3 tertiary segments up to 0.5 mm long. Sterile branchlets 5 or 6 in a whorl, up to 18 mm in total, 1(–2)×furcate; primary segments up to 6 mm long; 2 or 3 secondary segments, up to 2 mm long; rarely 2 or 3 tertiary segments, up to 2 mm long (Fig. 10c). Dactyl cells swollen or inflated 1–2 mm long, 0.5–1 mm wide, sometimes narrowing distally to be confluent with the mucronate end-cell. End-cell 75 µm long × 30–40 µm wide at the base (Fig. 10f). Fertile dactyls as for sterile; individual segments can be less inflated on male plants. Gametangia on separate plants (Fig. 10d, f). Oosporangia up to 230 µm long, 190 µm wide; coronula up to 18 µm high, 6 or 7 helical stripes (Fig. 10g). Oospores 200 µm long × 170 µm wide with 6 or 7 striae of thick ridges; the striae uniting in a small apical crest (Fig. 10h). Oospore membrane shallowly reticulate with (0–)1–2 smooth meshes across the fossa (Fig. 10i). Antheridia up to 450–470 µm in diameter. Chromosome numbers n = 9.
Nitella micklei (a, b) from P.K.Latz 16363 (DNA), (c–g) from holotype specimens M.T.Casanova PBS17: r114 (MEL), (h, i) from M.N.Lyons & D.A.Mickle 3063 (PERTH). (a) Three whorls of branchlets, scale: 1 cm. (b) Single whorl of antheridial branchlets, scale: 1 mm. (c) Sterile branchlet whorl, scale: 5 mm. (d) Fertile branchlet whorl, scale: 1 mm. (e) Apex of fertile branchlet, scale: 1 mm. (f) Fertile female branchlets with oogonium, scale: 1 mm. (g) Oogonium with 5 or 6 convolutions, scale: 100 µm. (h) Scanning electron microscope (SEM) image of oospore, scale: 50 µm. (i) SEM image of detail of the reticulum of the oospore wall, scale: 2 µm.

A low growing but distinctive shrubby species with somewhat inflated bicellulate dactyls and robust axes, not at all slender. The oospores are distinctively patterned. Nitella tumida Nordst., which has not yet been found in the Northern Territory, has much more inflated, even spherical dactyls, and slender axes.
WESTERN AUSTRALIA: Koodjeepindawarranna Pool, 19 Aug. 2006, M.T.Casanova t254 (MEL); Gnalka Gnoona, 19 Aug. 2006, M.T.Casanova t256 (MEL). Fortescue River at Millstream (Palm Pool), 21 Aug. 2006, M.T.Casanova t258 (MEL); Ethel Creek Claypan, 1 Aug. 2004, D.A.Mickle & M.N.Lyons 3073 (PERTH); Cooliarin Pool T1, 18 May 2004, M.N.Lyons & D.A.Mickle 3063 (PERTH). NORTHERN TERRITORY: Jimmy Dam, 23 km ESE Erldunda Homestead, 26 June 2000, P.K.Latz 16363 (DNA).
Nitella myriotricha A.Braun ex Kütz. Tab. Phycol. 7: 15, pl. 39. (1857)
Type: Prope McIvor, 1852?, F. v. Mueller 819 (holo: MEL!; iso: MEL!).
Dioecious. Plants elongate, fine and flabellate with thick mucus on the upper whorls; up to 210 mm high (Fig. 11a). Axes 800–950 µm in diameter; internodes 28–60 mm long; branchlets up to 30 mm long. Fertile branchlets contracted and covered in mucus (Fig. 11e); 5 or 6 in a whorl, usually 3 × but up to 4×furcate. Primary segments 1–2 mm long, furcate into 5–7 secondary segments up to 500 µm long; these again furcate into 4 or 5 tertiary segments ~500 µm long; these again furcate into 2–5 quaternary segments (dactyls), each 2 cells long and 500–1500 µm long. The end-cells are elongate (200–300 µm long) and briefly apiculate. Sterile branchlets 6 in a whorl, 3 or 4×furcate, to 30 mm long; primary segments approximately half the branchlet length, up to 15 mm long and 200 µm wide; secondary segments 5 or 6, 2–3 mm long and 100 µm wide; tertiary segments 2–4 to 2–3 mm long and 20 µm wide; quaternary segments 1–4, up to 2 mm long and 15 µm wide; quinary segments (where present) 3–5, up to 3 mm long and 10 µm wide (Fig. 11c). Sterile dactyls 3 or 4 cells long (Fig. 11b); end-cells variable but generally elongate and apiculate (Fig. 11d). Gametangia at all furcations. Oosporangia up to 310 µm long; coronula ~20 µm high, upper row of cells similar to lower row of cells (Fig. 11e). Oospores dark brown, 210–280 µm long, 190–210 µm wide with 7 or 8 striae with smooth flanges 10–30 µm high (Fig. 11f), uniting into a crest at the apex of the oospore. Ornamentation of a smooth reticulum, the ridges sometimes developing small papillae. The specimen illustrated appears to have an incomplete reticulum (Fig. 11g). Basal-cell impressions rectangular and triangular (Fig. 11h). Antheridia not known. Chromosome numbers not known.
Nitella myriotricha (a, b) from specimen J.Schult t842 (MEL), (c–h) from specimen P. Dostine 59DW73-2 (MEL). (a) Habit of shoot, scale: 10 cm. (b) Sterile branchlet tips, scale: 0.5 mm. (c) Sterile whorl, scale: 5 mm. (d) Branchlet tips, scale: 100 µm. (e) Female fertile branchlet, scale: 5 mm. (f) Scanning electron microscope (SEM) image of oospore, scale: 100 µm. (g) SEM image of detail of oospore wall, scale: 20 µm. (h) Impression of end-cells on oospore wall, scale: 50 µm.

Nitella nitida Casanova & Karol, sp. nov.
Type: Manton Dam Recreation Area, 0.45 m depth, 5 Sep. 2010, M.T.Casanova & B.M.Atkinson r754 (holo: DNA!; iso: MEL!, NY!).
Dioecious. Plants with uneven spreading branchlet whorls; up to 15 cm high; with reproductive structures on shorter whorls (Fig. 12a, d). Axes up to 350 µm wide; internodes up to 30 mm long, approximately as long as the branchlet whorls except in the fertile parts. Fertile branchlets 7 or 8 in a whorl, the male whorls more contracted and shorter than the sterile whorls (Fig. 12b); females similar to the sterile whorls (Fig. 12f). Primary segments on male branchlets up to 10 mm long, longer on female branchlets; secondary segments 3–6, some of them dactyls, 0.1–0.3 mm long; the shorter ones divided into up to 6 tertiary segments, up to 0.3 mm long; sterile branchlets 7 or 8 in a whorl, up to 20 mm long, with long primary segments and often more furcate than fertile branchlets; primary segments up to 13 mm long, unevenly furcate into 3–7 secondary segments which are 4–8 mm long; some again divided into 3–8 tertiary segments, up to 5 mm long (Fig. 12g). Fertile dactyls 3–7; bicellulate, the end-cell shortly conical and acute, confluent with the end of the penultimate cell (Fig. 12c). Sterile dactyls similar to fertile but often longer. Heads not formed, however the fertile whorls are often well separated along the upper axes. Gametangia on separate plants; antheridia single and central at the final 1 or 2 furcations (Fig. 12g); oosporangia single, lateral to the final 1 or 2 fertile branchlet nodes. Oosporangia up to 500 µm long, 300 µm wide with 7 helical stripes (Fig. 12e); coronula up to 30 µm high, the upper cells shorter than the lower cells. Oospores 230–250 µm long × 200–210 µm wide with 5 or 6 flanges; the flanges mostly free of ornamentation, 10–20 µm high (Fig. 12h); ornamentation verrucate-papillate (Fig. 12i), that is small papillae arising from the verrucae, nippled (Fig. 12j). Antheridia up to 100 µm in diameter (Fig. 12c). Chromosome numbers not known.
Nitella nitida (a–c) from isotype specimen M.T.Casanova & B.M.Atkinson r754 (MEL) and (d–j) from M.T.Casanova & B.M.Atkinson r753 (MEL). (a) Habit of whole male plant, scale: 10 mm. (b) Fertile male branchlet whorl, scale: 5 mm. (c) Male fertile final furcations, scale: 500 µm. (d) Habit of whole female plant, scale: 10 mm, (e) Oogonium, scale: 0.1 mm, (f) Fertile female branchlet, scale: 1 mm, (g) Fertile female branchlet whorl, scale: 5 mm, (h) Scanning electron microscope (SEM) image of oospore, scale: 50 µm. (i) SEM image of detail of oospore wall, scale: 10 µm, (j) SEM image of side view of oospore ornamentation, scale: 10 µm.

The oospores of this new species are very similar to those of a specimen collected in 1887 (W.A.Persieh 48, BM!) and labelled Nitella polyglochin A.Braun ex Nordst., which was Braun’s collective name for some species related to N. furcata (Roxb. ex Bruzelius) C.Agardh. However, N. polyglochin is described as monoecious, and N. nitida is clearly dioecious (Specimen r754 is male, Specimen r753 is female).
This species has interrupted fertile whorls, with very long primary branchlet segments; in some cases, the distal segments appear like little brushes at the tips of the branchlets. This feature is similar to Nitella oollooensis, but in that species the branchlet tips are more uneven and there is a central percurrent branchlet segment. The two can also be distinguished on the basis of oospore ornamentation. Other species of Nitella can be distinguished on the dactyl structure, oospore ornamentation, the presence of abundant mucus or the overall appearance of the branchlet whorls.
Nitella nitida was found in shallow water, in the vicinity of Darwin, but quite likely occurs across the wetter parts of the Northern Territory and possibly Queensland.
Nitella oollooensis Casanova & Karol, sp. nov.
Type: Site 5, Daly River, 0.15 m deep, 6 Sep. 2010, M.T.Casanova & J.Schult r776 (holo: DNA!).
Dioecious. Plants small, with spreading whorls and no mucus; up to 10 cm high; with bunches of furcations at the elongate fertile branchlet tips (Fig. 13a, b). Axes up to 350 µm wide; internodes up to 35 mm long, longer than the branchlet whorls. Fertile branchlets 6–8 in a whorl, with a central secondary segment (monopodial); primary segments up to 5–7 mm long; secondary segments 4–6, of which one is central and longer, up to 2 mm long; the shorter ones divided into 4–6 tertiary segments, up to 0.5 mm long, some of which are dactyls; the central one divided into 5–7 segments of which one is central; some again divided into 2–4 quaternary segments (dactyls) (Fig. 13d). Sterile branchlets 6–8 in a whorl, up to 15 mm long, with longer secondary and tertiary segments than in the fertile branchlets (approximately half the total length), but also with a central percurrent segment (Fig. 13f–h); primary segments up to 6 mm long, secondary segments 3–5, 3–5 mm long, unevenly divided into 3–5 tertiary segments, up to 1 mm long. Fertile dactyls bicellulate (Fig. 13d); the end-cell shortly conical and acute, confluent with the end of the penultimate cell. Sterile dactyls similar to fertile but often longer. Heads not formed; however, the fertile whorls are often well separated along the upper axes. Gametangia on separate plants, antheridia single and central at the final furcations (Fig. 13e), oosporangia single and lateral to the 2 upper-most fertile branchlet nodes (Fig. 13c). Oosporangia up to 400 µm long × 300 µm wide with ~8 helical stripes; coronula up to 20 µm high, the upper cells as long as the lower cells. Oospores 230–240 µm long × 200–230 µm wide with 7 or 8 flanges ~8 µm high (Fig. 13i), the ornamentation of interlocking sinuous verrucae (Fig. 13j), becoming linear onto the flanges. Antheridia up to 200 µm in diameter (Fig. 13e). Chromosome numbers not known.
Nitella oollooensis (a, c, g i j) from holotype specimen M.T.Casanova & J.Schult r776 (MEL), (b, h, e) from specimen M.T.Casanova & J.Schult r770 (MEL), and (d, f) from specimen M.T.Casanova & J. Schult r767 (MEL). (a, b) Habit of whole plant, scale: 10 mm. (c) Fertile female branchlet tip, scale: 500 µm (d) Dactyls, scale: 100 µm. (e) Fertile male branchlet tip, scale: 200 µm. (f, g, h) Sterile whorls, scale: 5 mm. (i) Scanning electron microscope (SEM) image of oospore in side view, scale: 50 µm. (j) SEM image of oospore ornamentation, scale: 10 µm.

This species would have been designated a form of Nitella pseudoflabellata var. imperialis (≡N. imperialis) in the past because of the central percurrent ray. However, N. imperialis differs in having an oospore with a fibrous construction. The central percurrent rays are reminiscent of N. monopodiata Raam from Tasmania; however, that species is monoecious, rather than dioecious. The presence of ‘little brushes’ of furcations at the branchlet tips is reminiscent of the description of N. robertsonii A.Braun by Nordstedt (1883), but the oospores are quite different.
The central rays and the spreading fertile branchlets with elongate primary segments are distinctive. In Nitella nitida, there are elongate primary segments on the fertile whorls; however, there is no central ray, and the branchlets are less furcate.
This species is named for the location of its habitat (Oolloo Crossing on the Daly River) and for the Oolloo aquifer that keeps the river flowing perennially.
NORTHERN TERRITORY: Oolloo Crossing, Daly River, 0.15 m deep, 6 Sep. 2010, M.T.Casanova & J.Schult r767 (DNA, MEL) r770 (MEL), r772 (DNA, MEL); Fish River station, near northern creek, 0.2 m depth, 26 Apr. 2012, I.D.Cowie 13207 (DNA 215653); Middle Creek, Douglas–Daly region, 0.25 m depth, 6 Sep. 2010, M.T.Casanova r763 (MEL).
Nitella silicea Casanova & Karol, sp. nov.
Type: Woodford Lagoon, in 0.35 m of water, 3 Sep. 2010. M.T.Casanova & J.Schult r741 (holo: DNA!; iso MEL!, NY!).
Dioecious. Plants occurring as isolated, elongate plants with flabellate whorls; up to 4×furcate (Fig. 14a). Axes up to 450 µm wide; internodes up to 100 mm long. Fertile branchlets 6 in a whorl, similar to the sterile branchlets but somewhat smaller (Fig. 14c); primary segments up to 10 mm long; secondary segments 6 or 7, up to 11 mm long; tertiary segments 3–5, up to 11 mm long; quaternary segments (usually dactyls) 2 or 3, up to 1 mm long, rarely further divided, 2 dactyls up to 0.5 mm long; sterile branchlets 6 in a whorl; up to 30 mm long; hardly distinguished from fertile branchlets (Fig. 14b). Fertile dactyls 2 or 3; bicellulate, the end-cell shortly conical and acute, confluent with the end of the penultimate cell; dactyls of variable lengths, up to 1 mm long (Fig. 14d). Sterile dactyls similar to fertile; however, where there are fewer furcations the dactyls can be up to 3 mm long. Heads not formed, the upper whorls not much contracted. Gametangia on separate plants, antheridia single and central at the final 2 furcations. Oosporangia geminate or single, lateral to the upper-most fertile branchlet nodes (Fig. 14d). Oosporangia up to 400 µm long × 350 µm wide with 8 helical stripes; coronula up to 35 µm high, the upper cells longer than the lower cells (Fig. 14e). Oospores 290–300 µm long × 270–290 µm wide with 7 or 8 smooth striae (Fig. 14f); the ornamentation consists of closely packed, rounded verrucae, similar to cobblestones (silice) (Fig. 14g). Antheridia up to 250 µm in diameter. Chromosome numbers not known.
Nitella silicea from isotype specimen M.T.Casanova & J.Schult r741 (MEL). (a) Habit of whole plant, scale 10 mm. (b) Sterile whorl, scale: 1 mm. (c) Female fertile banchlet, scale: 1 mm. (d) Fertile dactyls, scale: 1 mm. (e) Oogonium, scale: 100 µm. (f) Scanning electron microscope (SEM) image of oospore, scale: 100 µm. (g) SEM image of detail of oospore wall, scale: 20 µm.

These specimens would have been distinguished as Nitella furcata or N. mucronata (A.Braun) Miq. by Wood (1971) because of the short dactyls; however, Wood’s concept of those species was an amalgamation of morphologically similar monoecious (and ‘submonoecious’, in the case of N. flagelliformis A.Braun) species, including N. tumulosa Zaneveld, N. oligospira A.Braun, N. inversa Imahori, N. japonica T.F.Allen, N. axillaris A.Braun, N. axilliformis Imahori and N. orientalis, all of which are now recognised as distinct species (Sakayama et al. 2004, 2005; Sakayama 2008). Wood (1971) found that the herbarium specimens that he called N. furcata in Australia were almost invariably sterile or poor material. Nitella silicea is dioecious rather than monoecious, and appears to be restricted to the Northern Territory around Darwin. Other dioecious species in this group have different oospores (e.g. N. duthieae J.Groves & E.L.Stephens).
Nitella silicea is distinguished by its very short dactyls (terminal branchlet segments), gametangia at the distal-most furcations, a lack of mucus and distinctive oospores.
Nitella silicea grows in shallow lagoons at the end of the wet season around Darwin, possibly more widespread, fertile in September.
Nitella townsendii Casanova, sp. nov.
Type: Northern Territory: Parkin Rd, Livingston, 6 May 2010, P.Dostine 49DW73-4 (holo: DNA!).
Monoecious. Plants up to 8 cm high, with mucus; apparently rhizomatous (Fig. 15a). Axes 400 µm wide; internodes 10–20 mm long, often longer than the sterile branchlets. Fertile branchlets 6–8 in a whorl (Fig. 15e); 2×furcate; primary segments to 1.5–4 mm long; secondary segments 4–7, some without gametangia up to 3 mm long; some much shorter (0.8 mm) and with gametangia; 2–4 tertiary segments 0.6–1 mm long. Sterile branchlets 6 in a whorl (Fig. 15d); unevenly 2 or 3×furcate; primary segments up to 15 mm long; secondary segments 5–7, up to 6 mm long; tertiary segments 6–8, up to 8 mm long. Fertile and sterile dactyls similar, bicellulate; 0.5–0.8 μm long, tapering to a long, conical, acute end-cell (Fig. 15c, g). Heads not formed; fertile branchlets somewhat contracted; mucus abundant around the gametangia (Fig. 15e). Gametangia conjoined at the fertile branchlet nodes, but antheridia appear to dehisce before oogonia mature (Fig. 15b). Oosporangia single or geminate (Fig. 15b, g), up to 350 µm long; coronula up to 15 µm high, upper cells longer (Fig. 15f). Oospores dark brown, 190–230 µm long × 180–190 µm wide with 7 or 8 striae (Fig. 15h) with robust, figured, porate flanges ~5 μm high; ornamentation of ropy, sinuous vermiculae and verrucae (Fig. 15j). End-cell impressions quite small (Fig. 15i). Antheridia up to 150 µm in diameter (Fig. 15i). Chromosome numbers not known.
Nitella townsendii (a, b) from specimen J.Schult t841 (MEL), (c–i) from holotype specimen P.Dostine 49DW73-4 (DNA). (a) Habit of whole shoot, scale: 5 mm. (b) Fertile branchlet with central antheridia and lateral oogonia, scale: 1 mm. (c) Sterile dactyls, scale: 0.5 mm. (d) Sterile whorl, scale: 1 mm. (e) Fertile whorl, scale: 1 mm. (f) Oogonium, scale: 100 µm. (g) Branchlet tip with only oogonia and dactyls, scale: 100 µm. (h) Scanning electron microscope (SEM) image of oospore, scale: 100 µm. (i) SEM image of base of oospore, scale: 50 µm. (j) SEM image of detail of oospore wall, scale: 20 µm.

The oospores and other morphological characters of this species are most similar to Nitella vermiculata J.Groves from Madagascar; however, the diameter of the vermiculate elements on the oospore wall of N. vermiculata are much narrower, and N. townsendii has a more ropy and perforate flange.
Nitella tumulosa Zaneveld, Blumea 4: 86 (1940)
Nitella mucronata f. tumulosa (Zaneveld) R.D.Wood, Taxon 11: 18 (1962); Nitella furcata f. tumulosa (Zaneveld) R.D.Wood, Revis. Charac. 1: 526 (1965).
Type: Java, ?Korthals (lecto: L n.v. [specimen in a small packet adjacent to the ‘TYPE’ label], fide R.D.Wood in R.D.Wood and K.Imahori, Revis. Charac. 1: 527 (1965)).
Monoecious. Plants up to 25 cm high; tangled and spreading (Fig. 16a). Axes 750–1000 µm wide; internodes up to 33 mm long, as long as the sterile branchlets. Fertile branchlets 6 in a whorl; 2 or 3×furcate (Fig. 16d); primary segments up to 5 mm long; secondary segments 1–4, up to 4 mm long; 2–4 tertiary segments up to 4 mm long; 2–3 quarternary segments, very short, up to 200 μm long (=dactyls); sterile branchlets 6 in a whorl; 2× furcate (Fig. 16b); primary segments 15–20 mm long; secondary segments 1–3, up to 18 mm long; tertiary segments 2 or 3, up to 1 mm long. Fertile and sterile dactyls similar; bicellulate, 50–200 μm long, tapering to a conical, acute end-cell (Fig. 16e). Heads not formed; fertile branchlets somewhat contracted, no mucus present. Gametangia conjoined at the fertile branchlet nodes (Fig. 16f). Oosporangia single or geminate; coronula up to 15 µm high, upper cells longer (Fig. 16c). Oospores golden brown (Fig. 16g, h); up to 240 µm long × 225 µm wide with 7 prominent ridges (Fig. 16i), ornamentation a beaded incomplete reticulum (Fig. 16h, j). Antheridia up to 300 µm in diameter (Fig. 16f). Chromosome numbers not known.
Nitella tumulosa from specimen J.Schult t840 (MEL). (a) Habit of whole plant, scale: 5 mm. (b) Apical whorls, scale: 1 mm. (c) Oogonium, scale: 100 µm. (d) Fertile whorl, scale: 1 mm. (e) Dactyls, scale: 0.2 mm. (f) Placement of gametangia, scale: 1 mm. (g) Light micrograph (LM) of oospore, scale: 100 µm. (h) LM of detail of oospore wall, scale: 20 µm. (i) Scanning electron microscope (SEM) image of oospore, scale: 100 µm. (j) SEM image of detail of oospore wall, scale: 20 µm.

This determination is based on the illustration and description of the lectotype provided in Wood and Imahori (1964, icon 267) and on Sakayama et al. (2005) who illustrated a similar oospore under the name Nitella tumulosa. The specimens from the Northern Territory largely agree with the description of N. tumulosa var. typica (nom. inval.) by Zaneveld (1940), except for having smaller oospores and usually fewer segments at each furcation. However, Zaneveld’s illustration of the oospore (and perhaps the species name) was based on a second specimen (which he designated var. pumila, meaning ‘dwarfed’) (Zaneveld 1940; Wood and Imahori 1964, icon 268). The illustration shows small mounds or ‘tumuli’ on the oospore wall, rather than the ‘papillate or beaded imperfect reticulum’ described by Sakayama et al. (2005) or the papillate appearance illustrated by Imahori (in Wood and Imahori 1964, icon 267). On the weight of evidence, without having viewed the type material, but in agreement with the description of the oospore illustrated by Sakayama et al. (2005) the name N. tumulosa is applied here.
The short, usually paired dactyls on uneven branchlets, monoecy and clustered gametangia are diagnostic for this species.
Thailand, Malaysia, Indonesia and the Northern Territory, in shallow, shaded, slow-flowing or still water.
Named for the appearance of the oospore wall, interpreted by Zaneveld (1940) to have tumuli, or low mound-like structures. It seems that this description refers to the oospore of Nitella tumulosa var. pumila rather than to that of the type variety.
Additional taxa
Among the specimens collected in the Northern Territory, there are additional taxa that have been seen in insufficient quantity or quality to determine, or to use as the basis of new species descriptions.
A monoecious taxon similar to Nitella hyalina has been collected at Willoughby Bore (G.C.Taylor 7 (DNA, MEL)) and the Daly River at Mt Nancar (J.Schult A299 (MEL)); however, the Willoughby Bore specimen has oospores with a verrucate surface, rather than the usual fibrous structure of N. hyalina.
A single gathering has been made of a taxon with very smooth oospores and bicellulate dactyls (P.K.Latz 18111 (DNA)), but the specimen is so covered with mud and adherent particles that it is not a good candidate for identification, or description.
A number of specimens from Katherine River collected by J. Schult are distinctive, but infertile, and determination of these awaits further gatherings.
Discussion
This study of the Northern Territory charophytes has dealt with the tribe Nitelleae in family Characeae. It included the following seven previously described species: Nitella belangeri, N. biformis, N. congesta, N. heterophylla, N. micklei, N. myriotricha and N. tumulosa, of which N. tumulosa and N. belangeri are recorded as new for the Australian flora. In addition, nine new species (N. acanthospora, N. boreali-australis, N. crocodylus, N. limosa, N. martinii, N. nitida, N. oollooensis, N. silicea, N. townsendii) are described.
Of the five Nitella species listed by Wood (1971) for the Northern Territory, only N. myriotricha can be confirmed. Most of Wood’s taxa were either misidentifications, misplacements (erroneous localities) or amalgamations based on his monograph (Wood and Imahori 1965). Thus, the remaining 15 species listed here are either new records (6 spp.), or species newly described on the basis of the Northern Territory material (9 spp.). Nitella hyalina possibly occurs in the region, but was not confirmed in this study.
The charophyte flora of this region can be divided into species of the wet–dry tropics, with both endemic (i.e. restricted to Australia) and cosmopolitan taxa, and a suite of Australian species that probably occur throughout the arid and semi-arid zones (Nitella acanthospora, N. congesta, N. heterophylla, N. micklei). The species in the wet–dry tropics have affinities with those in South-East Asia (Zaneveld 1940), although there are few studies on the charophytes of our nearest neighbours (East Timor, Papua, Indonesia, Filarszky 1934; New Guinea, Leach and Osborne 1985). Species with a wider distribution are N. belangeri (South-East Asia) and N. tumulosa (Malaysia), and these are monoecious. Approximately 75% of the Northern Territory Nitelleae are dioecious, a character that is strongly linked with endemism in the Characeae (Proctor 1980).
In general, although Characeae have been collected from a large number of localities in Northern Territory, some of the specimens are virtually unusable for taxonomic work. Good presentation requires a degree of care, namely, decalcification if calcified, floating out onto herbarium sheets in the fresh state, sometimes shaking in water, or submitting to a spray of water to remove debris. ‘Less is more’ when it comes to mounting on sheets. Specimens that are poorly presented are sometimes impossible to determine. In the genus Nitella, if the material is fertile and female, oospores can be used to determine species. However, if the species is new or poorly known, well-presented vegetative material is required. If the time or tools for mounting on sheets are not available, then preservation in 70% alcohol is a reasonable alternative and supplement.
A number of specimens from arid-zone water bodies have been provisionally identified as Nitella lhotzkyi or N. hyalina, but they were so encumbered with sediment that it was not possible to tell whether they have secondary whorls or not. However, their overall appearance fits with those species that have an occasional secondary branchlet at the base of the whorls. These specimens have not been included in this treatment.
The perils of using previous descriptions are demonstrated when examining the group of species related to Nitella myriotricha. In Nordstedt (1883), N. myriotricha is segregated by having ultimate branchlet segments of 3–6 cells, dioecy, oosporangia absent from the base of the whorls, fertile whorls congested, but well separated (interrupted), sterile branchlets 3–4× furcate, and the last and penultimate cells of the same diameter. Braun’s description in Nordstedt (1883) is a combination of observations on the type material (from Victoria) and a specimen collected near Brisbane (Moreton Bay). Imahori’s illustration (in Wood and Imahori 1964, icon 218) and description (Wood and Imahori 1965) were based on the Moreton Bay material and Braun’s description, and Wood (1971) merely copied that description without modification (despite having seen additional material, including types). Examination of ~50 specimens in this group including type material of N. myriotricha (MEL), N. huillensis (A.Braun & Welw.) T.F.Allen (MEL), N. pulchella T.F.Allen (BM, NY), N. blowiana J.Groves (BM), N. mirabilis Nordst. ex J.Groves (LD), N. plumosa A.Braun (PC) and specimens of N. dualis Nordst. ex T.F.Allen and N. superba Pal, as well as specimens identified as N. myriotricha from Western Australia, South Australia, Queensland, Victoria and Tasmania, indicated that although all these species are vegetatively similar (and very difficult to examine when pressed and dried), their oospores are all different. The Australian material that has been grouped as N. myriotricha consists of at least three species (N. myriotricha sens. str., N. limosa and a taxon from Western Australia), and possibly more.
Several species remain problematic; Nitella phauloteles is poorly known, and it was not possible to obtain a ripe oospore from the type material. A monoecious species similar to N. arthroglochin (Nordst.) Casanova, probably related to N. hookeri A.Braun, has been collected from Waldapungu water hole (I.J.Powling v050 (MEL)), and mature material is needed to determine its identity.
On the basis of the ~35 specimens (some of which are listed above) that cannot be identified in the current treatment, there remains undocumented diversity in the Northern Territory charophyte flora, and new collections of good specimens from the arid and semi-arid zones are likely to allow additional species to be recognised.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Declaration of funding
Funds for this work were provided by the Australian Biological Resources Study, Bush Blitz Grant Number TTC216-10 and by Charophyte Services to M. T. Casanova. This study is also based on work supported by the United States National Science Foundation under Grant Numbers DEB-1020660 and DEB-1036466 to K. G. Karol.
Acknowledgements
Julia Schult and Simon Townsend are particularly thanked for their assistance with this project. For this study, access to type material in various American and European (B, BM, BP, GFW, GOET, HBG, L, LD, LE, NY, PC and W) and Australian (AD, BRI, CANB, DNA, HO, MEL, NSW, NT, PERTH) herbaria was essential. M. T. Casanova thanks the directors and staff of these institutions for their kind assistance and permission to remove oospores for examination. Many people have assisted with providing specimens, fresh and preserved, since 2000; some of their names are noted in the specimen lists. This study would have been impossible without their efforts. Beth Williams provided notes, translations and literature in an early part of this study. This paper has benefited from meticulous proof-reading and correction of nomenclature by Anna Monro (CANB).
References
Braun A (1849) Charae Indiae Orientalis et Insularum Maris Pacifici. [Charas of the East Indies and the Islands of the Pacific Sea.]. Hooker’s Journal of Botany and Kew Garden Miscellany 1, 292-301 [In Latin].
| Google Scholar |
Braun A (1859) Characeen aus Columbien, Guyana und Mittelamerika. [Characeae from Colombia, Guyana and Central America.]. Monatsberichte der Königlichen Preussischen Akademie der Wissenschaften zu Berlin 1858, 349-368 [In German].
| Google Scholar |
Casanova MT (2005) An overview of Chara L. in Australia (Characeae, Charophyta). Australian Systematic Botany 18, 25-39.
| Crossref | Google Scholar |
Casanova MT (2007) Typification and circumscription of Nitella sonderi (Characeae, Charophyceae). Australian Systematic Botany 20, 464-472.
| Crossref | Google Scholar |
Casanova MT (2009) An overview of Nitella (Characeae, Charophyceae) in Australia. Australian Systematic Botany 22, 193-218.
| Crossref | Google Scholar |
Casanova MT (2013) Lamprothamnium in Australia (Characeae, Charophyceae). Australian Systematic Botany 26, 268-290.
| Crossref | Google Scholar |
Casanova MT, Karol KG (2008) Monoecious Nitella species (Characeae, Charophyta) from south-eastern mainland Australia, including Nitella paludigena sp. nov. Australian Systematic Botany 21, 201-216.
| Crossref | Google Scholar |
Casanova MT, Karol KG (2014) A revision of Chara sect. Protochara, comb. et stat. nov. (Characeae: Charophyceae). Australian Systematic Botany 27, 23-37.
| Crossref | Google Scholar |
Casanova MT, Karol KG (2023) Charophytes of Australia’s Northern Territory – I Tribe Chareae. Australian Systematic Botany 36, 38-79.
| Crossref | Google Scholar |
Casanova MT, Nicol J (2009) Chara canescens (Characeae, Charophyceae) in the Southern Hemisphere. Charophytes 1, 55-60.
| Google Scholar |
Casanova MT, Porter JL (2013) Two new species of Nitella (Characeae, Charophyceae) from arid-zone claypan wetlands in Australia. Muelleria 31, 53-59.
| Crossref | Google Scholar |
Cirujano Bracamonte S, Guerrero Maldonado N, García Murillo P (2013) The genus Tolypella (A.Braun) A.Braun in the Iberian Peninsula. Acta Botanica Gallica 160, 121-129.
| Crossref | Google Scholar |
Crawford SA, Higgins MJ, Mulvaney P, Wetherbee R (2001) Nanostructure of the diatom frustule as revealed by atomic force and scanning electron microscopy. Journal of Phycology 37, 543-554.
| Crossref | Google Scholar |
Filarszky F (1934) Die Characeen der Deutschen Limnologischen Sunda-Expedition. [The Characea of the German Limnological Sunda Expedition.]. Archiv für Hydrobiologie Suppl.-Bd XII, Tropische Binnengewässer Band IV, 705-726 [In German].
| Google Scholar |
García A, Chivas AR (2006) Diversity and ecology of extant and Quaternary Australian charophytes. Cryptogamie, Algologie 27, 323-340.
| Google Scholar |
Groves J, Allen GO (1935) A review of the Queensland Charophyta. Proceedings of the Royal Society of Queensland 46, 34-59.
| Crossref | Google Scholar |
Karol KG (2004) Phylogenetic studies of the Charales: the closest living relatives of land plants. PhD dissertation, University of Maryland, College Park, MD, USA. Available at http://hdl.handle.net/1903/2074
Lyons MN (2015) The riparian flora and plant communities of the Pilbara region of Western Australia. Records of the Western Australian Museum, Supplement 78, 485-515.
| Crossref | Google Scholar |
Mueller F (1889) Notes on the geographical distribution of Australian Characeae. Transactions, Proceedings and Report of the Royal Society of South Australia 12, 149.
| Google Scholar |
Nordstedt CFO (1883) Fragmente einer Monographie der Characeen. [Fragments of a monograph of Characea.]. Abhandlungen der Königlichen Akademie der Wissenschaften in Berlin 1882, 1-211 [In German].
| Google Scholar |
Pérez W, Hall JD, McCourt RM, Karol KG (2014) Phylogeny of North American Tolypella (Charophyceae, Charophyta) based on plastid DNA sequences with a description of Tolypella ramosissima sp. nov. Journal of Phycology 50, 776-789.
| Crossref | Google Scholar |
Proctor VW (1980) Historical biogeography of Chara (Charophyta): an appraisal of the Braun–Wood classification plus a falsifiable alternative for future consideration. Journal of Phycology 16, 218-233.
| Crossref | Google Scholar |
Ramdani M, Flower RJ, Elkhiati N, Kraïem MM, Fathi AA, Birks HH, Patrick ST (2001) North African wetland lakes: characterization of nine sites included in the CASSARINA Project. Aquatic Ecology 35, 281-302.
| Crossref | Google Scholar |
Sakayama H (2008) Review: Taxonomy of Nitella (Charales, Charophyceae) based on comparative morphology of oospores and multiple DNA marker phylogeny using cultured material. Phycological Research 56, 202-215.
| Crossref | Google Scholar |
Sakayama H, Nozaki H, Kasaki H, Hara Y (2002) Taxonomic re-examination of Nitella (Charales, Charophyceae) from Japan, based on microscopical studies of oospore wall ornamentation and rbcL gene sequences. Phycologia 41, 397-408.
| Crossref | Google Scholar |
Sakayama H, Hara Y, Nozaki H (2004) Taxonomic re-examination of six species of Nitella (Charales, Charophyceae) from Asia, and phylogenetic relationships within the genus based on rbcL and atpB gene sequences. Phycologia 43, 91-104.
| Crossref | Google Scholar |
Sakayama H, Miyaji K, Nagumo T, Kato M, Hara Y, Nozaki H (2005) Taxonomic reexamination of 17 species of Nitella subgenus Tieffallenia (Charales, Charophyceae) based on internal morphology of the oospore wall and multiple DNA marker sequences. Journal of Phycology 41, 195-211.
| Crossref | Google Scholar |
Sakayama H, Arai S, Nozaki H, Kasai F, Watanabe MM (2006) Morphology, molecular phylogeny and taxonomy of Nitella comptonii (Charales, Characeae). Phycologia 45, 417-421.
| Crossref | Google Scholar |
Schubert H, Blindow I, Bueno NC, Casanova MT, Pełechaty M, Pukacz A (2018) Ecology of charophytes – permanent pioneers and ecosystem engineers. Perspectives in Phycology 5(1), 61-74.
| Crossref | Google Scholar |
Wallman J (1856) Essai d’une exposition de la famille des Characées. Traduit du Suédois par M. le Dr W. Nylander. [Essay of an exhibition of the Characeae family. Translated from the Swedish by Dr W. Nylander.]. Actes de la Société Linnéenne de Bordeaux 21, 1-90 [In French].
| Google Scholar |
Wood RD (1958) Some Characeae from Arnhem Land. Records of the American–Australian Scientific Expedition to Arnhem Land 3, 137.
| Google Scholar |
Wood RD (1962) New combinations and taxa in the revision of Characeae. Taxon 11, 7-25.
| Crossref | Google Scholar |
Wood RD (1971) Characeae of Australia. Nova Hedwigia 22, 1-120.
| Google Scholar |
Zaneveld JS (1940) The Charophyta of Malaysia and adjacent countries. Blumea 4, 1-223.
| Google Scholar |


