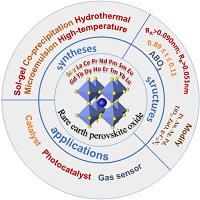
This paper reviews recent progress in rare-earth perovskite oxide materials, explores the effects of capacity factors, lattice defects, different metal active centers and perovskite modifications on crystal structure and applied properties, discusses the effects of synthesis processes (sol-gel, co-precipitation, microemulsion, hydrothermal, high-temperature) on their physical and chemical properties, and summarizes their applications in pollutant catalytic oxidation and photocatalytic degradation, VOCs gas sensing, and photocatalytic splitting of water for hydrogen production and carbon dioxide reduction and conversion. (Image credit: P. Li.)

Australian Journal of Chemistry
Volume 77 Number 3 2024
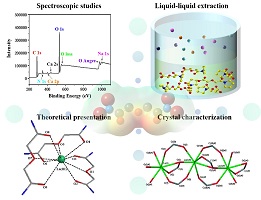
In the analytical methods for the extraction of rare-earth elements (REEs) by diglycolamide (DGA), liquid–liquid extraction experiments can demonstrate basic extraction properties, spectroscopic techniques can provide information on the inner-sphere coordination of REEIII–DGA complexes, and scattering techniques can be used to study the size and morphology of aggregates with nanoscale structures. (Image credits: bottom right, G. B. Deacon et al.; other images, A. Gong.)
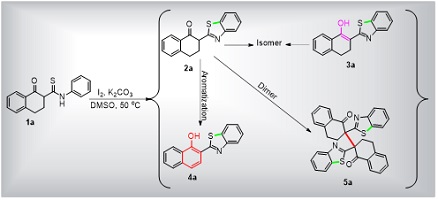
We have developed a facile oxidative cyclization of β-ketothioamides for the simultaneous formation of a compound library similar to natural product benzothiazole derivatives, which are important structural motifs that are prevalent in both natural and designed compounds and display interesting biological and physical properties. (Image credit: M. Cui.)
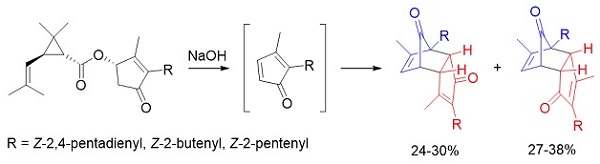
On treatment with base, the natural pyrethrins pyrethrin I, cinerin I and jasmolin I undergo elimination to transient cyclopentadienones that dimerise to afford regioisomeric endo cycloadducts. (Image credit: J. H. Ryan.)
CH23197 Abstract | CH23197 Full Text | CH23197PDF (1.4 MB) | CH23197Supplementary Material (2.3 MB) Open Access Article