How Australian mammals contributed to our understanding of sex determination and sex chromosomes
Jennifer A. Marshall GravesSchool of Life Sciences, La Trobe University, Melbourne, Vic. 3186, Australia, and Research School of Biology, Australian National University, Canberra, ACT 0200, Australia. Email: j.graves@latrobe.edu.au
Australian Journal of Zoology 64(4) 267-276 https://doi.org/10.1071/ZO16054
Submitted: 7 August 2016 Accepted: 21 October 2016 Published: 2 December 2016
Journal Compilation © CSIRO Publishing 2016 Open Access CC BY-NC-ND
Abstract
Marsupials and monotremes can be thought of as independent experiments in mammalian evolution. The discovery of the human male-determining gene, SRY, how it works, how it evolved and defined our sex chromosomes, well illustrates the value of comparing distantly related animals and the folly of relying on humans and mice for an understanding of the most fundamental aspects of mammalian biology. The 25th anniversary of the discovery of SRY seems a good time to review the contributions of Australian mammals to these discoveries.
The discovery of the mammalian sex determining gene, SRY, was a milestone in the history of human genetics. SRY opened up investigations into the pathway by which the genital ridge (bipotential gonad) becomes a testis. Studies of Australian mammals were important in the story of the discovery of SRY, not only in refuting the qualifications of the first candidate sex-determining gene, but also in confirming the ubiquity of SRY and raising questions as to how it works. Studies in marsupials also led to understanding of how SRY evolved from a gene on an autosome with functions in the brain and germ cells, and to identifying the ancestors of other genes on the human Y. The discovery that platypus have sex chromosomes homologous, not to the human XY, but to the bird ZW, dated the origin of the therian SRY and the XY chromosomes it defined. This led to important new models of how our sex chromosomes function, how they evolved, and what might befall this gene and the Y chromosome it defines.
Introduction – sex before SRY
For millennia, people wondered how a baby becomes a boy or a girl. The ancient Greeks had some very imaginative hypotheses, including the relatively rational notion that sperm from the left testicle determined a girl and from the right determined a boy.
The discovery of human sex chromosomes in the 1950s showed that females have two copies of a large X chromosome; males have a single X and a much smaller Y (they got their name, not from their shape at mitosis, but because the first sight of an X in a bug was so puzzling that it was called ‘X for unknown’). Females produce eggs that each have a single X (because they produce only one type of gametes, females are called ‘homogametic’). Males produce sperm, half of which receive an X, and half a Y (because they produce two kinds of gametes, males are called ‘heterogametic’). Thus the sex ratio of the offspring is expected to be half boys and half girls.
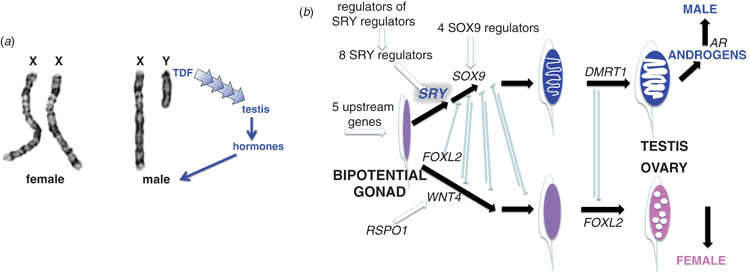
Initially, it was thought that what determines sex is the number of X chromosome a baby receives: two X chromosomes produce a female and a single X produces a male. This was not a crazy idea because it was already known in the 1950s that that’s the way the system works in fruit flies (Bridges 1925). What showed this to be wrong was a study of people born with aberrant numbers of sex chromosomes: babies born with a single X chromosome and no Y were girls with Turner’s syndrome (Ford et al. 1959), and babies with two X chromosomes as well as a Y were boys with Klinefelter’s syndrome (Jacobs and Strong 1959). The testis-determining factor (TDF) had to be a gene on the Y chromosome (Fig. 1).
TDF was shown to act as a male dominant entity by removing the testes from rabbit embryos and allowing them to complete development (Jost 1970); they all developed as females. Doing the opposite – removing the ovaries from XX rabbit embryos – did not switch their sex; again, they were all born female. This implied that a positive signal was required for male development, and in its absence a female develops. Females became known as the ‘default sex’ because of this result.
What did TDF do? The human embryo has a ridge of cells attached to the embryonic kidney. This genital ridge is the same in early XX and XY embryos, so it is called the bipotential gonad. In XY embryos the TDF gene kick-starts a cascade of genes that differentiates this genital ridge into a testis. The testis makes androgens, and the androgens make the baby male. In the absence of a Y chromosome and TDF (and androgens), nothing happens for a few more weeks, after which the ovary-determining pathway is established.
Sex chromosomes – the conserved X and the wimpy Y
Even the relatively primitive cytological techniques of the 1970s and 1980s were sufficient to reveal that mammalian sex chromosomes are quite weird.
The X chromosome is a relatively normal, middle-sized chromosome with a complex g-banding pattern. Sex-linkage studies showed that many genes were located on the X; these revealed themselves because boys, having only a single copy, expressed mutations such as colour blindness and haemophilia that were due to a missing or abnormal colour vision pigment or a clotting factor. Early gene mapping assigned to the X many classic enzyme loci that had no role in sex.
The Y chromosome was very different, being much smaller and showing very aberrant banding. When stained by fluorescent chemicals that reveal repetitive sequence, it literally glows in the dark. Attempts to pin genes on it were completely unsuccessful, despite early claims to have identified male-to-male transmission of characters such as hairy ears.
Early studies showed that sex chromosomes are monophyletic across placental mammals. The X chromosome is highly conserved in size (~5% of the genome) and gene content, giving rise to the concept (Ohno’s Law) that the X is completely conserved in mammals (Ohno 1967). Sequencing confirms that the gene content of the X is virtually invariant, and even gene order is conserved between human and the distantly related elephant (Delgado et al. 2009). In contrast, the size and gene content of the Y chromosome is more variable.
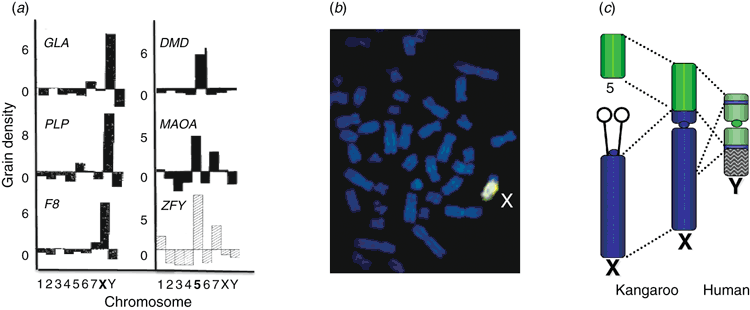
Marsupial mammals also have an XY system, and early gene mapping showed that the marsupial X shares genes with the eutherian X. The first genomic surprise to come out of marsupial genetics was the finding that, although the genes on the marsupial X are all homologous with genes on the human X, about a third of the X genes in eutherians are autosomal in marsupials (Fig. 2) (Wilcox et al. 1996). Comparison with the genomes of birds shows that the marsupial X and the autosomal regions represent separate conserved genomic blocks. The marsupial X represents an ancient X, to which the autosomal block was added in an ancient eutherian after the divergence from marsupials. The eutherian Y has homology to the same two ancestral blocks, but most of it derives from the recently added block.
Marsupials and the molecular search for the male-determining gene
With the realisation that TDF lay on the Y, efforts redoubled to characterise the DNA of the human Y and find the active gene. This was tough because the Y is largely composed of repetitive sequence. Since the discovery of non-coding RNAs with functions in gene regulation we are more careful about dismissing such repetitive sequences as ‘junk DNA’, but most of the long arm comprises simple sequences repeated many thousands of times – what I call hard core junk DNA. One of these sequences was identified on sex chromosomes of snakes as well as humans, and for a time was thought to act as the sex-determining signal (Singh et al. 1994).
The only other sighting of a gene was gained by immunising female mice with cells from males of the same strain – they made a weak antibody that suggested there was a male-specific gene called HYA (for Human Antigen on the Y) (Ohno 1978). The search for HYA went on for many years before it was ruled out as the sex-determining gene because it mapped at the wrong end of the human Y (Simpson et al. 1987). In fact, HYA turns out to be an amalgam of products, different in different animals; any product of a widely expressed Y gene will contribute male-specific determinants.
The hunt for TDF really revved up in the 1980s, when it became at least theoretically possible to positionally clone genes – that is, pinpoint them by finer and finer mapping, then capture the DNA in a virus or bacterial vector.
It’s hard to map the human Y chromosome. You can’t use ordinary genetic mapping procedures because the Y does not recombine; nor is it amenable to somatic cell mapping because the Y is usually lost from cell hybrids. The best approach was to study DNA from patients having only parts of a Y. It was discovered that some people lacked the whole long arm of the Y – and they were male (Affara et al. 1987). TDF therefore had to lie on the small short arm of the Y.
Deletion mapping was used to refine the position of TDF. Most informative were male patients who apparently had two X chromosomes. However, many of these patients had a tiny bit of the tip of the Y exchanged with the tip of the X, and this could be spotted by looking for Y-borne repetitive sequences. The search focussed on this bit of the Y (Fig. 1). It might be tiny, but it was full of repetitive sequences that made it hard to map and assemble sequence.
David Page’s group at MIT were the first to find a gene in this region (Page et al. 1987). ZFY looked like an excellent candidate; it was a zinc finger gene, related to many transcription factors, appropriate for a gene whose job it was to turn on a cascade of testis-differentiating genes. ZFY was conserved on the Y chromosome in other placental mammals, including chimps, mouse, cats and horses, as you would expect of a gene crucial for reproduction and survival. And it was expressed in the testis. The only puzzling attribute was that it had a close homologue (ZFX) on the short arm of the X.
This paper created quite a sensation. I waved it in front of my human genetics class as a splendid example – one of the first – of cloning a gene via its location (positional cloning), which was later to transform human genetics. But I was not then directly involved in sex and the Y chromosome, being more interested in the evolution of the X chromosome and epigenetic changes involved in dosage compensation (I still am: Graves 2015), using comparisons between humans, mice and the distantly related marsupial mammals.
All that changed with a phone call from David Page in Boston, requesting me to check out the position of ZFY in marsupials. Any decent candidate for a universal mammal sex-determining gene should rightly map to the Y in all mammals.
I gave the job to two of my Ph.D. students. Andrew Sinclair was finishing up his laboratory-work – literally in his last week – mapping the orthologues of human X-borne genes in marsupials. He was curious about ZFY because he had found that genes near ZFX on the short arm of the human X map, not to the X, but to chromosome 5 in kangaroos; they are part of the recently added region. Jamie Foster had just arrived in my laboratory, and proposed to work on marsupial ZFY and sex determination. I suggested that they collaborate on the mapping, thinking they might get a little ‘me too’ paper from the work.
Andrew and Jamie set about preparing a radioactive version of Page’s ZFY probe. Quite coincidentally, Andrew received another version of the ZFY probe from Peter Goodfellow, then working in London, to whom he had already applied for a postdoc position. They hybridised the radioactive probes in situ to the chromosomes of two marsupial species whose cells we can grow in the laboratory: our model kangaroo, the tammar wallaby (Macropus eugenii), and the fat-tailed dunnart (Sminthopsis crassicaudata). Then they covered the slides with autoradiographic film, and we waited.
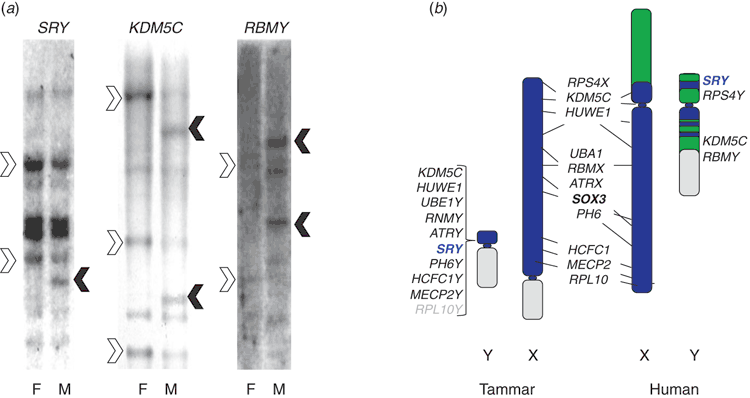
When the preparations were finally ready to be developed, Andrew and Jamie stayed late in the laboratory counting grains over chromosomes in hundreds of cells, a tedious task. Andrew called me at 1 : 00 a.m. to tell me, ‘ZFY is not on the Y. It is on chromosome 5 in kangaroo.’ Startled and excited, I advocated counting more grains produced by both probes in both species. This produced deep groans, but by morning it was clear. ZFY is autosomal in kangaroos (on chromosome 5) and the dunnart (on chromosome 3), precisely the locations of the other genes Andrew had mapped from the added region of the X. This was verified by Southern blot analysis: a ZFY probe produced male-specific bands in eutherians, but not in marsupials (Fig. 3). An autosome would be a strange place for a sex-determining gene, and the obvious implication was either that marsupials used a different gene for sex determination than did placental mammals, or that ZFY was the wrong gene.
Rather than the little ‘me too’ paper I had envisaged, Andrew and Jamie produced a cover story in Nature (Sinclair et al. 1988). Both students went on to make fundamental discoveries about human sex determination in Peter Goodfellow’s laboratory.
Our conclusion that ZFY was the wrong gene was soon confirmed independently by the finding (by another young Australian, Peter Koopman, a postdoc in Robin Lovell-Badge’s London laboratory), that ZFY was expressed in the germ cells of the mouse testis, but not in the somatic cells (Koopman et al. 1989), where the sex determination signal had to be received.
Discovery of SRY
Back to the drawing board on both sides of the Atlantic. Andrew Sinclair left Melbourne for London to join a renewed search for the human sex-determining gene in Peter Goodfellow’s laboratory. The group used DNA from patients with smaller and smaller fragments of a Y chromosome. It was a difficult and frustrating search, using the techniques of the day to isolate small fragments cloned in virus or bacteria. Repetitive sequence was always a barrier to progress. Jamie broke off a vacation in Europe to visit them – and stayed for the year’s search, despite phone calls from his hapless supervisor in Melbourne, pleading ‘Just another week …?’.
It took another year before Goodfellow’s team, led by Andrew Sinclair and Mark Palmer, found a tiny gene buried in the repetitive junk of the Y chromosome. Non-committally, they called it SRY for ‘Sex-determining Region on the Y’ (Sinclair et al. 1990).
Evidence began stacking up that SRY was the right gene. Most telling was the discovery of three girls who had a Y chromosome but a mutated version of SRY (Berta et al. 1990). And Peter Koopman produced XX mice transgenic for the mouse version of SRY to produce two XX male mice that featured on the cover of Nature (one the famous ‘Randy’) (Koopman et al. 1991).
As would be expected of the sex-determining factor, SRY was found to be expressed in the bipotential gonad of a mouse embryo in a narrow time window before testis differentiation. It was conserved on the Y chromosome in a range of placental mammals. Jamie returned to Melbourne and, with the help of talented Research Assistant Francine Brennan, showed that it was also on the Y in marsupials (Foster et al. 1992) – phew!
What does SRY do?
SRY is a small gene with no introns. Its sequence was initially a puzzle, with no immediate lookalikes in any databases. It turned out to be a member of a large family of SOX genes that all share the HMG box (an 80 amino acid domain that binds DNA) with SRY (hence SOX), and with a group of High Mobility Proteins (hence HMG) (Gubbay et al. 1990b). Many of them turn out to have important functions in development; for instance, SOX2 is one of the pluripotency factors that go into the mix that produces induced pluripotent stem cells (Avilion et al. 2003).
Biochemical tests on SRY were difficult and expensive because there is never much protein made and it is around for only a crucial day during mouse development. However, Vince Harley, another Australian postdoc in Peter Goodfellow’s laboratory, persevered and showed that the SRY protein bound DNA at a consensus sequence and bent it through a specific angle, which was disrupted in sex-reversing SRY mutations (Harley and Goodfellow 1994).
SRY was expected to be an on/off switch. Where SRY is present, downstream genes are activated to differentiate the genital ridge into a testis. In the absence of SRY, four weeks pass before an alternate ovary differentiation pathway is activated.
The gonad differentiation network
We all hoped that once the testis-determining gene had been isolated, it would be easy to walk down the testis differentiation pathway, and the alternative ovary-determining pathway. Other steps should give way their secrets, falling like a line-up of dominoes. But it has not been that simple. The pathway turns out to be more of a network, full of checks and balances; some genes promote testis differentiation or maintenance, others oppose it (Fig. 1b).
The immediate target of SRY remained elusive for four years, until SOX9 was discovered by two independent investigations of a rare sex-reversing condition (Foster et al. 1994; Wagner et al. 1994). Jamie Foster, now a postdoc in Goodfellow’s laboratory, which had just moved to Cambridge, had wearied of difficult biochemical investigations of SRY, and turned his attention, instead, to a condition called campomelic dysplasia (CD). CD is a lethal birth deformity in which the long bones are bent. XY babies with this condition often have female genitalia, suggesting a mutation that affects sex determination as well as bone formation.
Jamie investigated the DNA of CD babies who had a chromosome rearrangement and got a surprise – close by the break-point was one of the SOX genes. SOX9 was a highly conserved gene – with introns – that is one of the first genes expressed in the developing testis, as well as determining the cartilage that frames bone deposition. SOX9 turns out to be pivotal in sex determination in all vertebrates.
Other genes in the sex-determining network have since been identified by studying sex-reversal syndromes. The gene DMRT1 was identified at the tip of chromosome 9, deletion of which produces XY sex-reversed females (Raymond et al. 1999; Calvari et al. 2000). Interestingly, this gene also turns out to be vital for sex determination in birds, lying on the Z chromosome but not the W, and determining sex by its dosage: two copies are required for male development in ZZ eggs and a single copy permits female development of ZW eggs (Raymond et al. 1998; Smith et al. 1999, 2009). It has a very long association with sex, being involved even in fruitflies and worms, and the same gene, or a copy of it, turns out to be sex determining in a variety of vertebrates (Graves 2013).
Some genes, such as SF1 and WT4 were isolated from patients with syndromes that affected the pathway upstream, even before a bipotential gonad was formed. Other genes were clearly downstream; for instance, mutation of the AR gene on the X chromosome that makes a nuclear receptor for androgens (Lyon and Hawkes 1970) produces patients who are outwardly female, although they have internal testes and make, but cannot use, androgen (Migeon et al. 1981).
Other genes in the network were identified by sex reversal in other animals: mice, dogs, even goats (Meyers-Wallen et al. 1999; Pailhoux et al. 2002). It’s the same pathway in all mammals, so a gene cloned from one animal will have a homologue, doing much the same job, in humans.
Classical studies that showed the Y chromosome to have a male-dominant effect gave rise to the concept that female development was the ‘default’ position in humans and other mammals. This dismissive attitude was hardened by the observation that mutations in several genes in the testis pathway – right down to the androgen receptor – produced a female phenotype. However, not surprisingly, making an ovary in an XX embryo is every bit as demanding as making a testis. We now know of mutations in several genes, such as RSPO1, that block or destabilise ovary determination (Parma et al. 2006), and find that several gene pairs exert a yin/yang influence on the direction of gonad development (Fig. 1b).
The checks and balances in the gonad differentiation network are complex, with several gene pairs working to promote or reverse a step (Sekido and Lovell-Badge 2013; Wilhelm et al. 2013; Eggers et al. 2014). Besides DMRT1, several other genes are dosage sensitive; for instance, too little SOX9 produces XY females (with campomelic dysplasia), but too much produces XX males.
The network now boasts more than 30 genes, and ongoing research turns up more. Are all organs – liver, heart, brain – regulated in such a complex way? Or is sex special because of the unique evolutionary history of SRY?
Platypus and the origin of SRY and mammal sex chromosomes
It is tempting to believe that sex in other animals works much the same as it does in humans, with a male-dominant SRY gene on the male-specific Y calling the shots.
This is decidedly not the case. There is no SRY outside mammals, and other genes control gonad differentiation and sex determination (Smith et al. 2009; Kikuchi and Hamaguchi 2013; Chen et al. 2014). Nor are the sex chromosomes homologous outside mammals. For instance, comparative gene mapping, and, more recently, whole genome sequencing, shows that the bird ZW pair is homologous, not to the human XY, but to parts of human chromosomes 5 and 9 (Nanda et al. 1999). The snake ZW pair is different again, and so is the sex pair in the Australian dragon lizard (Ezaz et al. 2009).
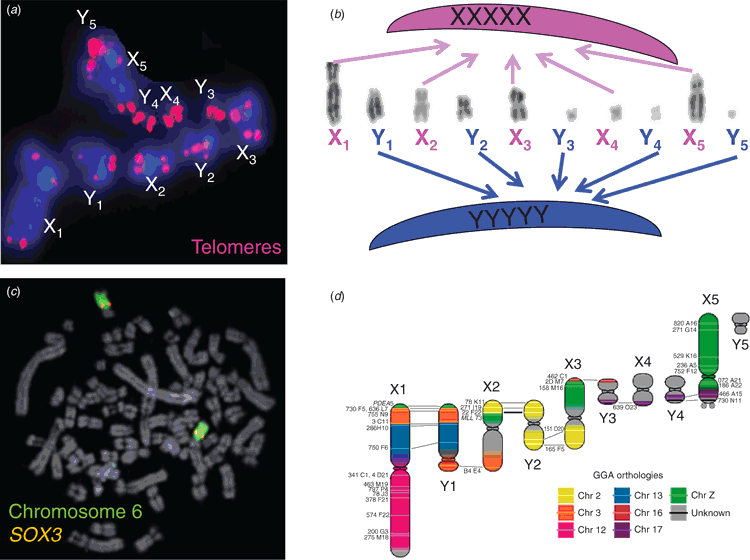
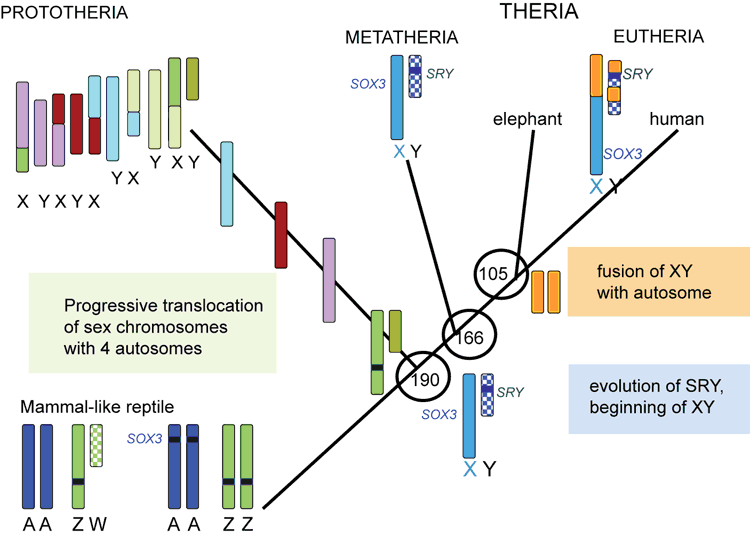
Astonishingly, our work with the more distantly related platypus showed that even monotreme mammals have no SRY (Fig. 4c) (Wallis et al. 2007). Their bizarre multiple sex chromosomes (Fig. 4a, b) (Grutzner et al. 2004; Waters et al. 2005) are homologous, not to the human XY, but to the bird ZW (Fig. 4d) (Veyrunes et al. 2008). This allows us to date the beginnings of our sex chromosomes to after the divergence of therian mammals from monotremes 190 million years ago, but before the marsupial–eutherian divergence 160 million years ago.
There has therefore been considerable turnover of sex chromosomes in vertebrate evolution, and this has been driven by different sex-determining genes (Graves 2013). We know something of this process, which is parallel in all lineages of mammals, other vertebrates and even insects.
It all starts when a new sex-determining gene, SRY for instance, arises on one member of a pair of autosomes. This proto-Y still pairs and recombines with its partner, now a proto-X. But other genes nearby on the Y are now selected for a male function, and pretty soon recombination in this region is driven lower by selection to keep together a male-specific package of genes.
Low recombination is the kiss of death for any genome region because it prevents reconstitution of a mutant-free Y, so the region of the Y including SRY rapidly degenerates as genes mutate and are ultimately lost (Charlesworth 1991; Graves 2006). This explains why the Y is such a wimp; it retains only 45 genes, compared with the 1600-odd genes it started with. And it explains why most of the genes on the male-specific part of the Y have partners on the X from which they obviously evolved.
The ultimate endpoint – complete loss of the Y and replacement of SRY by a novel system, is predicted to occur in a few million years (Aitken and Graves 2002). It has already occurred in two rodent lineages (Just et al. 1995; Kuroiwa et al. 2010), although the Y in humans and other primates appears to be more stable (Hughes and Rozen 2012).
The same process in reverse is observed with the degradation of the female-specific W chromosome in snakes and birds. We can see evolutionary intermediates in several lineages; for instance, boid snakes and the ancient flightless ratite birds such as emus and ostriches have W chromosomes that are undifferentiated or only partly degraded.
Thus the evolution of SRY was the crucial event that initiated the evolution of the mammal XY sex chromosomes. And platypuses provided the critical start-point of the evolution of our XY pair and SRY.
Marsupials and the evolution of SRY
How did SRY evolve? The answer came in the form of another SOX gene, and again from work done in marsupials.
In Jamie Foster’s hunt for marsupial SRY, he demonstrated many related genes in the tammar and dunnart genomes, the homologues, presumably, of the Sox family that had been described in the mouse (Gubbay et al. 1990a). One of these (SOX3) showed clear dosage differences in marsupials: twice as much in XX females as in XY males, an indication that it was on the X chromosome (Fig. 3a).
We found that SOX3 was the SOX gene most closely related to SRY, and we suggested that it represented the ancestor of SRY (Foster and Graves 1994). SOX3 is normally expressed in the central nervous system and in germ cells, but not in the somatic cells of the testis, so it has no normal role in sex determination.
Twenty years later, this idea gains support from the finding of XX male babies with no SRY, in whom SOX3 is expressed, not just in the central nervous system and germ cells, but in the somatic cells of the testis (Sutton et al. 2011). Evidently, SOX3 can substitute for SRY if it is misexpressed in the right tissue. So, too, can it when expressed in the genital ridge in XX mice transgenic for SOX3.
This suggests that SRY arose by a simple rearrangement that truncated SOX3 and substituted a promotor that drove its expression into the genital ridge. A similar thing has happened in several fish, in which change of the tissue – or the timing or the amount – of an autosomal gene that upstaged the reigning sex-determining gene (Graves 2013; Kikuchi and Hamaguchi 2013).
Kangaroos and Y chromosome degradation
It turns out that SRY is typical of genes on the Y chromosome in humans and other mammals, most of which have partners on the X from which they obviously evolved. Kangaroos were crucial to these discoveries.
Studies in the tammar wallaby identified X-borne partners, not only to SRY, but also to other genes on the Y chromosome (Fig. 3a). For instance, a gene RBMY on the human Y was thought to be a critical spermatogenesis gene lying in an interval, deletion of which caused azoospermia. It had been classed by Page as a ‘Type II’ Y gene that was unique to males (in contradistinction from Type I genes that had copies on the X: Lahn and Page 1997). However, our attempts to clone its homologue from the tammar wallaby and the dunnart revealed a homologue on the marsupial X chromosome, and we soon found that the human X, too, contained a copy of this RBMX gene. This gene, expressed in brain and germ cells, is highly conserved throughout vertebrates and critical for brain development in zebrafish (Tsend-Ayush et al. 2005). It is clearly the ancestor of RBMY. So too is a gene TSPX on the X the ancestor for the gonadoblastoma gene TSPY on the Y (Delbridge et al. 2004).
It turns out that most (20 of the 27) of the unique protein-coding genes on the human Y have copies on the X from which they clearly diverged. Some genes on the Y keep their original function, suggesting that they are dosage sensitive (Bellott et al. 2014). Others have adopted male-specific functions.
This implies that the human Y chromosome is essentially a degraded X, and genes on the Y are mostly degraded copies of genes on the X that have been selected for a male-specific function, usually in spermatogenesis (Delbridge et al. 1999; Delbridge et al. 2004). There is no distinction between Class I and II genes, since many of the male-specific genes have homologues on the X. This finding is consistent with the hypothesis that the Y chromosome is essentially a degraded X (Graves 2006).
The Y chromosome of marsupials is very tiny, sometimes just a dot under the microscope. This is not surprising because it is derived from the smaller X of ancient mammals which did not include the added region. Surprisingly, however, it retains several genes, some shared with the human Y, others unique to marsupials (Fig. 3b).
The first unique marsupial Y gene was discovered in the attempt to isolate the marsupial version of a sex-reversing gene on the human X, ATRX. Surprisingly, sequences from human ATRX detected, as well as an ATRX orthologue on the marsupial X, a sequence on the tammar wallaby Y we called ATRY (Pask et al. 2000). A strategy of screening genes with DNA from physically isolated tammar wallaby Y chromosomes netted 11 more (Murtagh et al. 2012). Four of these (including SRY and RBMY) are shared with the human XY. The others are not present on the Y chromosome in eutherians. But all have copies on the X chromosome in tammar wallabies, as well as in humans.
The mammal Y chromosome has therefore degraded very rapidly since it was initiated 166–190 million years ago. Since divergence 166 million years ago, degradation has occurred independently in marsupials and eutherians, so that the gene content of their Y chromosomes is different.
Degradation of the Y in rodents has proceeded to the state that it contains only two genes that are required to produce fertile males (Yamauchi et al. 2016); even these can be easily substituted. There are many rodent species with variant sex chromosomes and modes of sex determination, including inactive SRY alleles in XY* females, suppressor loci on the X that produce XY* females, translocations and rearrangements that might add novel sex determining genes. Two rodent lineages have completely lost the Y chromosome.
I have suggested that the rodent Y chromosome has degraded to a point at which the sex-determining system is no longer stable, providing a selective advantage to novel systems. The same fate may eventually overtake the human Y chromosome, promoting similar experiments in sex chromosome evolution. It has recently been proposed that this type of sex chromosome turnover is associated with the major mammal divergences (Fig. 5) and may have promoted mammal speciation (Graves 2016).
Conclusions – the value of ‘independent experiments in mammal evolution’
The discovery of SRY was historically important. It was an early demonstration of the power of positional cloning, which has since unlocked the secrets of many human genetic diseases. SRY is an important gene that regulates the switch of a complex network of genes that control the direction of differentiation of the undifferentiated gonad into either a testis or an ovary.
Like most of mammal genetics, the focus of discovery in this field has been on humans and mice. There is no doubt that these species provide excellent models; humans because we cherish, diagnose and treat our mutants, and mice because their genomes are so manipulable. Sensible scientists work on these two species because they are given the lion’s share of funding (even in Australia).
However, Australian mammals offer special insights because they are so distantly related to humans and mice. Marsupials have been evolving independently from eutherians for 166 million years, and monotremes for 190 million years. In this time, many fundamental differences, even in the most basic functions, like sex, are likely to have evolved (Fig. 5) (Luo et al. 2011).
It was the ‘marsupial test’ that disqualified ZFY from a role in sex determination because it was autosomal in tammar wallabies and dunnarts, and the same species that offered confirmation of the role of SRY. It was the same two species that provided the first glimpse of an X-borne partner of SRY that was the key to discovering how this critical gene evolved. And the X-borne partners of other human Y genes that led to the theory that the human Y is a degenerate X, and Y genes evolved from X genes. It was the platypus, with its bizarre bird-like sex chromosomes, that provided a (surprisingly recent) time-zero point for the evolution of SRY and the therian XY pair.
SRY is important also in evolutionary history. Its birth 190–166 million years ago defined the mammal XY chromosome pair, and its eventual death may lead to the evolution of novel systems. SRY is a paradigm of genes isolated on the Y chromosome, which diverge and take on male-specific roles.
References
Affara, N. A.,, et al (1987). Mapping the testis determinants by an analysis of Y-specific sequences in males with apparent XX and XO karyotypes and females with XY karyotypes. Nucleic Acids Research 15, 7325–7342.| Mapping the testis determinants by an analysis of Y-specific sequences in males with apparent XX and XO karyotypes and females with XY karyotypes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2sXls12qsrY%3D&md5=e28d50ae18e0072acea1f3d7901c354dCAS |
Aitken, R. J., and Graves, J. A. M. (2002). The future of sex. Nature 415, 963.
| The future of sex.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xhsl2ltL8%3D&md5=d8012e392dc4d86027af6b93672121afCAS |
Avilion, A. A.,, et al (2003). Multipotent cell lineages in early mouse development depend on SOX2 function. Genes & Development 17, 126–140.
| Multipotent cell lineages in early mouse development depend on SOX2 function.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXktlKqtg%3D%3D&md5=63c6c17d666b80a2ecae48562c90bdddCAS |
Bellott, D. W.,, et al (2014). Mammalian Y chromosomes retain widely expressed dosage-sensitive regulators. Nature 508, 494–499.
| Mammalian Y chromosomes retain widely expressed dosage-sensitive regulators.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXmslaktbg%3D&md5=6b046b635e0f727dc58100561764f6ccCAS |
Berta, P.,, et al (1990). Genetic evidence equating SRY and the testis-determining factor. Nature 348, 448–450.
| Genetic evidence equating SRY and the testis-determining factor.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXhvVOnsg%3D%3D&md5=cf6723f289c373c790d8bda4cb3ebfceCAS |
Bridges, C. B. (1925). Sex in relation to chromosomes and genes. American Naturalist 59, 127–137.
| Sex in relation to chromosomes and genes.Crossref | GoogleScholarGoogle Scholar |
Calvari, V.,, et al (2000). A new submicroscopic deletion that refines the 9p region for sex reversal. Genomics 65, 203–212.
| A new submicroscopic deletion that refines the 9p region for sex reversal.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXktFarsr4%3D&md5=aea3aba18e8b8c588bc50fc06adb0a2bCAS |
Charlesworth, B. (1991). The evolution of sex chromosomes. Science 251, 1030–1033.
| The evolution of sex chromosomes.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3M7kvF2jtw%3D%3D&md5=b60cc263c65280411f5322150429217aCAS |
Chen, S.,, et al (2014). Whole-genome sequence of a flatfish provides insights into ZW sex chromosome evolution and adaptation to a benthic lifestyle. Nature Genetics 46, 253–260.
| Whole-genome sequence of a flatfish provides insights into ZW sex chromosome evolution and adaptation to a benthic lifestyle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhs1Snu7k%3D&md5=e4fa5103366092925309c2192f7a192cCAS |
Delbridge, M. L.,, et al (1999). The candidate spermatogenesis gene RBMY has a homologue on the human X chromosome. Nature Genetics 22, 223–224.
| The candidate spermatogenesis gene RBMY has a homologue on the human X chromosome.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXkt1eqsLg%3D&md5=73305735d5d252264b911b79af17524aCAS |
Delbridge, M. L.,, et al (2004). TSPY, the candidate gonadoblastoma gene on the human Y chromosome, has a widely expressed homologue on the X – implications for Y chromosome evolution. Chromosome Research 12, 345–356.
| TSPY, the candidate gonadoblastoma gene on the human Y chromosome, has a widely expressed homologue on the X – implications for Y chromosome evolution.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXlsVOmt7c%3D&md5=fdda059c582301107242523346290868CAS |
Delgado, C. L. R.,, et al (2009). Physical mapping of the elephant X chromosome: conservation of gene order over 105 million years. Chromosome Research 17, 917–926.
| Physical mapping of the elephant X chromosome: conservation of gene order over 105 million years.Crossref | GoogleScholarGoogle Scholar |
Eggers, S., Ohnesorg, T., and Sinclair, A. (2014). Genetic regulation of mammalian gonad development. Nature Reviews: Endocrinology 10, 673–683.
| Genetic regulation of mammalian gonad development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhsFyksrfE&md5=37627ca9d11ad74c03c7dcbe92cb1339CAS |
Ezaz, T.,, et al (2009). The ZW sex microchromosomes of an Australian dragon lizard share no homology with those of other reptiles or birds. Chromosome Research 17, 965–973.
| The ZW sex microchromosomes of an Australian dragon lizard share no homology with those of other reptiles or birds.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsFGrurbI&md5=b85be7250d39c0c2b74e897515e73488CAS |
Ford, C. E.,, et al (1959). A sex-chromosome anomaly in a case of gonadal dysgenesis (Turner’s syndrome). Lancet 273, 711–713.
| A sex-chromosome anomaly in a case of gonadal dysgenesis (Turner’s syndrome).Crossref | GoogleScholarGoogle Scholar |
Foster, J. W., and Graves, J. A. M. (1994). An SRY-related sequence on the marsupial X chromosome: implications for the evolution of the mammalian testis-determining gene. Proceedings of the National Academy of Sciences of the United States of America 91, 1927–1931.
| An SRY-related sequence on the marsupial X chromosome: implications for the evolution of the mammalian testis-determining gene.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXivFWisr0%3D&md5=4d1cd1aab2c130e1f7f1e5fd763e6406CAS |
Foster, J. W.,, et al (1992). Evolution of sex determination and the Y chromosome: SRY-related sequences in marsupials. Nature 359, 531–533.
| Evolution of sex determination and the Y chromosome: SRY-related sequences in marsupials.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXltlamt70%3D&md5=e73bcd86a0f8b650ad617f8f8a99302dCAS |
Foster, J. W.,, et al (1994). Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature 372, 525–530.
| Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXisFKkt7k%3D&md5=20b70c55b8d6f383ed999b4e4077605aCAS |
Graves, J. A. M. (2006). Sex chromosome specialization and degeneration in mammals. Cell 124, 901–914.
| Sex chromosome specialization and degeneration in mammals.Crossref | GoogleScholarGoogle Scholar |
Graves, J. A. M. (2013). How to evolve new vertebrate sex determining genes. Developmental Dynamics 242, 354–359.
| How to evolve new vertebrate sex determining genes.Crossref | GoogleScholarGoogle Scholar |
Graves, J. A. M. (2015). Evolution of vertebrate sex chromosomes and dosage compensation. Nature Reviews. Genetics 17, 33–46.
| Evolution of vertebrate sex chromosomes and dosage compensation.Crossref | GoogleScholarGoogle Scholar |
Graves, J. A. M. (2016). Sex chromosome turnover and mammalian divergence. BioEssays 38, 734–743.
| Sex chromosome turnover and mammalian divergence.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28XhtF2ntr%2FN&md5=45cb7e7727833e8b52a5a5a8213a93e9CAS |
Grutzner, F.,, et al (2004). In the platypus a meiotic chain of ten sex chromosomes shares genes with the bird Z and mammal X chromosomes. Nature 432, 913–917.
| In the platypus a meiotic chain of ten sex chromosomes shares genes with the bird Z and mammal X chromosomes.Crossref | GoogleScholarGoogle Scholar |
Gubbay, J.,, et al (1990a). A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature 346, 245–250.
| A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXkvFaltrk%3D&md5=d72a5608d0da069cafa02108c1c5e249CAS |
Gubbay, J.,, et al (1990b). Normal structure and expression of Zfy genes in XY female mice mutant in Tdy. Development 109, 647–653.
| 1:CAS:528:DyaK3cXlvVGrsLw%3D&md5=24da45bddc86b0c81fe81a4f284b3e76CAS |
Harley, V. R., and Goodfellow, P. N. (1994). The biochemical role of SRY in sex determination. Molecular Reproduction and Development 39, 184–193.
| The biochemical role of SRY in sex determination.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXht1aktbw%3D&md5=057e64ab11ed004e6a43555d476a0750CAS |
Hughes, J. F., and Rozen, S. (2012). Genomics and genetics of human and primate Y chromosomes. Annual Review of Genomics and Human Genetics 13, 83–108.
| Genomics and genetics of human and primate Y chromosomes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsVGit77P&md5=1b9996b0c191ace3ba549a3bae45ff4eCAS |
Jacobs, P. A., and Strong, J. A. (1959). A case of human intersexuality having a possible XXY sex-determining mechanism. Nature 183, 302–303.
| A case of human intersexuality having a possible XXY sex-determining mechanism.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaG1M%2Fmt1aqug%3D%3D&md5=241fab6728bdc4615556216c56e4a1e0CAS |
Jost, A. (1970). Hormonal factors in the sex differentiation of the mammalian foetus. Philosophical Transactions of the Royal Society of London, Series B: Biological Sciences 259, 119–131.
| Hormonal factors in the sex differentiation of the mammalian foetus.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE38%2FjsFSmuw%3D%3D&md5=132f61b2e467548f02c63e5605f30479CAS |
Just, W.,, et al (1995). Absence of Sry in species of the vole Ellobius. Nature Genetics 11, 117–118.
| Absence of Sry in species of the vole Ellobius.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXosF2rsbo%3D&md5=6059cdec03f00d148d6959f8906fd39eCAS |
Kikuchi, K., and Hamaguchi, S. (2013). Novel sex-determining genes in fish and sex chromosome evolution. Developmental Dynamics 242, 339–353.
| Novel sex-determining genes in fish and sex chromosome evolution.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXksFWhtb8%3D&md5=940bca2e3430b7c92616cfeec7115d63CAS |
Koopman, P.,, et al (1989). Zfy gene expression patterns are not compatible with a primary role in mouse sex determination. Nature 342, 940–942.
| Zfy gene expression patterns are not compatible with a primary role in mouse sex determination.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXpt12ksw%3D%3D&md5=5e28b90d411d04787c518626a486586cCAS |
Koopman, P.,, et al (1991). Male development of chromosomally female mice transgenic for Sry. Nature 351, 117–121.
| Male development of chromosomally female mice transgenic for Sry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXktVagsrk%3D&md5=0624619e1faf10c0c387837140221c20CAS |
Kuroiwa, A.,, et al (2010). The process of a Y-loss event in an XO/XO mammal, the Ryukyu spiny rat. Chromosoma 119, 519–526.
| The process of a Y-loss event in an XO/XO mammal, the Ryukyu spiny rat.Crossref | GoogleScholarGoogle Scholar |
Lahn, B., and Page, D. C. (1997). Functional coherence of the human Y chromosome. Science 278, 675–680.
| Functional coherence of the human Y chromosome.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXmvVygu70%3D&md5=957356daed5492a32ddd6f678d917afeCAS |
Luo, Z. X.,, et al (2011). A Jurassic eutherian mammal and divergence of marsupials and placentals. Nature 476, 442–445.
| A Jurassic eutherian mammal and divergence of marsupials and placentals.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtVOnu7jO&md5=02fe6a3dee4dc95b8bee8d6264792c0aCAS |
Lyon, M. F., and Hawkes, S. G. (1970). X-linked gene for testicular feminization in the mouse. Nature 227, 1217–1219.
| X-linked gene for testicular feminization in the mouse.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE3c3ntVSmsA%3D%3D&md5=a9f608cb065673cf4303da781fd47c38CAS |
Meyers-Wallen, V. N.,, et al (1999). Sry-negative XX sex reversal in purebred dogs. Molecular Reproduction and Development 53, 266–273.
| Sry-negative XX sex reversal in purebred dogs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXjsFGhtb4%3D&md5=1b4e6f6b3bd5c219b22b2f8f2717e0c7CAS |
Migeon, B. R.,, et al (1981). Studies of the locus for androgen receptor: localization on the human X chromosome and evidence for homology with the Tfm locus in the mouse. Proceedings of the National Academy of Sciences of the United States of America 78, 6339–6343.
| Studies of the locus for androgen receptor: localization on the human X chromosome and evidence for homology with the Tfm locus in the mouse.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3MXmtVWgsrg%3D&md5=3615cc97d0a09526d7735322a8e84bfaCAS |
Murtagh, V. J.,, et al (2012). Evolutionary history of novel genes on the tammar wallaby Y chromosome: implications for sex chromosome evolution. Genome Research 22, 498–507.
| Evolutionary history of novel genes on the tammar wallaby Y chromosome: implications for sex chromosome evolution.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XjsV2lsb0%3D&md5=443b6c43315b0b7aa56671dcf6d7f92cCAS |
Nanda, I.,, et al (1999). 300 million years of conserved synteny between chicken Z and human chromosome 9. Nature Genetics 21, 258–259.
| 300 million years of conserved synteny between chicken Z and human chromosome 9.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXitVCit7k%3D&md5=76ab5a2dc83c6639719812e33b405255CAS |
Ohno, S. 1967. ‘Sex Chromosomes and Sex Linked Genes.’ (Springer-Verlag: New York.)
Ohno, S. (1978). The role of H-Y antigen in primary sex determination. Journal of the American Medical Association 239, 217–220.
| The role of H-Y antigen in primary sex determination.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE1c%2FmvFGhtQ%3D%3D&md5=3d4f7af2d7ad485fb8c31c7d9c4f16dcCAS |
Page, D. C.,, et al (1987). The sex-determining region of the human Y chromosome encodes a finger protein. Cell 51, 1091–1104.
| The sex-determining region of the human Y chromosome encodes a finger protein.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1cXntVSiuw%3D%3D&md5=32f3232ffeee8d4edf0500991bfe0e3cCAS |
Pailhoux, E.,, et al (2002). Ontogenesis of female-to-male sex-reversal in XX polled goats. Developmental Dynamics 224, 39–50.
| Ontogenesis of female-to-male sex-reversal in XX polled goats.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XktlCisLs%3D&md5=382dce2784b7fa1b0595dbd691c8116cCAS |
Parma, P.,, et al (2006). R-spondin1 is essential in sex determination, skin differentiation and malignancy. Nature Genetics 38, 1304–1309.
| R-spondin1 is essential in sex determination, skin differentiation and malignancy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtFeisbjF&md5=6503d62ee1ae175e7686ddf41bea7193CAS |
Pask, A., Renfree, M. B., and Graves, J. A. M. (2000). The human sex-reversing ATRX gene has a homologue on the marsupial Y chromosome, ATRY: implications for the evolution of mammalian sex determination. Proceedings of the National Academy of Sciences of the United States of America 97, 13198–13202.
| The human sex-reversing ATRX gene has a homologue on the marsupial Y chromosome, ATRY: implications for the evolution of mammalian sex determination.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXosVKqurY%3D&md5=91662c6f8a176dda4ef9deca0a3782cfCAS |
Raymond, C. S.,, et al (1998). Evidence for evolutionary conservation of sex-determining genes. Nature 391, 691–695.
| Evidence for evolutionary conservation of sex-determining genes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXht1GmtLw%3D&md5=da453ecaf41715562de6d88f075c4a2aCAS |
Raymond, C. S.,, et al (1999). A region of human chromosome 9p required for testis development contains two genes related to known sexual regulators. Human Molecular Genetics 8, 989–996.
| A region of human chromosome 9p required for testis development contains two genes related to known sexual regulators.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXjs1WntLg%3D&md5=0fe7c0e4c04e4a38c458a98460e85e4aCAS |
Sekido, R., and Lovell-Badge, R. (2013). Genetic control of testis development. Sexual Development: Genetics, Molecular Biology, Evolution, Endocrinology, Embryology, and Pathology of Sex Determination and Differentiation 7, 21–32.
| Genetic control of testis development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhvVClsb3J&md5=409ff1da9f7e402a312e675ab8e644f4CAS |
Simpson, E.,, et al (1987). Separation of the genetic loci for the H-Y antigen and for testis determination on human Y chromosome. Nature 326, 876–878.
| Separation of the genetic loci for the H-Y antigen and for testis determination on human Y chromosome.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2sXktVGnsbc%3D&md5=6e78372cc70371506c3aa2e778537f2cCAS |
Sinclair, A. H.,, et al (1988). Sequences homologous to ZFY, a candidate human sex-determining gene, are autosomal in marsupials. Nature 336, 780–783.
| Sequences homologous to ZFY, a candidate human sex-determining gene, are autosomal in marsupials.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1MXovFOjtw%3D%3D&md5=ee9e3b9b9e0bc37b24981ca62b47b459CAS |
Sinclair, A. H.,, et al (1990). A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature 346, 240–244.
| A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXkvFaltrg%3D&md5=ad0faf0d3b7fb940e95ba592cd08af75CAS |
Singh, L.,, et al (1994). Banded krait minor-satellite (Bkm)-associated Y chromosome-specific repetitive DNA in mouse. Nucleic Acids Research 22, 2289–2295.
| Banded krait minor-satellite (Bkm)-associated Y chromosome-specific repetitive DNA in mouse.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXlt1Klsb4%3D&md5=d1636318ff4b74cb176fb398c9c732a7CAS |
Smith, C. A.,, et al (1999). Conservation of a sex-determining gene. Nature 402, 601–602.
| Conservation of a sex-determining gene.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXjsVCm&md5=f10a1d98624936d22d2a06c09aec0acdCAS |
Smith, C. A.,, et al (2009). The avian Z-linked gene DMRT1 is required for male sex determination in the chicken. Nature 461, 267–271.
| The avian Z-linked gene DMRT1 is required for male sex determination in the chicken.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtVGiurvN&md5=07c3ba3db278e38efb906a485b63701bCAS |
Sutton, E.,, et al (2011). Identification of SOX3 as an XX male sex reversal gene in mice and humans. The Journal of Clinical Investigation 121, 328–341.
| Identification of SOX3 as an XX male sex reversal gene in mice and humans.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXis1ylsA%3D%3D&md5=800432feb553402b0c42b79e72a637f4CAS |
Tsend-Ayush, E.,, et al (2005). RBMX gene is essential for brain development in zebrafish. Developmental Dynamics 234, 682–688.
| RBMX gene is essential for brain development in zebrafish.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXht1Cru7%2FJ&md5=d25d7cfb22102728527d2546b9c9cbe1CAS |
Veyrunes, F.,, et al (2008). Bird-like sex chromosomes of platypus imply recent origin of mammal sex chromosomes. Genome Research 18, 965–973.
| Bird-like sex chromosomes of platypus imply recent origin of mammal sex chromosomes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXnsVygsbY%3D&md5=d58ebbe0971088ca8e955df58017baa2CAS |
Wagner, T.,, et al (1994). Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell 79, 1111–1120.
| Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2M%2FpsVaktg%3D%3D&md5=25658ced6ee53cb5fb0b1ab1f36a66daCAS |
Wallis, M. C.,, et al (2007). Sex determination in platypus and echidna: autosomal location of SOX3 confirms the absence of SRY from monotremes. Chromosome Research 15, 949–959.
| Sex determination in platypus and echidna: autosomal location of SOX3 confirms the absence of SRY from monotremes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXkslWrsA%3D%3D&md5=f76537d5694ab2fe93ecdd4903724ef1CAS |
Waters, P. D.,, et al (2005). Autosomal location of genes from the conserved mammalian X in the platypus (Ornithorhynchus anatinus): implications for mammalian sex chromosome evolution. Chromosome Research 13, 401–410.
| Autosomal location of genes from the conserved mammalian X in the platypus (Ornithorhynchus anatinus): implications for mammalian sex chromosome evolution.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXltl2mtLY%3D&md5=1b9e281c9f24d444a60a8ab5991d0669CAS |
Wilcox, S. A., Watson, J. M., Spencer, J. A., and Graves, J. A. M. (1996). Comparative mapping identifies the fusion point of an ancient mammalian X-autosomal rearrangement. Genomics 35, 66–70.
| 1:CAS:528:DyaK28Xkt12qs7c%3D&md5=0ce51b0e8eba5fe881cf9270b533ab47CAS |
Wilhelm, D., Yang, J. X., and Thomas, P. (2013). Mammalian sex determination and gonad development. Current Topics in Developmental Biology 106, 89–121.
| Mammalian sex determination and gonad development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXktlertr0%3D&md5=d36665a283552732b10a95a0f27fbb95CAS |
Yamauchi, Y.,, et al (2016). Two genes substitute for the mouse Y chromosome for spermatogenesis and reproduction. Science 351, 514–516.
| Two genes substitute for the mouse Y chromosome for spermatogenesis and reproduction.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28XhtlyntLc%3D&md5=54b20b140919b1b3153c6b0b7fd93741CAS |