The CLAW hypothesis: a review of the major developments
Greg P. Ayers A and Jill M. Cainey B CA CSIRO Marine and Atmospheric Research, Aspendale, Vic. 3195, Australia.
B Cape Grim Baseline Air Pollution Station, Bureau of Meteorology, Smithton, Tas. 7330, Australia.
C Corresponding author. Email: j.cainey@bom.gov.au
Environmental Chemistry 4(6) 366-374 https://doi.org/10.1071/EN07080
Submitted: 29 October 2007 Accepted: 12 November 2007 Published: 6 December 2007
Environmental context. Understanding the role of clouds in the warming and the cooling of the planet and how that role alters in a warming world is one of the biggest uncertainties climate change researchers face. Important in this regard is the influence on cloud properties of cloud condensation nuclei, the tiny atmospheric particles necessary for the nucleation of every single cloud droplet. The anthropogenic contribution to cloud condensation nuclei is known to be large in some regions through knowledge of pollutant emissions; however, the natural processes that regulate cloud condensation nuclei over large parts of the globe are less well understood. The CLAW hypothesis provides a mechanism by which plankton may modify climate through the atmospheric sulfur cycle via the provision of sulfate cloud condensation nuclei. The CLAW hypothesis was published over 20 years ago and has stimulated a great deal of research.
Abstract. The CLAW hypothesis has for 20 years provided the intriguing prospect of oceanic and atmospheric systems exhibiting in an intimately coupled way a capacity to react to changing climate in a manner that opposes the change. A great number of quality scientific papers has resulted, many confirming details of specific links between oceanic phytoplankton and dimethylsulfide (DMS) emission to the atmosphere, the importance of DMS oxidation products in regulation of marine atmospheric cloud condensation nucleus (CCN) populations, and a concomitant influence on marine stratocumulus cloud properties. However, despite various links in the proposed phytoplankton–DMS–CCN–cloud albedo climate feedback loop being affirmed, there has been no overall scientific synthesis capable of adequately testing the hypothesis at a global scale. Moreover, significant gaps and contradictions remain, such as a lack of quantitative understanding of new particle formation processes in the marine atmospheric boundary layer, and of the extent to which dynamical, rather than microphysical, cloud feedbacks exist. Nevertheless, considerable progress has been made in understanding ‘Earth System Science’ involving the integration of ocean and atmospheric systems inherent in the CLAW hypothesis. We present here a short review of this progress since the publication of the CLAW hypothesis.
Introduction
It is not often that a scientific paper such as that published in 1987 by Charlson, Lovelock, Andreae and Warren[1] remains immortalised 20 years on by an acronym that has become irretrievably embedded in the lingua franca of ongoing scientific banter. In this case the acronym is CLAW, taken from the authors’ names. The CLAW hypothesis is an early manifestation of what today would be described as Earth Systems Science, a terminology coined in response to the recognition that the grand challenges facing the earth and its inhabitants are not issues on which science can offer neat, single-disciplinary answers. Today when we think of challenges at a global scale in such areas as biodiversity conservation, drought and water resources management, agricultural production, fisheries management, or climate change, it is evident that none of these issues can be addressed as scientific problems by single disciplines such as biology, chemistry, physics, meteorology and the like. What is required is a multidisciplinary response in which the systemic links and dependencies between biological, chemical, physical and other systems are recognised explicitly and researched as a whole. Moreover, we can expect increasingly that optimum input of scientific knowledge into policy aimed at meeting these global challenges will see more explicit coupling of these traditional ‘hard’ disciplines to other disciplines that directly reflect the human dimensions of global change. This will require explicit coupling of biophysical sciences to social and economic sciences, which is sometimes labelled ‘socioeconomic integration’.
In the present short review, we explain and discuss the CLAW hypothesis from the original ideas that seeded it, through the major scientific breakthroughs contributing to our present understanding – in ocean science, atmospheric chemistry, clouds and cloud formation, and attempts to measure feedback – to why we think that we are now in a good position for rapid progress towards the necessary overall synthesis needed to test the hypothesis.
What makes CLAW a compelling, long-lived hypothesis?
There is a compelling simplicity to the overall picture painted in the CLAW hypothesis that has been part of its longevity and iconic status. The hypothesis posits a planetary scale homeostatis or dynamic equilibrium in which biological productivity in ocean surface waters is coupled to cloud properties over the ocean in a manner that acts to oppose variations in climate. The simple word-picture painted in the abstract of the original Charlson et al.[1] paper remains as evocative today as it was 20 years ago:
The major source of cloud condensation nuclei (CCN) over the oceans appears to be dimethylsulphide, which is produced by planktonic algae in sea water and oxidises in the atmosphere to form sulfate aerosol. Because the reflectance (albedo) of clouds (and thus the earth’s radiation budget) is sensitive to CCN density, biological regulation of the climate is possible through the effects of temperature and sunlight on phytoplankton populations and dimethylsulfide production.
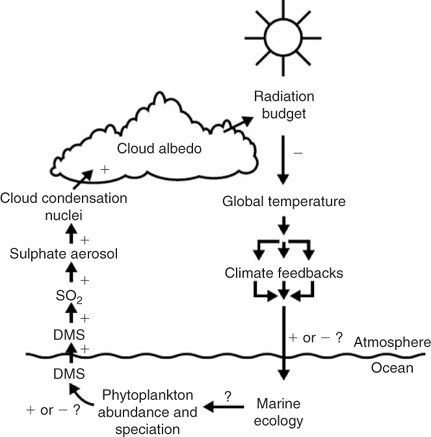
Put even more simply, the hypothesis is that oceanic phytoplankton when pushed to either extreme of their coping range (too hot and sunny or too cold and dim) will respond by altering their dimethylsulfide (DMS) emissions so as to decrease or increase solar input to the ocean surface by regulating marine stratiform cloud reflectivity (Fig. 1), hence driving the system back towards the middle of the phytoplankton coping range.
|
Although it was the CLAW hypothesis[1] that captured the imagination and interest of scientists, spurring significant research in this area, this was not the first publication to suggest a role for the sulfur cycle in modifying climate. The earlier work of Lovelock[2] and Lovelock and Marguilis[3] proposed the ‘Gaia hypothesis’ suggesting that the remarkably stable climate and atmospheric composition on Earth, over very long time scales, was the result of active intervention by life, while Twomey had drawn the connection between cloud reflectivity (albedo) and climate.[4,5]
Later work by Walker et al.[6] and Lovelock and Whitfield[7] suggested the carbon cycle, through carbon dioxide, as the means by which a climate feedback loop might operate and although Lovelock et al.[8] did discuss the near-ubiquitous presence of DMS throughout the marine environment, it was Shaw[9] who made the link between a climate feedback loop and the sulfur cycle.
The devil is in the detail
Despite the profundity inherent in the 1500 or more publications on CLAW since 1987, the challenge of proving the CLAW hypothesis remains: the devil is indeed in the detail. Dropping down from the high-level picture of the proposed feedback loop to the detail of how the phytoplankton dynamics, air–sea exchange, atmospheric chemistry and cloud microphysical and dynamical systems interact is non-trivial, even when specific details of each subsystem have been characterised in various ways in individual experiments. The system is neatly summarised by the diagram drawn by Charlson et al.[1] conceptually reproduced in Fig. 1. We will discuss developments below as they relate to this diagram.
The ocean
DMS in the atmosphere originates in the ocean, and DMS in the ocean is the result of biological activity. Dimethylsulfoniopropionate (DMSP) is the precursor compound to DMS[10] and DMSP is synthesised by phytoplankton, and the amount of DMSP is highly dependent on the species of plankton present.[11] Although direct emission of DMS from plankton in the laboratory has been observed,[12] it has not been observed in the oceans and more typically it is the action of grazers and viruses on the plankton community that results in significant levels of DMSP in the water column;[13,14] typically, the highest levels of DMSP and DMS are associated with the senescence of a plankton bloom.[15,16]
The role of DMSP or DMS in plankton physiology remains unclear.[17] DMSP conversion to DMS may be a mechanism to relieve oxidative stress[17] resulting from exposure to UV radiation, increasing surface temperatures or nutrient limitation,[17] although definitive evidence for an antioxidant role is still lacking.[12,17] DMSP has also been suggested to act as an osmoregulator and as a carrier in organic sulfur cycling.[18]
Because the role for DMSP and DMS in plankton is unclear, it is difficult to determine how emission of either DMSP or DMS from the plankton community would change as the surface water environment changed in response to climate change. It may be that changing ocean conditions alter the plankton community to promote species that are a high emitters of either DMSP or DMS, rather than altering the response of a given community to stress.[11]
The increasing acidity[19] of the ocean is expected to affect the ability of certain plankton species to construct calcium carbonate skeletons, with deformed plankton being observed.[20] Emiliania huxleyi is one such affected species of plankton that also contains high levels of DMSP,[13,21] but it is not yet clear what effect this damage has on E. huxleyi as a source of either DMSP or DMS.
Recent mesocosm studies in Norway[22] suggest that a doubling of carbon dioxide (CO2) leads to a 26% increase in the emission of DMS, whereas a tripling of CO2 leads to only an 18% increase in DMS emissions. However, other measurements during the same experiment[23] did not see a similar increase in DMS emissions for the same increase in CO2, suggesting that the response of biology to changes in CO2 levels is not consistent. Wingenter et al.[22] could not be sure if the increase in DMS emission was due to the response of the plankton, including E. huxleyi, to higher carbon dioxide or due to increased lysis or viral attack.
In the case of nutrient limitation, the series of iron addition experiments[24,25] have demonstrated that in high-nutrient, low-chlorophyll waters, the addition of iron promotes the formation of plankton blooms that enhance the draw-down of carbon dioxide. In some cases, but not all, the resulting bloom results in increased DMS in the water column[24] and it is unclear whether any climatic effect of large-scale fertilised blooms would be more strongly related to the removal of carbon dioxide from the atmosphere or any sulfate–CCN-induced effect on cloud properties.[26]
In order to understand how any CLAW-related feedback mechanism (positive or negative) between the plankton community and atmosphere might operate, it is essential that the biological role of DMSP/DMS within plankton cells is clarified, because this is inextricably tied to how the feedback between biology and atmosphere may respond to environmental changes, and an understanding of this feedback process in the CLAW hypothesis (the ‘right-hand’ side of Fig. 1) remains elusive.[12,27]
Ocean–atmosphere exchange
Once DMSP is in the water, there are several complex processes that convert the DMSP to DMS[16] in the water column. There are many loss processes in the water that control the amount of DMS that is then available to cross the sea–air interface to enter the atmosphere.
The air–sea interface is a very complex environment and much early work on determining the flux of DMS from the ocean to the atmosphere was strongly based in physics, using the solubility of DMS, concentrations of DMS in surface waters and air above, and wind speed to parameterise the flux according to the best available physical understanding.[28] One of the largest difficulties was obtaining DMS measurements in the water and air at wind speeds typical of the oceans commonly associated with large blooms (mid-to-high latitudes). For reasons related to platform and scientist safety, measurements in high wind speeds remained difficult to obtain, but over many years of research, various techniques including improvements to the wind speed-based parameterisations have been made,[29–31] as the complexities of the role of the surface microlayer, skin temperature and of breaking waves became evident. Ayers et al. in 1995[32] used high time-resolved measurements of atmospheric DMS at Cape Grim, the current wind speed parameterisations and a simple model of atmospheric DMS chemistry to infer a DMS flux.
More recently, it has been possible to directly measure the flux of DMS from the ocean using micrometeorological techniques such as relaxed eddy accumulation and eddy covariance.[33,34] This has required the development of highly sensitive analytical techniques to monitor DMS at high frequency.[34] The direct flux measurements of DMS are useful for determining the ability of wind speed-based parameterisations to predict fluxes.[33]
Atmospheric chemistry
Observations of atmospheric sulfur and sulfate species have increased the understanding the atmospheric processes that influence the sulfur cycle. In the atmosphere, it is most clear that the atmospheric sulfur cycle is distinctly split into two hemispheres; the northern hemisphere sulfur cycle is largely influenced by anthropogenic processes, through the burning of fossil fuels, and the emissions of sulfur dioxide from anthropogenic activities came to prominence through acid precipitation and resulting foliar damage in the 1980s.[35] Such is the burden of anthropogenic sulfur (and associated pollutants), it has been shown to have a strong surface cooling effect in regions of high emission, leading to a partial offset of greenhouse warming.[36–38]
In the southern hemisphere, with less land and fewer humans, it was possible to study the unperturbed sulfur cycle in the atmosphere. Long-term measurements at Cape Grim, Australia, showed the strong seasonal variation in non-sea-salt sulfate (NSS-sulfate) and methanesulfonate (MSA) aerosol, both products of the atmospheric oxidation of DMS, related to seasonal changes in the biologically driven source of DMS.[39] Later, continuous measurements of DMS at Cape Grim demonstrated a strong seasonal cycle, with interannual variability that was matched in the sulfate aerosol.[40]
The critical intermediate sulfur dioxide was hard to measure at Cape Grim,[41] but a modelling study encouraged the use of a novel technique[42] to monitor extremely low levels of sulfur dioxide,[43] showing that the most comprehensive modelling scheme for atmospheric sulfur available at the time[44,45] did an excellent job of reproducing the atmospheric sulfur cycle at Cape Grim in summer and demonstrated the influence of location (latitude), temperature, biology and photochemistry on the sulfur cycle.[43] Later work has shown that in some locations the OH radical is not the only oxidant and that halogen chemistry, in particular BrO, is probably involved in DMS oxidation. However, there still remains uncertainty as to the overall oxidation process and the importance of particular mechanisms in determining the balance of the stable intermediates and the final end products of DMS oxidation.[46,47]
Work in the Southern Ocean, including that of the First Aerosol Characterisation Experiment (ACE-1), advanced understanding of the role of the sulfur cycle in providing aerosol in the remote marine boundary layer (MBL),[48] with the remote Southern Ocean, particularly in the waters around Antarctica, providing a large source of DMS.[49,50]
However, although it was possible to demonstrate coherence in the seasonal cycles of DMS, sulfur dioxide, NSS-sulfate, MSA and CCN at Cape Grim,[41] this alone does not quantitatively confirm a role for biogenically derived sulfur in CCN. The aerosol chemistry measurements could only be made on particles between 0.1 and 12 µm in diameter, although CCN are typically smaller than 0.1 µm diameter.[51] Further, the aerosol chemistry measurements demonstrated that both MSA and NSS-sulfate had a strong relationship with surface area, suggesting heterogeneous processes rather than homogeneous nucleation.[41,52]
In the presence of pre-existing aerosol, sulfur dioxide will preferentially react on the surface of these particles, especially sea salt, rather than nucleate to form new particles.[52] The reaction of ozone with sulfur dioxide on sea salt also ensures a ready loss pathway for DMS-derived sulfur before it can nucleate to form CCN.[53] Recent work by von Glasow and Crutzen[47] suggests that the presence of even low levels of BrO reduces the conversion efficiency of DMS to sulfur dioxide, reducing the potential of DMS to provide a source of new CCN.[47]
In addition to the long-term monitoring at Cape Grim,[39,40] there have been short-term measurements made around the Southern Ocean[48–50,54–56] and the role of the sulfur cycle has been studied extensively at other locations including the tropics,[57–60] the Arctic,[61] Amsterdam Island[62–65] and Europe.[66–68] At each location, the sulfur cycle operates differently and exhibits different dependencies, further increasing the difficulty of understanding the role of the sulfur cycle in providing CCN.
Clouds and cloud formation
At Cape Grim, Ayers and Gras[69] demonstrated that the coherence in the seasonal cycles of atmospheric sulfur species matched the seasonal cycle in CCN numbers,[70] suggesting a role for DMS-derived sulfur in CCN chemistry. Further work using satellite-derived cloud optical depth (COD) downwind of Cape Grim and a model incorporating sulfur chemistry showed seasonal coherence between COD and CCN at this site,[71] while airborne measurements in summer and winter demonstrated a seasonal cycle in cloud droplet number and size exactly as expected based on the seasonal CCN cycle.[72]
However, despite the observed coherence of DMS and CCN number at Cape Grim, Raes[73] showed that CCN populations in the MBL could be supplied largely from above the boundary layer and the measurements made during ACE-1 further supported this suggestion.[74] Further work at Cape Grim[77] has provided more evidence that post-frontal entrainment of free troposphere air is largely the source of CCN at Cape Grim,[75] rather than DMS-derived CCN in the MBL.
DMS may not be responsible for controlling CCN numbers in the MBL at Cape Grim, but measurements in the free troposphere[55,76] suggest that DMS and DMS-derived sulfur dioxide could play a role in CCN production in the free troposphere,[52,73] which could then supply CCN numbers, through entrainment, to the MBL below, as proposed by Shaw et al.[77]
Cloud microphysics is highly complicated and non-linear, and CCN need to be activated before forming a cloud, and the chemistry of the CCN and the mode of activation and growth will affect the optical properties of the cloud formed.[78] Six years of global satellite measurements of aerosol optical depth (AOD) and cloud optical thickness (COT) have shown a decreasing trend in AOD, with a minor increase in COT,[78] but these global trends in COT and AOD showed regional variation and there was no strong relationship between AOD and COT except in the tropics[79] and rather than an increasing global trend in albedo, there is an increasing trend in the amount of radiation reaching the surface,[80] the opposite of what might be expected with a negative feedback with the CLAW hypothesis.
Feedbacks
Determining the strength and even the direction, positive or negative, of the feedbacks in the CLAW hypothesis has proved one of the most challenging aspects of research into the role of the sulfur cycle on climate modification.
Contrary to the suggestion that the CLAW hypothesis would lead to a cooling effect, Lovelock[81] suggested that in a warming world and with a warming ocean, the role of plankton in climate modification is much reduced and as such represents a positive feedback such that, as the ocean warms, there is less DMS and less potential for the cooling influence of clouds. Modelling by Lovelock and Kump[82] indicated that the sulfur cycle could exert its greatest influence over climate during glacial periods and in the absence of an anthropogenic influence. Gabric et al.[83] also used modelling to understand the DMS production in surface waters and then extended this work to assess the response of marine biology to a changing climate,[84,85] showing the expected increase in wind speed would enhance DMS fluxes to atmosphere.[86]
More recent modelling studies[87–89] suggest that climate change will result in a small, 1–2%, global increase in DMS emissions, with the potential for a small negative feedback (cooling). Although the global increase in DMS is small, there is much variation regionally, which could have local impacts on climate. The causes of the changes in DMS emissions are related to productivity, species changes in the plankton community and changes in wind speed.[87]
Curran et al.[90] used MSA in an Antarctic ice core to demonstrate the extent of sea ice from c. 1840 to present. MSA is thought to be a more or less conservative marker for DMS and lower MSA correlates with a lower extent of sea ice, because waters covered by sea ice in winter tend to be highly biologically active after sea ice melt, and a major source of DMS and hence MSA.[90,91] Satellite observations have shown that since 1950 the extent of the sea ice has diminished, corresponding to a decrease in MSA in the ice core,[90] which could imply a reduction in DMS emissions.[91,92]
The modelling study of Sarmiento et al.[93] suggests that as surface temperatures increase with increasing carbon dioxide, there will be an increase in primary productivity of between 0.7 and 8.1%. However, there would be major shifts from currently productive regions to other ocean regions.[93] Using satellite observations of ocean colour, Gregg and Conkright[94] have shown a reduction in global net primary productivity of ~10% since 1979, and this reduction is related to decreased availability of nutrients, through increased stratification.[95]
Observations of DMS have not been made over a consistently long timeframe at one location to allow the determination of a response to climate change, but research[22,23] indicates that the response of plankton communities to increasing carbon dioxide levels and to warming surface waters is highly complicated owing to many interrelated processes, including but not limited to fertilisation by carbon dioxide, the response of bacteria and viruses, changes in nutrient availability, changes in surface pH and changes in ocean chemistry due to temperature and light levels.[22,23]
Research using remote sensing has become an increasingly important approach to assessing potential feedbacks. Gabric et al.[96] assessed the coupling of ocean colour and AOD using satellite data and found strong correlations on short timescales. Modelling by Vallina et al.,[97] assessing the relative contribution to CCN by biogenic-derived sulfur and sea salt over the Southern Ocean, found that DMS-derived CCN were important in summer. In more recent work by Vallina and Simó,[98] again using remote sensing measurements, a strong relationship between DMS in the water column and solar radiation dose was found, suggesting a negative feedback such that DMS increases as solar radiation dose increases. However, significant difficulties remain in determining whether positive or negative feedbacks dominate owing to the complexities of the various processes involved.[99]
Not just sulfur?
DMS is not the only species evolved from plankton and other work has suggested a role for iodine at Mace Head,[100] isoprene off South Georgia[101] and ammonia off Macquarie Island,[102] and there are many other compounds that may be important in providing a source of new particles that result from micro- and macro-algae.[103] The role of isoprene has been linked to increased cloudiness, suggesting an alternative mechanism for biological control of climate,[101] but this effect may be highly localised.[104] It may be that these other precursor gases are a useful marker for DMS,[103] or DMS could be a useful marker for biogenic precursors to particle formation, even if DMS itself is not involved in nucleation.
In addition to precursor gases resulting from biological activity, primary particles from the surface of the ocean also act as CCN. Sea salt particles have the necessary properties to act very efficiently as CCN.[105,106] Other work confirms the role of sea salt as CCN,[107,108] with sea salt particles representing between 5 and 90% of CCN in the MBL.[109] It is now possible to directly measure the flux of sea salt from the surface of the ocean[110] and this along with laboratory studies of breaking bubbles[111] is improving the understanding of the relative contribution of sea salt to CCN number concentrations.
In the Arctic, primary particles often provide a reactive or condensing surface for precursor gases, allowing growth to CCN sizes.[112,113] These primary particles are fragments of biological material, including viruses.[107] Biogenic primary particles have now been seen at the centre of CCN at Cape Grim[114] and in the tropics.[115] As well as providing the nucleus for CCN, biogenic material has an effect on the chemistry of the aerosol. Excess calcium, likely to be related to biogenic calcium carbonate fragments, is believed to enhance alkalinity in marine aerosol, promoting the reaction of sulfur dioxide and ozone on sea salt, further reducing the role for DMS-derived sulfur in nucleation.[116] Recent chamber experiments[117] involving sea water have confirmed the surface microlayer to be a source of calcium.
Future directions
The many years of studying of the CLAW hypothesis have not led to a definitive answer to the question ‘Does CLAW work as proposed?’ The CLAW hypothesis led to the development of Earth Systems Science and cross-disciplinary tools and techniques, and an interdisciplinary scientific culture necessary to address such an overarching feedback loop. It provided a huge advance in our understanding of coupled marine systems, but the overall synthesis and integration required to fully assess the CLAW hypothesis remains to be achieved. The many papers (>1500) citing CLAW, only a few of which could be included here, provide a valuable platform on which to advance research in this area.
There is reason to be optimistic about the future. The rapid advances in in-situ remote measurement and characterisation coupled to the advances in remote sensing applications, both from space, increasingly allow for comprehensive observation in real time of atmospheric and ocean phenomena, physical, chemical and biological. In parallel, major advances in computing power and data assimilation provide the basis for development of improved Earth Systems simulations that will not only improve the understanding of the role of sulfur emission in providing CCN, but will drive understanding of wider global climate issues.
Conclusions
Despite the major advances in understanding of specific marine environmental systems to date, the coupled systems inherent in the CLAW hypothesis are so complex that we have yet to learn ‘enough to give a defensible answer’. There is much empirical evidence, such as the coherence in seasonal cycles of DMS, NSS-sulfate, CCN and AOD, but quantitative descriptions of key processes and direct links have yet to be achieved.
A consequence of this somewhat equivocal position is that the CLAW hypothesis (while mentioned briefly in its Chapter 7) does not figure heavily in this year’s Working Group 1 Report from the Intergovernmental Panel on Climate Change,[118], entitled ‘Climate Change 2007, The Physical Science Basis’. In this there is perhaps some sense of the line attributed to a quizzical cartoon strip character some time ago that ‘the more we learn, the more we know, but the more we know, the more we realise how much we don’t know’. However, unless we fully understand the biological–physical–chemical–dynamic–microphysical connections inherent in the CLAW hypothesis, we will be unable properly to assess climate-change risk to coupled marine systems, or consider appropriate mitigation or adaptation strategies.
This lack of quantitative understanding has important consequences for how natural sources of CCN can be handled in models, when attempting to determine how they affect and are affected by climate change. Nevertheless, it is clear that micro- and macro-algae do have an important role in the climate system by providing precursor gases (whether sulfur-based or involving other elemental cycles), for new particle formation, and provide a source of CCN, without which we would have no clouds at all.

|
[1]
R. Charlson ,
J. Lovelock ,
M. Andreae ,
S. Warren ,
Oceanic phytoplankton, atmospheric sulphur, cloud albedo and climate.
Nature 1987
, 326, 655.
| Crossref | GoogleScholarGoogle Scholar |

[2]
[3]
J. E. Lovelock ,
L. Margulis ,
Atmospheric homeostatis for and by the biosphere: the Gaia Hypothesis.
Tellus 1974
, 26, 2.

[4]
S. Twomey ,
The influence of pollution on the shortwave albedo of clouds,
J. Atmos. Sci. 1977
, 34, 1149.
| Crossref | GoogleScholarGoogle Scholar |

[5]
S. Twomey ,
Pollution and planetary albedo.
Atmos. Environ. 1974
, 8, 1251.
| Crossref | GoogleScholarGoogle Scholar |

[6]
J. C. G. Walker ,
P. B. Hays ,
J. F. Kasting ,
A negative feedback mechanism for the long-term stabilization of Earth’s surface temperature.
J. Geophys. Res. 1981
, 86, 9776.

[7]
J. E. Lovelock ,
M. Whitfield ,
Life span of the biosphere.
Nature 1982
, 296, 561.
| Crossref | GoogleScholarGoogle Scholar |

[8]
J. E. Lovelock ,
J. Maggs ,
R. A. Rasmussen ,
Atmospheric dimethylsulphide and the natural sulphur cycle.
Nature 1972
, 237, 452.
| Crossref | GoogleScholarGoogle Scholar |

[9]
G. E. Shaw ,
Bio-controlled thermostasis involving the sulfur cycle.
Clim. Change 1983
, 5, 297.
| Crossref | GoogleScholarGoogle Scholar |

[10]
S. M. Turner ,
G. Malin ,
P. S. Liss ,
D. S. Harbour ,
P. M. Holligan ,
The seasonal variation of dimethylsulfide and dimethylsulfoniopropionate concentrations in nearshore waters.
Limnol. Oceanogr. 1988
, 33, 364.

[11]
P. W. Boyd ,
S. C. Doney ,
Modelling regional responses by marine pelagic marine ecosystems to climate change.
Geophys. Res. Lett. 2002
, 29, 1806.
| Crossref | GoogleScholarGoogle Scholar |

[12]
S. D. Archer ,
Few short-cuts to predicting biological control of DMS emissions.
Environ. Chem. 2007
, 4, 404.
| Crossref | GoogleScholarGoogle Scholar |

[13]
G. V. Wolfe ,
M. S. Steinke ,
G. O. Kirst ,
Grazing-activated chemical defense in a unicellular marine alga.
Nature 1997
, 387, 894.
| Crossref | GoogleScholarGoogle Scholar |

[14]
G. Malin ,
W. H. Wilson ,
G. Bratbak ,
P. S. Liss ,
N. H. Mann ,
Elevated production of dimethylsulfide resulting from viral infection of cultures of Phaeocystis pouchetii.
Limnol. Oceanogr. 1998
, 43, 1389.

[15]
B. C. Nguyen ,
S. Belviso ,
N. Mihalopoulos ,
J. Gostan ,
P. Nival ,
Dimethylsulfide production during natural phytoplanktonic blooms.
Mar. Chem. 1988
, 24, 133.
| Crossref | GoogleScholarGoogle Scholar |

[16]
S. M. Turner ,
P. D. Nightingale ,
L. J. Spokes ,
M. I. Liddicoat ,
P. S. Liss ,
Increased dimethylsulphide concentrations in sea water from in situ iron enrichment.
Nature 1996
, 383, 513.
| Crossref | GoogleScholarGoogle Scholar |

[17]
J. Stefels ,
Physiological aspects of the production and conversion of DMSP in marine algae and higher plants.
J. Sea Res. 2000
, 43, 183.
| Crossref | GoogleScholarGoogle Scholar |

[18]
R. Simó ,
S. D. Archer ,
C. Pedros-Alio ,
L. Gilpin ,
C. E. Stelfox-Widdicombe ,
Coupled dynamics of dimethylsulfoniopropionate and dimethylsulfide cycling and the microbial food web in surface waters of the North Atlantic.
Limnol. Oceanogr. 2002
, 47, 53.

[19]
K. Caldeira ,
M. E. Wickett ,
Anthropogenic carbon and ocean pH.
Nature 2003
, 425, 365.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[20]
J. C. Orr ,
V. J. Fabry ,
O. Aumont ,
L. Bopp ,
S. C. Doney ,
R. A. Feely ,
A. Gnanadesikan ,
N. Gruber ,
et al. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms.
Nature 2005
, 437, 681.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[21]
U. Riebesell ,
I. Zondervan ,
B. Rost ,
P. D. Tortell ,
R. E. Zeebe ,
F. M. Morel ,
Reduced calcification of marine plankton in response to increased atmospheric CO2.
Nature 2000
, 407, 364.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[22]
O. W. Wingenter ,
K. B. Haase ,
M. Zeigler ,
D. R. Blake ,
F. S. Rowland ,
B. C. Sive ,
A. Paulino ,
R. Thyrhaug ,
et al. Unexpected consequences of increasing CO2 and ocean acidity on marine production of DMS and CH2ClI: potential climate impacts.
Geophys. Res. Lett. 2007
, 34, L05710.
| Crossref | GoogleScholarGoogle Scholar |

[23]
M. Vogt ,
M. Steinke ,
S. Turner ,
A. Paulino ,
M. Meyerhofer ,
U. Riebesell ,
C. LeQuere ,
P. Liss ,
Dynamics of dimethylsulphoniopropionate and dimethylsulphide under different CO2 concentrations during a mesocosm experiment.
Biogeosciences Discuss. 2007
, 4, 3673.

[24]
P. W. Boyd ,
T. Jickells ,
C. S. Law ,
S. Blain ,
E. A. Boyle ,
K. O. Buesseler ,
K. H. Coale ,
J. J. Cullen ,
et al. Mesoscale iron enrichment experiments 1993–2005: synthesis and future directions.
Science 2007
, 315, 612.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[25]
P. W. Boyd ,
A. J. Watson ,
C. S. Law ,
E. R. Abraham ,
T. Trull ,
R. Murdoch ,
D. C. E. Bakker ,
A. R. Bowie ,
et al. A mesoscale phytoplankton bloom in the polar Southern Ocean stimulated by iron fertilization.
Nature 2000
, 407, 695.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[26]
P. Liss ,
A. Chuck ,
D. Bakker ,
S. Turner ,
Ocean fertilization with iron: effects on climate and air quality.
Tellus B 2005
, 57, 269.
| Crossref | GoogleScholarGoogle Scholar |

[27]
R. Cropp ,
J. Norbury ,
Plankton modelling and CLAW.
Environ. Chem. 2007
, 4, 388.
| Crossref | GoogleScholarGoogle Scholar |

[28]
[29]
R. Wanninkhof ,
Relationship between wind speed and gas exchange over the ocean.
J. Geophys. Res. 1992
, 97, 7373.

[30]
P. D. Nightingale ,
G. Malin ,
C. S. Law ,
A. J. Watson ,
P. S. Liss ,
M. I. Liddicoat ,
J. Boutin ,
R. C. Upstill-Goddard ,
In situ evaluation of air–sea gas exchange parameterizations using novel conservative and volatile tracers.
Global Biogeochem. Cy. 2000
, 14, 373.
| Crossref | GoogleScholarGoogle Scholar |

[31]
D. T. Ho ,
C. S. Law ,
M. J. Smith ,
P. Schlosser ,
M. Harvey ,
P. Hill ,
Measurements of air–sea gas exchange at high wind speeds in the Southern Ocean: implications for global parameterizations.
Geophys. Res. Lett. 2006
, 33, L16611.
| Crossref | GoogleScholarGoogle Scholar |

[32]
G. P. Ayers ,
R. W. Gillett ,
J. P. Ivey ,
B. Schafer ,
A. Gabric ,
Short-term variability in marine atmospheric dimethylsulfide concentration.
Geophys. Res. Lett. 1995
, 22, 2513.
| Crossref | GoogleScholarGoogle Scholar |

[33]
B. W. Blomquist ,
C. W. Fairall ,
B. J. Huebert ,
D. J. Kieber ,
G. R. Westby ,
DMS sea–air transfer velocity: direct measurements by eddy covariance and parameterization based on the NOAA/COARE gas transfer model.
Geophys. Res. Lett. 2006
, 33, L07601.
| Crossref | GoogleScholarGoogle Scholar |

[34]
C. A. Marandino ,
W. J. De Bruyn ,
S. D. Miller ,
E. S. Saltzman ,
Eddy correlation measurements of the air/sea flux of dimethylsulfide over the North Pacific Ocean.
J. Geophys. Res. 2007
, 112, D03301.
| Crossref | GoogleScholarGoogle Scholar |

[35]
[36]
M. O. Andreae ,
C. D. Jones ,
P. M. Cox ,
Strong present-day aerosol cooling implies a hot future.
Nature 2005
, 435, 1187.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[37]
J. E. Kristjansson ,
T. Iversen ,
A. Kirkevag ,
Ø. Seland ,
J. Debernard ,
Response of the climate system to aerosol direct and indirect forcing: role of cloud feedbacks.
J. Geophys. Res. 2005
, 110, D24206.
| Crossref | GoogleScholarGoogle Scholar |

[38]
[39]
G. P. Ayers ,
J. P. Ivey ,
R. W. Gillett ,
Coherence between seasonal cycles of dimethylsulfide, methanesulphonate and sulphate in marine air.
Nature 1991
, 349, 404.
| Crossref | GoogleScholarGoogle Scholar |

[40]
[41]
G. P. Ayers ,
J. M. Cainey ,
R. W. Gillett ,
J. P. Ivey ,
Atmospheric sulphur and cloud condensation nuclei in marine air in the southern hemisphere.
Phil. Trans. R. Soc. London B 1997
, 352, 203.
| Crossref | GoogleScholarGoogle Scholar |

[42]
E. S. Saltzman ,
S. A. Yvon ,
P. A. Matrai ,
Low-level atmospheric sulfur dioxide measurement using HPLC/fluorescence detection.
J. Atmos. Chem. 1993
, 17, 73.
| Crossref | GoogleScholarGoogle Scholar |

[43]
G. P. Ayers ,
J. M. Cainey ,
H. Granek ,
C. Leck ,
Dimethylsulfide oxidation and the ratio of methanesulfonate to non-sea-salt sulfate in marine aerosol.
J. Atmos. Chem. 1996
, 25, 307.
| Crossref | GoogleScholarGoogle Scholar |

[44]
F. Yin ,
D. Grosjean ,
J. H. Seinfeld ,
Photooxidation of dimethylsulfide and dimethyldisulfide. I: mechanism development.
J. Atmos. Chem. 1990
, 11, 309.
| Crossref | GoogleScholarGoogle Scholar |

[45]
F. Yin ,
D. Grosjean ,
J. H. Seinfeld ,
Photooxidation of dimethylsulfide and dimethyldisulfide. II: mechanism evaluation.
J. Atmos. Chem. 1990
, 11, 365.
| Crossref | GoogleScholarGoogle Scholar |

[46]
G. P. Ayers ,
R. W. Gillett ,
J. M. Cainey ,
A. L. Dick ,
Chloride and bromide loss from sea-salt particles in Southern Ocean air.
J. Atmos. Chem. 1999
, 33, 299.
| Crossref | GoogleScholarGoogle Scholar |

[47]
R. von Glasow ,
P. Crutzen ,
Model study of multiphase DMS oxidation with a focus on halogens.
Atmos Chem. Phys. 2004
, 4, 589.

[48]
T. S. Bates ,
B. J. Huebert ,
J. L. Gras ,
F. B. Griffiths ,
P. A. Durkee ,
International Global Atmospheric Chemistry (IGAC) project’s First Aerosol Characterization Experiment (ACE-1): overview.
J. Geophys. Res. 1998
, 103, 16297.
| Crossref | GoogleScholarGoogle Scholar |

[49]
M. A. J. Curran ,
G. B. Jones ,
H. Burton ,
Spatial distribution of dimethylsulfide and dimethylsulfoniopropionate in the Australasian sector of the Southern Ocean.
J. Geophys. Res. 1998
, 103, 16677.
| Crossref | GoogleScholarGoogle Scholar |

[50]
W. J. De Bryun ,
T. S. Bates ,
J. M. Cainey ,
E. S. Saltzman ,
Shipboard measurements of dimethylsulfide and SO2 south-west of Tasmania during the First Aerosol Characterization Experiment (ACE-1).
J. Geophys. Res. 1998
, 103, 16703.
| Crossref | GoogleScholarGoogle Scholar |

[51]
S. Twomey ,
T. A. Wojciechowski ,
Observation of the geographical variation of cloud condensation nuclei.
J. Atmos. Sci. 1969
, 26, 648.
| Crossref | GoogleScholarGoogle Scholar |

[52]
J. M. Cainey ,
M. J. Harvey ,
Dimethylsulfide, a limited contributor to new particle formation in the clean marine boundary layer.
Geophys. Res. Lett. 2002
, 29, 1128.
| Crossref | GoogleScholarGoogle Scholar |

[53]
H. Sievering ,
J. Boatman ,
E. Gorman ,
Y. Kim ,
L. Anderson ,
G. Ennis ,
M. Luria ,
S. Pandis ,
Removal of sulphur from the marine boundary layer by ozone oxidation in sea-salt aerosols.
Nature 1992
, 360, 571.
| Crossref | GoogleScholarGoogle Scholar |

[54]
H. Berresheim ,
M. O. Andreae ,
G. P. Ayers ,
R. W. Gillett ,
J. T. Merrill ,
V. J. Harris ,
W. L. Chameides ,
Airborne measurements of dimethylsulfide, sulfur dioxide and aerosol ions over the Southern Ocean south of Australia.
J. Atmos. Chem. 1990
, 10, 341.
| Crossref | GoogleScholarGoogle Scholar |

[55]
W. De Bruyn ,
D. Wylie ,
E. Saltzman ,
M. Harvey ,
J. Cainey ,
Dimethylsulfide and sulfur dioxide measurements at Baring Head, New Zealand.
J. Atmos. Chem. 2002
, 41, 189.
| Crossref | GoogleScholarGoogle Scholar |

[56]
H. Berresheim ,
J. W. Huey ,
R. P. Thorn ,
Measurements of dimethylsulfide, dimethylsulfoxide, dimethylsulfone and aerosol ions at Palmer Station, Antarctica.
J. Geophys. Res. 1998
, 103, 1629.
| Crossref | GoogleScholarGoogle Scholar |

[57]
A. D. Clarke ,
Z. Li ,
M. Litchy ,
Aerosol dynamics in the equatorial Pacific marine boundary layer: microphysics, diurnal cycles and entrainment.
Geophys. Res. Lett. 1996
, 23, 733.
| Crossref | GoogleScholarGoogle Scholar |

[58]
T. S. Bates ,
J. A. Calhoun ,
P. K. Quinn ,
Variations in the methanesulfonate to sulfate molar ratio in submicrometre marine aerosol particles over the Pacific Ocean.
J. Geophys. Res. 1992
, 97, 9859.

[59]
A. Broadbent ,
G. B. Jones ,
Seasonal and diurnal cycles of dimethylsulphide, dimethylsulphoniopropionate and dimethylsulphoxide at One Tree Reef lagoon.
Environ. Chem. 2006
, 3, 260.
| Crossref | GoogleScholarGoogle Scholar |

[60]
B. J. Huebert ,
D. J. Wylie ,
L. Zhuang ,
J. A. Heath ,
Production and loss of methanesulfonate and non-sea salt sulfate in the equatorial Pacific marine boundary layer.
Geophys. Res. Lett. 1996
, 23, 737.
| Crossref | GoogleScholarGoogle Scholar |

[61]
C. Leck ,
C. Persson ,
Seasonal and short-term variability in dimethylsulfide, sulfur dioxide and biogenic sulfur and sea salt aerosol particles in the arctic marine boundary layer, during summer and autumn.
Tellus B 1996
, 48, 272.
| Crossref | GoogleScholarGoogle Scholar |

[62]
B. C. Nguyen ,
N. Mihalopoulos ,
S. Belviso ,
Seasonal variation of atmospheric dimethylsulfide at Amsterdam Island in the southern Indian Ocean.
J. Atmos. Chem. 1990
, 11, 123.
| Crossref | GoogleScholarGoogle Scholar |

[63]
J. P. Putaud ,
N. Mihalopoulos ,
B. C. Nguyen ,
J. M. Campin ,
S. Belviso ,
Seasonal variations of atmospheric sulfur dioxide and dimethylsulfide concentrations at Amsterdam Island in the southern Indian Ocean.
J. Atmos. Chem. 1992
, 15, 117.
| Crossref | GoogleScholarGoogle Scholar |

[64]
J. Sciare ,
E. Baboukas ,
N. Mihalopoulos ,
Short-term variability of atmospheric DMS and its oxidation products at Amsterdam Island during summer time.
J. Atmos. Chem. 2001
, 39, 281.
| Crossref | GoogleScholarGoogle Scholar |

[65]
J. Sciare ,
M. Kanakidou ,
N. Mihalopoulos ,
Diurnal and seasonal variation of atmospheric dimethylsulfoxide at Amsterdam Island in the southern Indian Ocean.
J. Geophys. Res. 2000
, 105, 17257.
| Crossref | GoogleScholarGoogle Scholar |

[66]
B. Davison ,
C. Hewitt ,
Natural sulphur species from the North Atlantic and their contribution to the United Kingdom sulfur budget.
J. Geophys. Res. 1992
, 97, 2475.

[67]
B. Davison ,
C. N. Hewitt ,
C. D. O’Dowd ,
J. A. Lowe ,
M. H. Smith ,
M. Schwikowski ,
U. Baltensperger ,
R. M. Harrison ,
Dimethylsulfide, methanesulfonic acid and physiochemical aerosol properties in Atlantic air from the United Kingdom to Halley Bay.
J. Geophys. Res. 1996
, 101, 22855.
| Crossref | GoogleScholarGoogle Scholar |

[68]
G. Kouvarakis ,
N. Mihalopoulos ,
Seasonal variation of dimethylsulfide in the gas phase and of methanesulfonate and non-sea-salt sulfate in the aerosol phase in the Eastern Mediterranean atmosphere.
Atmos. Environ. 2002
, 36, 929.
| Crossref | GoogleScholarGoogle Scholar |

[69]
G. P. Ayers ,
J. L. Gras ,
Seasonal relationship between cloud condensation nuclei and aerosol methanesulphonate in marine air.
Nature 1991
, 353, 834.
| Crossref | GoogleScholarGoogle Scholar |

[70]
J. L. Gras ,
Baseline atmospheric condensation nuclei at Cape Grim 1977–1987.
J. Atmos. Chem. 1990
, 11, 89.
| Crossref | GoogleScholarGoogle Scholar |

[71]
R. Boers ,
G. P. Ayers ,
J. L. Gras ,
Coherence between seasonal variation in satellite-derived cloud optical depth and boundary layer CCN concentrations at a mid-latitude southern hemisphere station.
Tellus B 1994
, 46, 123.
| Crossref | GoogleScholarGoogle Scholar |

[72]
R. Boers ,
J. B. Jensen ,
P. B. Krummel ,
Microphysical and short-wave radiative structure of stratocumulus clouds over the Southern Ocean: summer results and seasonal differences.
Q. J. R. Meteorol. Soc. 1998
, 124, 151.
| Crossref | GoogleScholarGoogle Scholar |

[73]
F. Raes ,
Entrainment of free tropospheric aerosols as a regulating mechanism for cloud condensation nuclei in the remote marine boundary layer.
J. Geophys. Res. 1995
, 100, 2893.
| Crossref | GoogleScholarGoogle Scholar |

[74]
T. S. Bates ,
V. N. Kapustin ,
P. K. Quinn ,
D. S. Covert ,
D. J. Coffman ,
C. Mari ,
P. A. Durkee ,
W. J. de Bruyn ,
et al. Processes controlling the distribution of aerosol particles in the lower marine boundary layer during the First Aerosol Characterization Experiment (ACE-1).
J. Geophys. Res. 1998
, 103, 16369.
| Crossref | GoogleScholarGoogle Scholar |

[75]
[76]
C. Mari ,
K. Suhre ,
R. Rosset ,
T. S. Bates ,
B. J. Huebert ,
A. R. Bandy ,
D. C. Thornton ,
S. Businger ,
One-dimensional modeling of sulfur species during the First Aerosol Characterization Experiment (ACE-1) Lagrangian B.
J. Geophys. Res. 1999
, 104, 21733.
| Crossref | GoogleScholarGoogle Scholar |

[77]
G. E. Shaw ,
R. L. Benner ,
W. Cantrell ,
A. D. Clarke ,
On the regulation of climate: a sulphate particle feedback loop involving deep convection.
Clim. Change 1998
, 39, 23.
| Crossref | GoogleScholarGoogle Scholar |

[78]
P. Kishcha ,
B. Starobinets ,
P. Alpert ,
Latitudinal variations of cloud and aerosol optical thickness trends based on MODros. Inf. Serv. satellite data.
Geophys. Res. Lett. 2007
, 34, L05810.
| Crossref | GoogleScholarGoogle Scholar |

[79]
[80]
R. T. Pinker ,
B. Zhang ,
E. G. Dutton ,
Do satellites detect trends in surface solar radiation?
Science 2005
, 308, 850.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[81]
J. E. Lovelock ,
A geophysiologist’s thoughts on the natural sulphur cycle.
Philos. Trans. R. Soc. London B 1997
, 352, 143.
| Crossref | GoogleScholarGoogle Scholar |

[82]
J. E. Lovelock ,
L. R. Kump ,
Failure of climate regulation in a geophysiological model.
Nature 1994
, 369, 732.
| Crossref | GoogleScholarGoogle Scholar |

[83]
A. J. Gabric ,
N. Murray ,
L. Stone ,
M. Kohl ,
Modelling the production of dimethylsulfide during a phytoplankton bloom.
J. Geophys. Res. 1993
, 98, 22805.

[84]
A. J. Gabric ,
P. Whetton ,
R. Cropp ,
Dimethylsulphide production in the subantarctic Southern Ocean under enhanced greenhouse conditions.
Tellus B 2001
, 53, 273.
| Crossref | GoogleScholarGoogle Scholar |

[85]
A. J. Gabric ,
R. Simó ,
R. A. Cropp ,
J. Dachs ,
T. Hirst ,
Global estimates of the oceanic emission of dimethylsulfide under enhanced greenhouse conditions.
Global Biogeochem. Cy. 2004
, 18, GB2014.
| Crossref | GoogleScholarGoogle Scholar |

[86]
A. J. Gabric ,
P. Whetton ,
R. Boers ,
G. P. Ayers ,
The impact of GCM predicted climate change on the air-to-sea flux of dimethylsulphide in the subantarctic Southern Ocean.
Tellus B 1998
, 50, 388.
| Crossref | GoogleScholarGoogle Scholar |

[87]
L. Bopp ,
O. Aumont ,
S. Belviso ,
P. Monfray ,
Potential impact of climate change on marine dimethylsulfide emissions.
Tellus B 2003
, 55, 11.
| Crossref | GoogleScholarGoogle Scholar |

[88]
S. Kloster ,
K. D. Six ,
J. Feichter ,
E. Maier-Reimer ,
E. Roeckner ,
P. Wetzel ,
P. Stier ,
M. Esch ,
Response of dimethylsulfide (DMS) in the ocean and atmosphere to global warming.
J. Geophys. Res. 2007
, 112, G03005.
| Crossref | GoogleScholarGoogle Scholar |

[89]
J. R. Gunson ,
S. A. Spall ,
T. R. Anderson ,
A. Jones ,
I. J. Totterdell ,
M. J. Woodage ,
Climate sensitivity to ocean dimethylsulphide emissions.
Geophys. Res. Lett. 2006
, 33, L07701.
| Crossref | GoogleScholarGoogle Scholar |

[90]
M. A. J. Curran ,
T. D. van Ommen ,
V. I. Morgan ,
K. L. Phillips ,
A. S. Palmer ,
Ice core evidence for Antarctic sea ice decline since the 1950s.
Science 2003
, 302, 1203.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[91]
A. J. Gabric ,
J. M. Shephard ,
J. M. Knight ,
G. Jones ,
A. J. Trevena ,
Correlations between the satellite-derived seasonal cycles of phytoplankton biomass and aerosol optical depth in the Southern Ocean: evidence for the influence of sea ice.
Global Biogeochem. Cy. 2005
, 19, GB4018.
| Crossref | GoogleScholarGoogle Scholar |

[92]
M. Legrand ,
Ice-core records of atmospheric sulphur.
Philos. Trans. R. Soc. London B 1997
, 352, 241.
| Crossref | GoogleScholarGoogle Scholar |

[93]
J. L. Sarmiento ,
R. Slater ,
R. Barber ,
L. Bopp ,
S. C. Doney ,
A. C. Hirst ,
J. Kieypas ,
R. Matear ,
et al. Response of ocean ecosystems to climate warming.
Global Biogeochem. Cy. 2004
, 18, GB3003.
| Crossref | GoogleScholarGoogle Scholar |

[94]
W. W. Gregg ,
M. E. Conkright ,
Decadal changes in global ocean chlorophyll.
Geophys. Res. Lett. 2002
, 29, 1730.
| Crossref | GoogleScholarGoogle Scholar |

[95]
M. J. Behrenfeld ,
E. Boss ,
D. A. Siegel ,
D. M. Shea ,
Carbon-based ocean productivity and phytoplankton physiology from space.
Global Biogeochem. Cy. 2005
, 19, GB1006.
| Crossref | GoogleScholarGoogle Scholar |

[96]
A. J. Gabric ,
R. Cropp ,
G. P. Ayers ,
G. McTainsh ,
R. Braddock ,
Coupling between cycles of phytoplankton biomass and aerosol optical depth as derived from SeaWiFS time series in the Subantarctic Southern Ocean.
Geophys. Res. Lett. 2002
, 29, 1112.
| Crossref | GoogleScholarGoogle Scholar |

[97]
S. M. Vallina ,
R. Simó ,
S. Gassó ,
What controls CCN seasonality in the Southern Ocean? A statistical analysis based on satellite-derived chlorophyll and CCN and model-estimated OH radical and rainfall.
Global Biogeochem. Cy. 2006
, 20, GB1014.
| Crossref | GoogleScholarGoogle Scholar |

[98]
S. M. Vallina ,
R. Simó ,
Strong relationship between DMS and the solar radiation dose over the global surface ocean.
Science 2007
, 315, 506.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[99]
M. J. Harvey ,
The iron CLAW.
Environ. Chem. 2007
, 4, 396.
| Crossref | GoogleScholarGoogle Scholar |

[100]
C. D. O’Dowd ,
M. Geever ,
M. K. Hill ,
M. H. Smith ,
S. G. Jennings ,
New particle formation: nucleation rates and spatial scales in the clean marine coastal environment.
Geophys. Res. Lett. 1998
, 25, 1661.
| Crossref | GoogleScholarGoogle Scholar |

[101]
N. Meskhidze ,
A. Nenes ,
Phytoplankton and cloudiness in the Southern Ocean.
Science 2006
,
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[102]
R. J. Weber ,
P. H. McMurry ,
L. Mauldin ,
D. J. Tanner ,
F. L. Eisele ,
F. J. Brechtel ,
S. M. Kreidenweis ,
G. L. Kok ,
et al. A study of new particle formation and growth involving biogenic and trace gases during the First Aerosol Characterization Experiment (ACE-1).
J. Geophys. Res. 1998
, 103, 16385.
| Crossref | GoogleScholarGoogle Scholar |

[103]
P. S. Liss ,
J. E. Lovelock ,
Climate change, the effect of DMS emissions.
Environ. Chem. 2007
, 4, 377.
| Crossref | GoogleScholarGoogle Scholar |

[104]
O. W. Wingenter ,
Isoprene, cloud droplets and phytoplankton.
Science 2007
, 317, 42.
| Crossref | GoogleScholarGoogle Scholar |

[105]
C. D. O’Dowd ,
M. H. Smith ,
Physico-chemical properties of aerosols over the North-east Atlantic: evidence for wind-speed-related submicron sea-salt aerosol production.
J. Geophys. Res. 1993
, 98, 1137.

[106]
Y. J. Yoon ,
P. Brimblecombe ,
Modelling the contribution of sea salt and dimethylsulfide-derived aerosol to marine CCN.
Atmos. Chem. Phys. Discuss. 2001
, 1, 93.

[107]
C. D. O’Dowd ,
J. A. Lowe ,
M. H. Smith ,
Coupling sea-salt and sulphate interactions and its impact on cloud droplet concentration predictions.
Geophys. Res. Lett. 1999
, 26, 1311.
| Crossref | GoogleScholarGoogle Scholar |

[108]
M. Smith ,
Sea-salt particles and the CLAW Hypothesis.
Environ. Chem. 2007
, 4, 391.
| Crossref | GoogleScholarGoogle Scholar |

[109]
A. D. Clarke ,
S. R. Owens ,
J. Zhou ,
An ultrafine sea-salt flux from breaking waves: implications for CCN in the remote marine atmosphere.
J. Geophys. Res. 2006
, 111, D06202.
| Crossref | GoogleScholarGoogle Scholar |

[110]
E. D. Nilsson ,
E. M. Martensson ,
J. S. van Ekeren ,
G. de Leeuw ,
M. Moerman ,
C. D. O’Dowd ,
Primary marine aerosol emissions: size-resolved eddy covariance measurements with estimates of the sea salt and organic carbon fractions.
Atmos. Chem. Phys. Discuss. 2007
, 7, 13345.

[111]
E. M. Martensson ,
E. D. Nilsson ,
G. de Leeuw ,
L. H. Cohen ,
H.-C. Hansson ,
Laboratory simulations and parameterizations of the primary marine aerosol productions.
J. Geophys. Res. 2003
, 108, 4297.
| Crossref | GoogleScholarGoogle Scholar |

[112]
C. Leck ,
E. K. Bigg ,
Biogenic particles in the surface microlayer and overlaying atmosphere in the central Arctic Ocean during summer.
Tellus B 2005
, 57, 305.
| Crossref | GoogleScholarGoogle Scholar |

[113]
C. Leck ,
E. K. Bigg ,
Source and evolution of marine aerosol – a new perspective.
Geophys. Res. Lett. 2005
, 32, L19803.
| Crossref | GoogleScholarGoogle Scholar |

[114]
E. K. Bigg ,
Sources, nature and influence on climate of marine airborne particles.
Environ. Chem. 2007
, 4, 155.
| Crossref | GoogleScholarGoogle Scholar |

[115]
C. Leck ,
E. K. Bigg ,
Comparison of sources and nature of the tropical aerosol with summer high Arctic aerosol.
Tellus B 2007
,
| Crossref | GoogleScholarGoogle Scholar |

[116]
H. Sievering ,
J. Cainey ,
M. Harvey ,
J. McGregor ,
S. Nichol ,
P. Quinn ,
Non-sea salt sulfate (NSS) budget in the remote marine boundary layer under clear sky and normal cloudiness conditions: evidence for enhanced NSS production by O3 oxidation in seasalt aerosols.
J. Geophys. Res. 2004
, 109, D19317.
| Crossref | GoogleScholarGoogle Scholar |

[117]
W. C. Keene ,
H. Maring ,
J. R. Maben ,
D. J. Kieber ,
A. A. P. Pszenny ,
E. E. Dahl ,
M. A. Izaguirre ,
A. J. Davis ,
et al. Chemical and physical characteristics of nascent aerosols produced by bursting bubbles at a model air–sea interface.
J. Geophys. Res. 2007
, 112, D21202.
| Crossref | GoogleScholarGoogle Scholar |

[118]
[119]
M. O. Andreae ,
Ocean-atmosphere interactions in the global biogeochemical sulfur cycle.
Mar. Chem. 1990
, 30, 1.
| Crossref | GoogleScholarGoogle Scholar |



