Life-history of masked water snakes (Homalopsis buccata) in Java: implications for the sustainability of harvesting
Evy Arida A B , Noor Laina Maireda C , Alamsyah Elang Nusa Herlambang B D , Mumpuni B D , Awal Riyanto B D , Amir Hamidy B D , Richard Shine B E and Daniel J. D. Natusch
B D , Richard Shine B E and Daniel J. D. Natusch  B E *
B E *
A
B
C
D
E
Abstract
Masked water snakes (Homalopsis buccata, Homalopsidae) thrive in the muddy edges of agricultural ponds and canals in densely populated areas of West Java, Indonesia, and are harvested by local farmers to protect fish stocks and to provide meat, skins, and medicines for commercial use.
Here, we aimed to quantify sexual dimorphism and reproductive biology of H. buccata, so as to deepen our knowledge of the species’ inherent ability to withstand commercial harvests.
We examined carcasses of 4286 snakes at six processing sites to quantify biological attributes (e.g. sexual dimorphism in body size and shape, seasonality of reproduction, fecundity, reproductive frequency), with emphasis on traits that affect the ability of snake populations to withstand this intensive harvesting.
The snakes we examined were primarily adults (<1% juvenile), with approximately equal numbers of males and females except in January (when females comprised >90% of specimens). Females grow larger than males, and they are more heavy-bodied but shorter-tailed than are males of the same snout–vent length. Reproduction is seasonal in both sexes, with testis volumes decreasing to a minimum over the period August to November (late dry season) when most adult-size females were gravid. Litter sizes ranged from 1 to 37 (mean 12), increasing with maternal body size, with ~75% of females reproducing each year.
On the basis of these results, we infer that the life history of H. buccata (viviparity, high fecundity, frequent reproduction, rapid maturation) renders it inherently resilient to harvesting, especially because that offtake is based on males as well as females. Because a lack of sustainability is evident only in hindsight, regular monitoring of the trade could assure that any problems are detected rapidly.
To further buffer these populations from the impact of harvest, hunting could be restricted during January (a time when gravid females are disproportionately vulnerable) and the largest snakes (females, with high fecundity and reproductive frequency) could be excluded from harvests.
Keywords: CITES non-detriment findings, harvest, Homalopsinae, Indonesia, offtake, population monitoring, puff-faced water snake, wildlife trade.
Introduction
Wildlife populations are harvested in many parts of the world, providing valuable resources for local people; however, excessively high rates of offtake can imperil viability of the populations being exploited (e.g. Campos-Silva et al. 2017). An extensive literature explores the sustainability of wildlife harvesting, and clarifies traits of the species involved (such as high rates of reproduction) and of the harvest (such as the degree to which hunters target critical cohorts such as reproductive females) that affect sustainability (e.g. Webb 2002; Leao et al. 2017). However, such analyses are available primarily for harvests of mammals, birds, and fish in temperate-zone habitats (Dobson et al. 2019; Natusch et al. 2019a). In many parts of the tropics, local people harvest reptiles (e.g. turtles, snakes, lizards) for skin, meat and medicinal use, but sustainability of those harvests is less well known. Part of the problem lies in the location of these harvests (far from major research facilities) and, in part, from the attributes of the harvested animals. For example, ectotherms in lush tropical forests or swamps can be difficult to survey, especially if they remain inactive for long periods of time (Shine et al. 1999; Natusch et al. 2019a). As a result, it can be difficult to quantify underlying abundances and demographic traits (e.g. growth rates, ages at maturation) of such animals, precluding the application of mathematical models such as population viability analyses (Natusch et al. 2016, 2019a, 2020a).
In the absence of robust data on underlying population structures and abundances, one logistically feasible alternative is to gather data on specimens that have been harvested. With the benefits of a large sample size, fundamental traits of the harvested species can be documented in detail, providing a basis from which to infer the population’s ability to withstand intense harvesting, and to identify modifications of harvest methods that might enhance sustainability (Shine et al. 1999; Natusch et al. 2019a). If repeated over significant time periods, a consistency in offtake rates and in key attributes (such as mean body sizes and sex ratios) of the harvested specimens can give assurance that the harvest is sustainable (e.g. Fitzgerald 1994; Natusch et al. 2019b), whereas changes in those parameters suggest that the harvest is affecting population structure and/or abundance (e.g. Natusch et al. 2020a). The information from such studies also is of interest from the standpoint of basic biology, because most tropical reptiles, even species subject to longterm intensive harvests, are poorly known (e.g. Keogh et al. 2001).
In the present study, we visited six processing facilities in West Java, Indonesia, to measure morphological and reproductive traits of snakes collected by local people, which were then killed and skinned at local processing sites. In addition to quantifying traits (both of the snakes and of the patterns of offtake) that are likely to affect harvest sustainability, we took the opportunity to analyse basic biological traits of this abundant tropical species. Importantly, our sample size is larger than has been available for previous analyses of this taxon (Bergman 1951; Berry and Lim 1967; Brooks et al. 2007a, 2007b).
Study area
We examined specimens of H. buccata in the city of Cirebon in the province of West Java, Indonesia (6°43′S, 108°34′E). The landscape around Cirebon primarily comprises coastal lowlands with very little topographic relief. Historically, the major habitats were lowland rainforest and swamps, but today the landscape is dominated by rice cultivation and associated urban development. Cirebon experiences high temperatures year-round (mean monthly temperatures 25–27°C), with the annual rainfall (2120 mm) being broadly distributed among months, except for a short dry season (May to September) (https://en.climate-data.org).
Methods
Study species
A freshwater species, H. buccata is one of the largest snake species within the family Homalopsidae (previously regarded as subfamily Homalopsinae within the Colubridae: Bernstein et al. 2021), with some specimens attaining more than a metre in length and over 2 kg in mass (Bergman 1951; present study). Heavy-bodied and with a relatively large head, most specimens are strongly banded (especially during juvenile life; Murphy (2007); see Fig. 1). Reflecting its abundance and wide geographic distribution through Southeast Asia, H. buccata has several common names (e.g. masked water snake, puff-faced water snake, see Murphy (2007) for an extensive list). The taxonomy remains confused, with multiple species-level lineages being likely to be occurring across the area from Bangladesh through Myanmar, Thailand, and the Indo-Malaysian Peninsula to at least as far east as Sulawesi (Murphy 2007; Bernstein et al. 2021). Commonly encountered at the muddy edges of waterbodies, H. buccata feeds primarily on fish and anurans (Murphy 2007). Females are viviparous, producing a litter of 2–37 neonates (see review by Murphy 2007). The seasonal timing of reproduction appears to differ among locations, being relatively aseasonal in Malaysia (Berry and Lim 1967), but with pregnancy being most common in the period February to May in Cambodia (Saint Girons and Pfeffer 1972) and Thailand (Brooks et al. 2009) and in the period June to December in Java (Bergman 1951).
Homalopsis buccata is a major pest for village-based aquaculture, consuming fish that are being raised for food and for commercial sale (e.g. Tweedie 1954; Hoesel 1959; Kusrini et al. 2022). For this reason, and reflecting the commercial value of skins and meat from this relatively large snake, the species is intensively harvested in Indonesia where the current annual quota is one million snakes. The Indonesian province of West Java is the focus of this harvest. Similarly intensive harvesting in Tonle Sap, Cambodia, has raised questions about population viability, with reports of declining abundances of snakes (Stuart 2004; Brooks et al. 2007a, 2007b). In West Java, these snakes are taken by a variety of methods, including nets, traps, electrostunning, and hand-capture at night (Kusrini et al. 2022). The live snakes are then taken to local processing centres to be killed and skinned (see Kusrini et al. 2022 for details).
Methods for collecting data
We visited six processing centres in and around the city of Cirebon, over the period from August 2019 to June 2021, to sample the commercial offtake of snakes across most months of the year. Sample sizes for snakes varied among locations and months (see Table 1). After a snake was humanely killed via brain destruction, we measured its snout–vent length (SVL) and tail length with a steel ruler, and mass with digital scales. Large prey items were removed from the gut before weighing, or the prey item was removed later, weighed, and its mass subtracted from the body mass of the snake. The snakes were then skinned, and we examined the carcass to record the sizes and numbers of ovarian follicles and oviductal embryos in females, the length and width of the right testis in males and vitellogenic follicles in females, and the thickness and appearance of oviducts and vas deferens to assess whether the animal had attained sexual maturity (see Natusch et al. 2016, 2019b, 2020a for details). The amount of body fat was scored on a four-point scale (0–3), and treated as an ordinal variable in analyses. The carcass was then returned to the processing-facility staff. Our research took advantage of an existing trade; no snakes were harmed for the purpose of our study.
| Month | Pak Asep | Pak Durali | Pak Mukti | Pak Tasrip | Pak Tosin | Pak Wasir | |
|---|---|---|---|---|---|---|---|
| January | 117 | 77 | 249 | 39 | – | – | |
| February | – | – | 719 | – | – | – | |
| March | – | 39 | 253 | – | – | – | |
| April | – | – | 288 | – | – | – | |
| May | 300 | 93 | 59 | – | – | – | |
| June | 245 | – | – | – | – | – | |
| July | 117 | 55 | 5 | 58 | 40 | 82 | |
| August | – | 174 | 27 | 6 | – | – | |
| September | 66 | 66 | 37 | 2 | 9 | 24 | |
| October | 65 | – | 17 | – | 26 | – | |
| November | – | 29 | 226 | – | 46 | 5 | |
| December | 249 | 82 | 217 | 30 | – | – |
The table shows the numbers of snakes examined at each facility in each month.
Methods for analysis of data
We analysed this dataset by using the statistical software program JMP Pro 15 (SAS Institute, Cary, NC, USA; see https://www.jmp.com/en_au/software/predictive-analytics-software.html). Variables were checked for normality and variance homogeneity, and ln-transformed when needed to improve fit to these assumptions of parametric statistical tests. We excluded animals with incomplete tails from analyses of relative tail length. We measured the right testis of dissected males, and secondary follicles of reproductive females, and estimated the volume of those organs from their length and width on the basis of the formula for a prolate spheroid (4/3π (half testis length) × (half testis width))2 (Harlow and Taylor 2000).
We compared the observed sex ratio (overall numbers of males vs females) by using a contingency-table test, against a null hypothesis of equal numbers per sex. We compared relative numbers of males versus females across the six processing facilities, and among the 12 months of the year, using nominal logistic regression.
We used ANOVA to compare mean body sizes (ln SVL and ln mass) between males and females, including location as a random factor. We used ANCOVA to document a sex-based divergence in ln mass relative to ln SVL, and in tail length relative to SVL, again with location as a random factor. To assess inter-facility variation in the degree of sexual-size dimorphism, we included location (processing facility) and sex (plus their interaction) as factors, and either ln SVL or ln mass as the dependent variable.
To look for seasonal variation in reproductive traits of female snakes, we conducted nominal logistic regression with month as the factor and reproductive state (reproductive vs not) as the dependent variable. To look for monthly variation in volumes of vitellogenic follicles and testes, we used ANOVA with month as the factor, organ volume as the dependent variable, and location as a random factor. To explore size-dependency of reproductive output in females, we used ANOVA with maternal SVL as the covariate, fecundity as the dependent variable, and location as a random factor. To find out whether a female’s body size affected the probability of her reproducing, we used nominal logistic regression with maternal SVL as the covariate, reproductive state (reproductive vs not, within the main breeding season of August to October) as the dependent variable, and location as a random factor.
Results
Demography
Our sample almost entirely comprised adult snakes, with only 21 juvenile females (of 2615 female snakes in total, so <1%) and 11 juvenile males (of 1671 male snakes, so <1%).
The overall sex ratio was female-biased (2615 females vs 1671 males, i.e. 61% female), being significantly different from 50:50 (χ2 = 103.96, 1 d.f., P < 0.0001). Females outnumbered males in all locations except Tosin, with the proportion of males varying from 20% at Durali to 56% at Tosin. Logistic regression confirmed significant differences among locations in sex ratios of the snakes that were processed (χ2 = 79.39, 5 d.f., P < 0.0001).
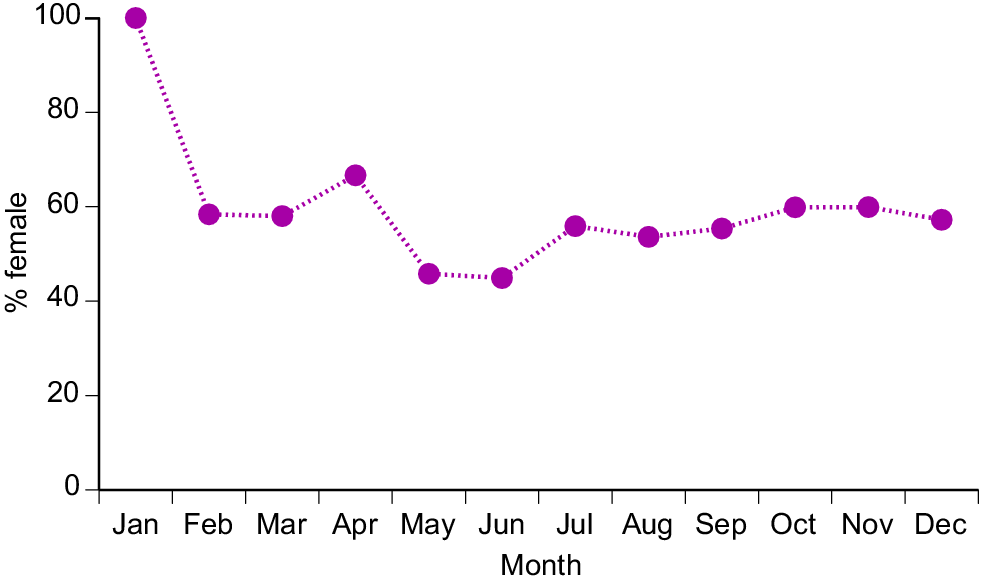
Sex ratios also varied among months (χ2 = 388.85, 11 d.f., P < 0.0001), primarily due to a highly female-biased sample in January (479 of 482 snakes, i.e. 99.4%), whereas sex ratios (% female) ranged between 45% and 67% in all other months (Fig. 2). The highly female-biased ratio was evident in all three sites sampled in January (Pak Asep, 100% female; Pak Durali, 99%; Pak Tasrip, 95%).
Sexual dimorphism
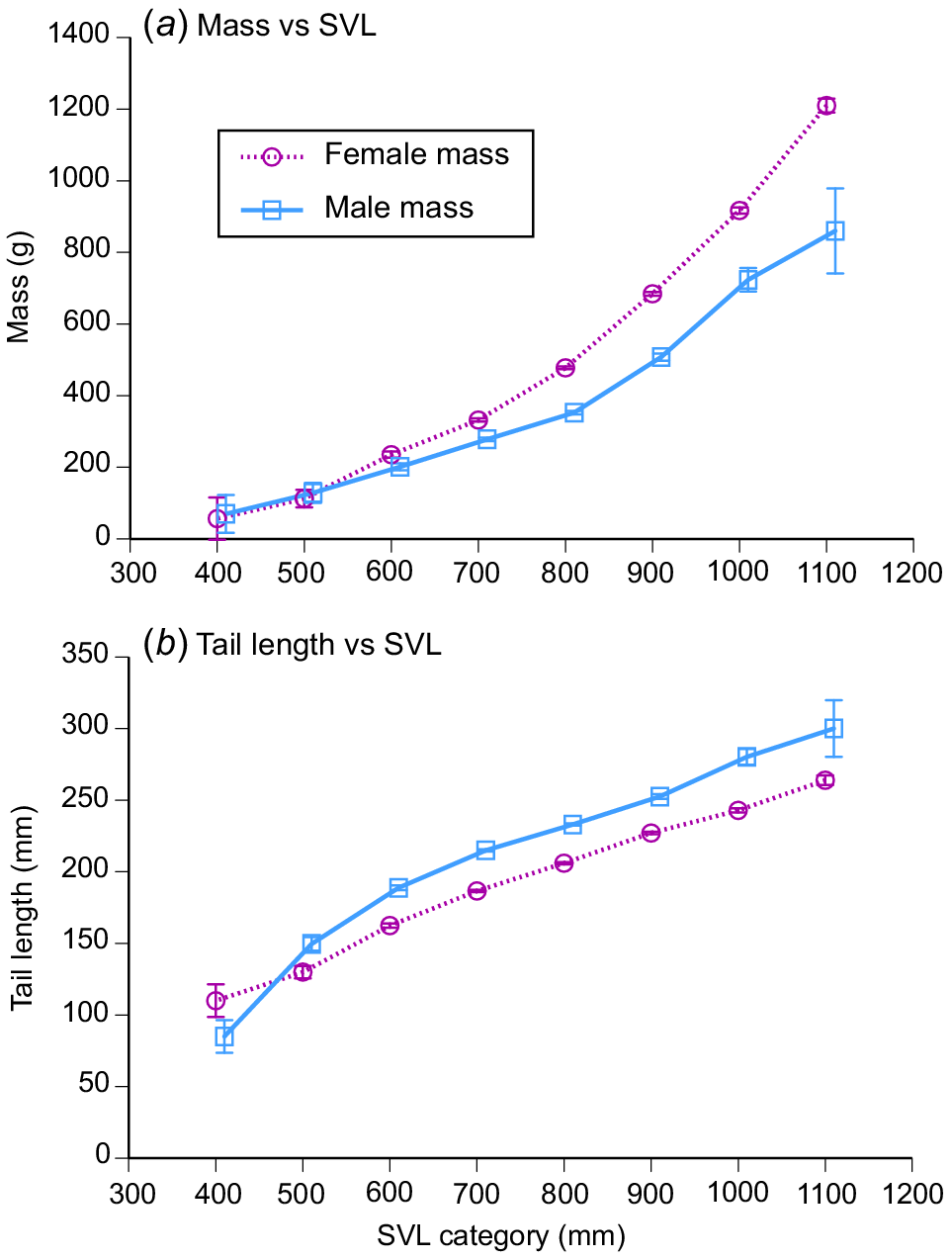
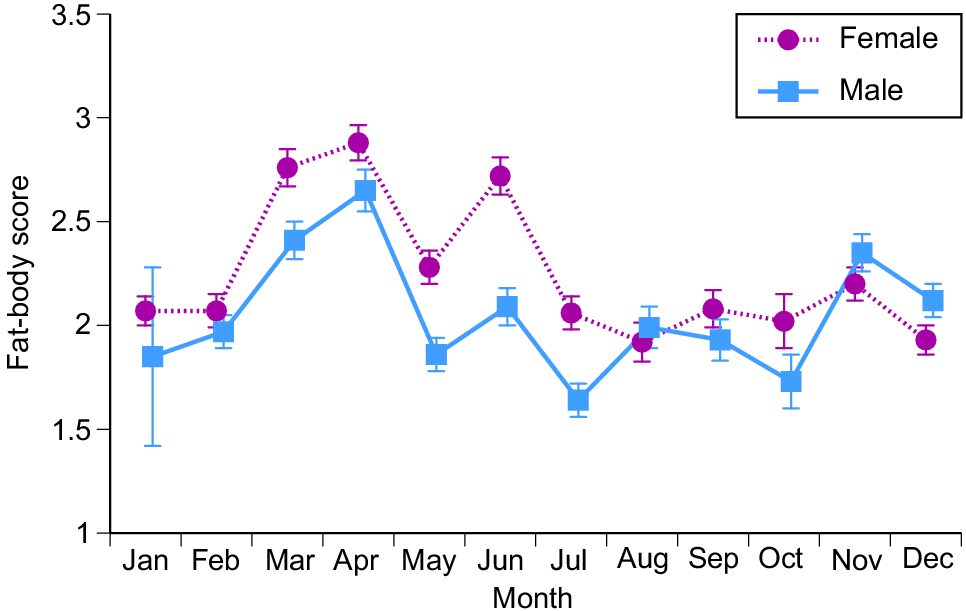
Female snakes attained larger average and maximum body sizes than did conspecific males (for ln SVL, F1,4208 = 221.63, P < 0.0001; for ln mass, F1,4198 = 788.36, P < 0.0001; see Fig. 3). The disparity was greater in mass than in SVL, reflecting the fact that females were heavier-bodied than were males of the same body length (ANCOVA interaction term, sex × ln SVL, F1,4197 = 84.05, P < 0.0001; see Fig. 4a). Our scores of fat-body mass during dissections showed that part of this sex disparity in body shape was due to larger fat bodies in females. Because fat-body size varied seasonally as well as differing between the sexes, we analysed variation in fat-body mass with a two-factor ANOVA that included month as well as sex. The interaction term was highly significant (F11,4127 = 9.72, P = 0.02), but the main effect of sex is interpretable because fat-body scores for females were consistently higher than for males (Fig. 5). Both sexes had larger fat bodies in the first half of the year, when males had enlarged testes and females were commencing vitellogenesis (Fig. 5). Males had longer tails than did females of the same SVL (ANCOVA, sex × ln SVL, F1,4065 = 5.43, P < 0.02; see Fig. 4b).
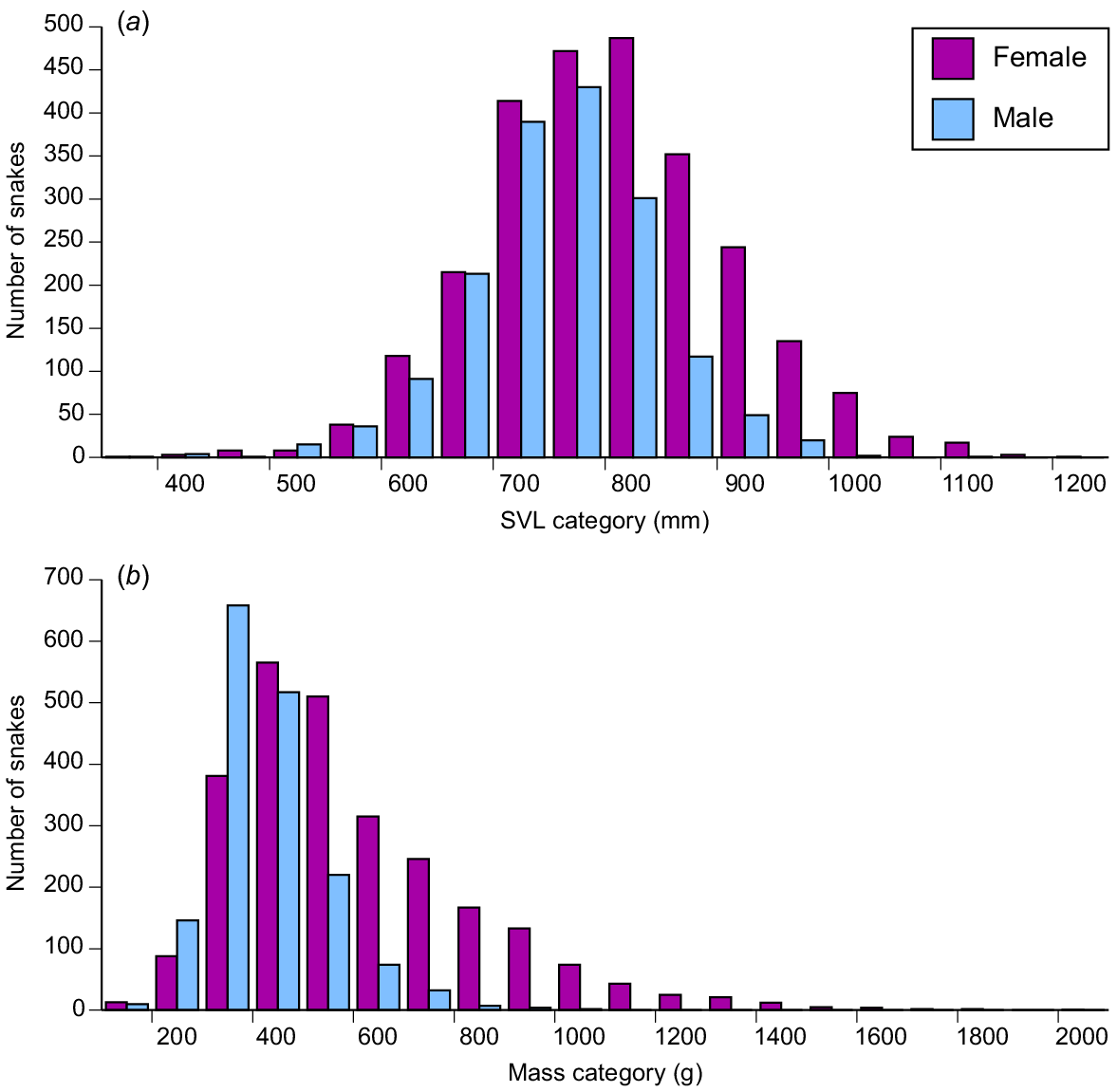
Frequency distributions of body sizes ((a) snout–vent length, and (b) mass) of masked water snakes (Homalopsis buccata) collected from the wild and killed at processing facilities near the city of Cirebon in West Java between August 2019 and June 2021.
Sexual dimorphism in body shape of masked water snakes (Homalopsis buccata) collected from the wild and killed at processing facilities near the city of Cirebon in West Java between August 2019 and June 2021. (a) Females were heavier-bodied than were males and (b) had a shorter tails than the snout–vent length. The graphs show mean values and associated standard errors for 100-mm intervals of snout–vent length, to facilitate depiction of patterns, but statistical analyses in the text treat body length as a continuous variable.
Monthly variation in fat-body sizes (on the basis of a 4-point scale) of masked water snakes (Homalopsis buccata) collected from the wild and killed at processing facilities near the city of Cirebon in West Java between August 2019 and June 2021. Data (means and standard errors per month) are shown separately for male and female snakes.
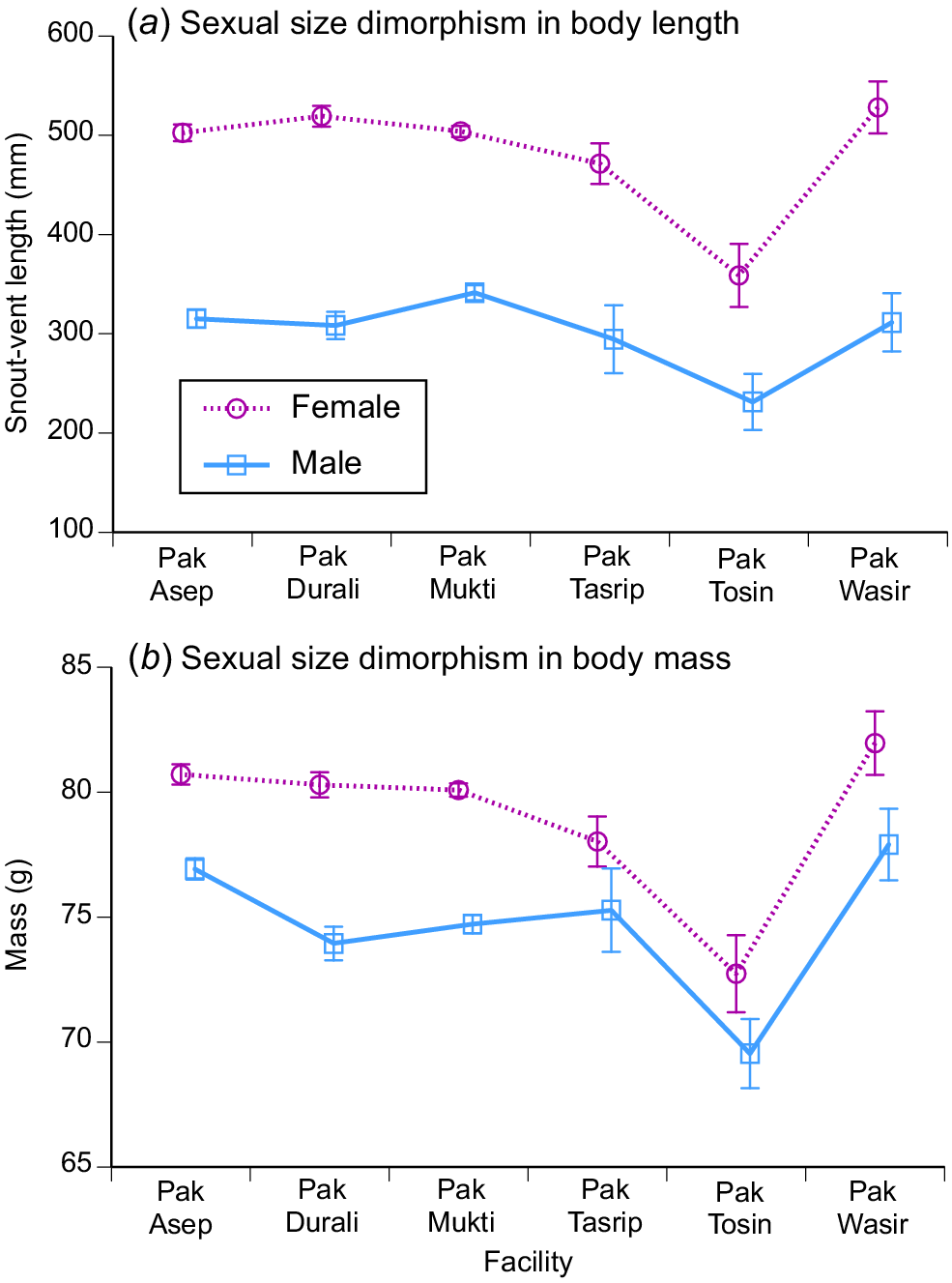
The patterns in sexual dimorphism were relatively consistent across the six processing facilities, with mean body sizes differing between the sexes and among facilities for both length and mass, with no significant interaction term for ln mass (F5,2727 = 0.48, P = 0.79) but a significant interaction term for ln SVL (F15,2735 = 2.74, P < 0.02). The degree of divergence between males and females in mean body sizes was similar among facilities, despite differences in absolute body sizes (Fig. 6). The statistical significance of the among-facility difference in body-length dimorphism reflects an unusually large mean body size of males as well as females in a small sample (N = 6 males, 23 females) from one facility (Wasir) (Fig. 6).
Seasonality of reproduction
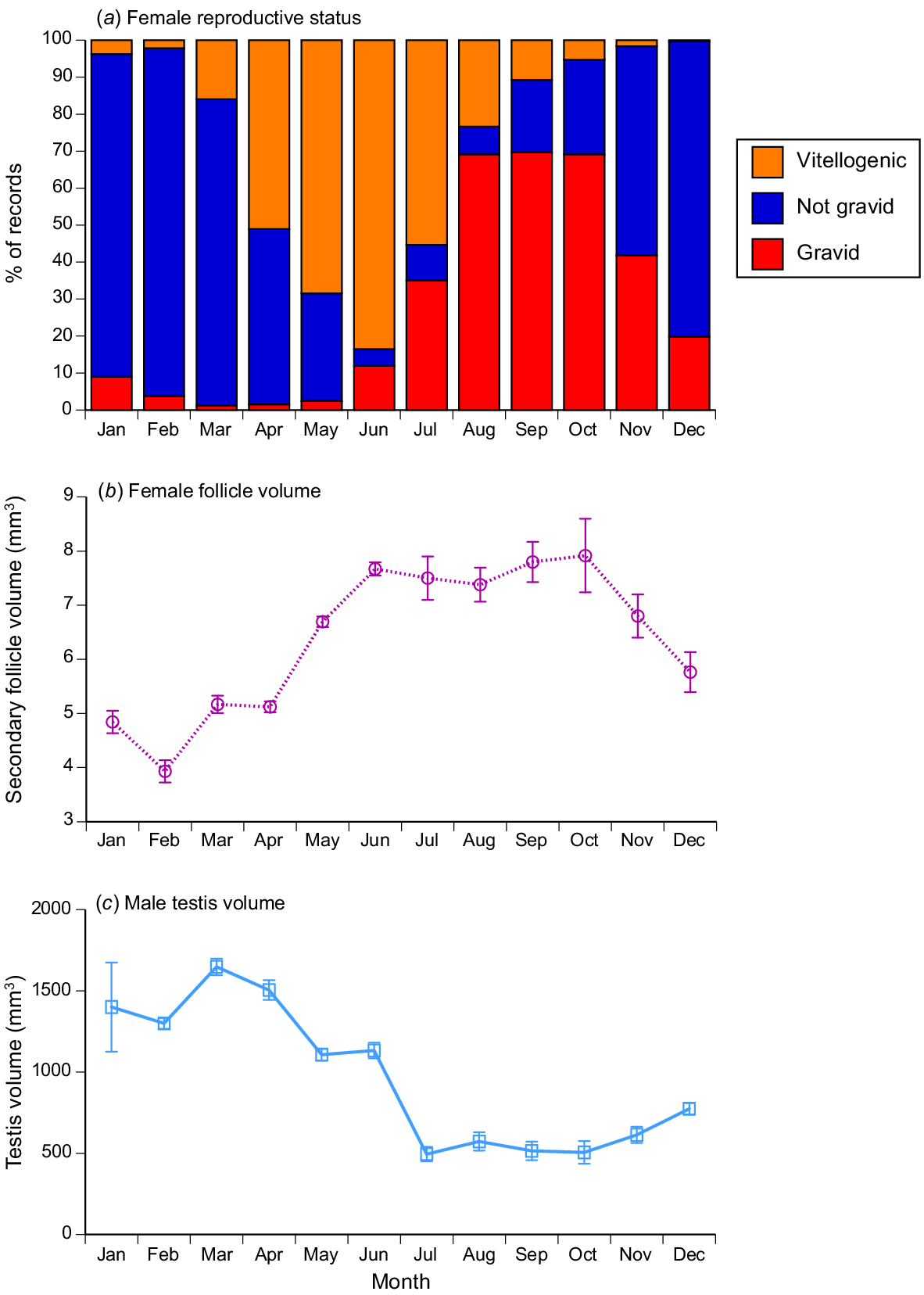
As for sex ratios (Fig. 2) and fat-body mass (Fig. 5), seasonality was apparent in the incidence of gravid snakes, and in the sizes of reproductive organs in both sexes. The proportion of females with enlarged vitellogenic follicles increased over the period from March to June, with ovulation in July–August and pregnancy often continuing through into December–January (Fig. 7a). Vitellogenesis thus occurs in the dry season, and parturition in the wet season. Vitellogenic follicles were small early in the year, increasing up to the size at ovulation by mid-year (Fig. 7b). Testis volumes showed the opposite pattern, with larger size in the first half of the year (Fig. 7c). Statistical analysis showed that this monthly variation was significant for the proportion of reproductive females (logistic regression, χ2 = 1109.95, 27 d.f., P < 0.0001), follicle volumes (ANOVA, F8,31.97 = 26.05, P < 0.0001) and testis volumes (ANOVA, F9,481.8 = 33.08, P < 0.0001).
Variation among months in reproductive state of masked water snakes (Homalopsis buccata) collected from the wild and killed at processing facilities near the city of Cirebon in West Java between August 2019 and June 2021. (a) Proportion of female snakes that were reproductively active (i.e. had large vitellogenic follicles or were gravid); (b) estimated volumes of vitellogenic follicles per month (means and s.e.); (c) estimated volume of the right hand-side testis per month (means and s.e.).
Body size at maturity
Among our small sample of juvenile snakes, SVLs ranged from 39 to 53 cm (males) and 39 to 55 cm (females). The smallest adult snakes were 41 cm SVL (male) and 49 cm SVL (female).
Fecundity
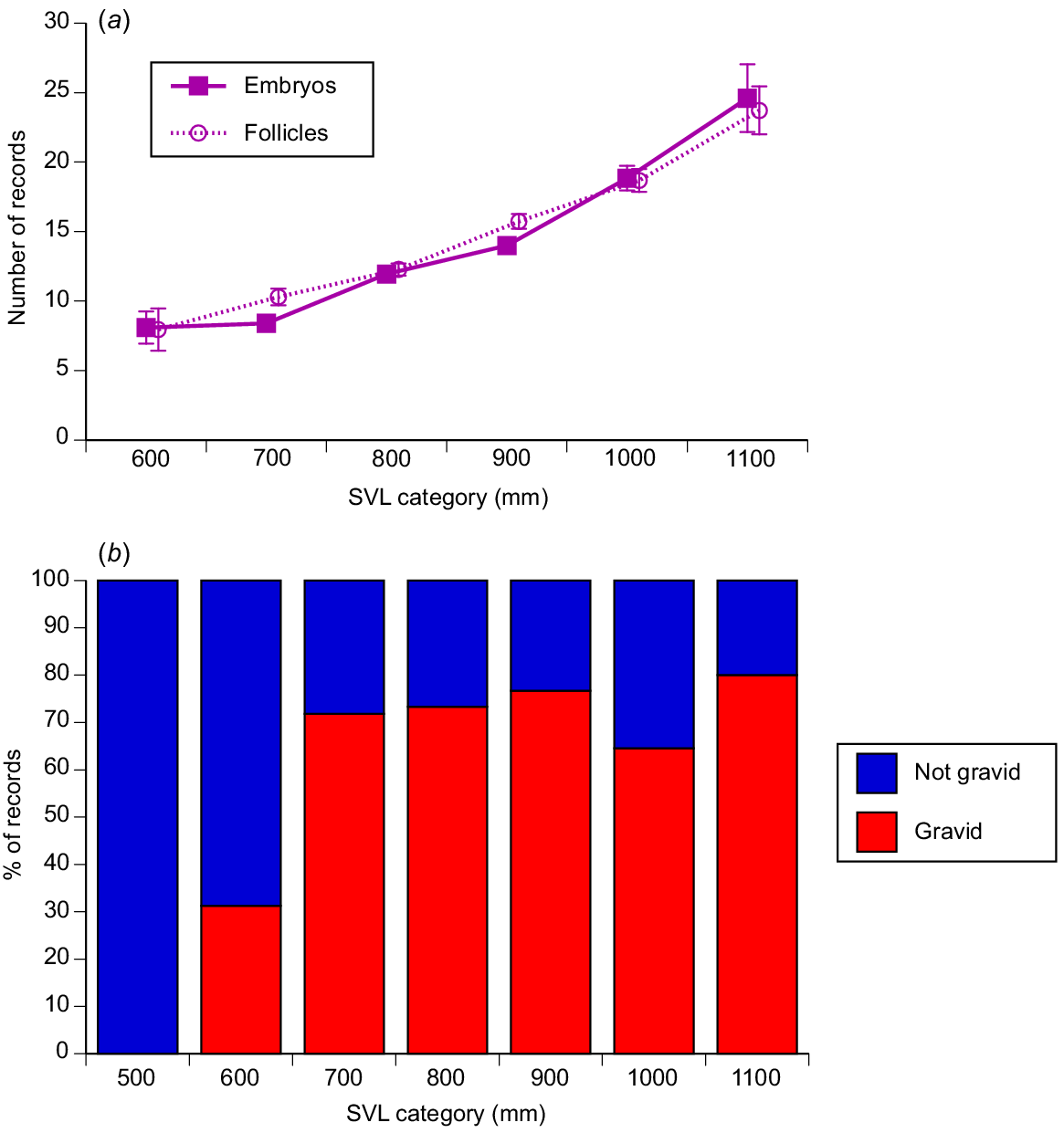
Litter sizes ranged from 1 to 37 offspring (mean = 12.05, s.e = 0.30, median = 11) and increased with maternal body length (r2 = 0.25, P < 0.0001; see Fig. 8a).
The relationship between maternal snout–vent length and reproductive output in masked water snakes (Homalopsis buccata) collected from the wild and killed at processing facilities near the city of Cirebon in West Java between August 2019 and June 2021. The graphs show data for 100-mm intervals of snout–vent length (SVL), to facilitate depiction of patterns, but statistical analyses in the text treat body length as a continuous variable. (a) The upper panel shows the relationship between maternal snout–vent length (SVL) and fecundity (litter size, or number of vitellogenic follicles. (b) The lower panel shows the relationship between maternal body size and whether or not a female was gravid, on the basis of data from the main reproductive season only (August–October).
Reproductive frequency
Some females had thin straplike oviducts and small follicles, even during the time of year (August–October) when most females were reproductive. The proportion of these non-reproductive animals during the August–October period was high for females <600 mm SVL, but consistently low for all larger size categories (Fig. 8b; nominal logistic regression χ2 = 22.91, 6 d.f., P = 0.0008). These data suggest that around three quarters of adult-size females breed each year, although that may be an underestimate if some females breed earlier or later in the year.
Discussion
Our data on >4000 field-collected H. buccata support the results of previous studies on this species complex in other parts of its wide geographic range. These snakes are abundant in disturbed areas, where they are pests of commercial fish farms, and are harvested in large numbers (Kusrini et al. 2022). Reproduction is seasonal in West Java (as also reported by Bergman 1951), with testes maximal in size in the wet season, and females ovulating late in the dry season and carrying offspring through the first part of the wet season (see Fig. 7). That timing differs from that seen in previously studied populations of H. buccata, most of which (unlike Cirebon) are north rather than south of the equator. As a result, seasonal schedules of precipitation differ (and are affected by local topography, such as monsoon-induced backflow of water into Tonle Sap Lake in Thailand: Brooks et al. 2009). Both rainfall patterns and temperatures may influence reproductive seasonality. Thus, for example, testes are largest in the latter part of the year in H. buccata from Cambodia, Malaysia and Thailand (Berry and Lim 1967; Saint Girons and Pfeffer 1972; Brooks et al. 2009), whereas the reverse timing occurs in Java (Fig. 7). Similar reversals across the equator in reproductive timing occur in other Indonesian snakes such as Malayopython reticulatus (Natusch et al. 2019a). If selection favours synchronisation of parturition, with the abundant food resources generated by wet-season flooding (as suggested by several authors: e.g. Brooks et al. 2009), then we might expect to see substantial geographic variation in the timing of pregnancy within tropical snakes.
Despite that variation in seasonality, most aspects of the morphology and reproductive biology of H. buccata in Cirebon accord well with the results of previous studies. Thus, for example, females grow larger than males, are more heavy-bodied, and have relatively shorter tails (Bergman 1951; Murphy 2007). Reports of a lack of sexual size dimorphism in this species (e.g. Karns et al. 2010) is likely to reflect inadequate sampling, exacerbated by habitat differences in body sizes and sex ratios. Thus, for example, Kusrini et al. (2022) reported that larger snakes of this species were generally collected from fish farms rather than irrigation canals. Our data on litter sizes, on the relationship between litter size and maternal body size, and on the high proportion of adult-size females reproductive each year, also are consistent with the results of earlier studies (Bergman 1951; Murphy 2007; Brooks et al. 2009).
Our results clarify factors that render this species resilient to the heavy harvesting pressure to which it is exposed over much of its range. First, the commercial offtake that we quantified was focused almost entirely (99%) on adult rather than juvenile snakes. That situation is likely to reflect aspects both of the snake species, and the nature of our sampling. Maturation in both sexes is attained at a relatively small body size relative to maximum adult sizes (at body masses of ~50 g, vs 2 kg maximum size). Thus, only a relatively small proportion of the population comprises juvenile snakes, except (presumably) in the period immediately after parturition. However, our sampling doubtless underestimated the numbers of juveniles, because individuals too small for the commercial trade may be released, or are never captured using the methods employed by harvesters. Kusrini et al. (2022) reported that professional harvesters captured many juvenile snakes along irrigation canals by using electrostunning equipment, but that these animals were released. In the Cirebon region, harvesters took approximately equal numbers of male and female snakes in most months of the year, a pattern that should be more sustainable than an offtake comprising primarily females (Dalerum et al. 2008).
Viviparity may also affect the species’ resilience to harvesting, depending on the timing of offtake and the determinants of embryonic survival in eggs laid in external nests. By retaining the developing embryos within her body, a female protects them from mortality owing to desiccation or flooding of the nest, or predation on eggs. However, viviparity means that if a gravid female is collected and killed, all of her offspring die as well. Indeed, oviductal embryos can be highly prized food items for local people, encouraging culling of gravid females (e.g. Shine 1986; Brooks et al. 2007a, 2007b). Because the harvest of H. buccata is motivated by a desire to reduce predation on fish as well as the commercial value of the snakes, it seems unlikely that gravid animals are specifically targeted.
Less ambiguously, the high reproductive output of H. buccata enhances its resilience to harvesting. Consistent with data on other populations, a high proportion of adult-size females reproduce each year, and individual females (especially large ones) produce large litters (Figs 7, 8). Maturation also occurs at a small body size, and likely at a young age; for example, Bergman (1951) inferred growth to 450 mm SVL in the first year of life for this species in Java (on the basis of seasonal distributions of body sizes), suggesting that some individuals may mature at 1 year of age (as do males in other tropical semi-aquatic snakes such as Tropidonophis mairii: Brown and Shine 2002). This ‘fast’ life-history of rapid maturation, frequent reproduction and high fecundity results in a system that can withstand high rates of mortality (e.g. Caughley 1985).
The most intensive examination of harvesting impacts of homalopsid snakes centres on the massive offtake of animals from Tonle Sap in central Cambodia (e.g. Stuart 2004, Brooks et al. 2007a, 2007b). That harvest is a relatively recent phenomenon, possibly owing to an increase in snake populations linked to the loss of large predatory fish and waterbirds in the lake (Stuart et al. 2000), and a shift by local people to capitalise on this abundant resource (J. Murphy, pers. comm. 2014). In contrast, the harvest of homalopsids in many other regions of Asia (including in West Java) has been ongoing for a long period, to protect farmed fish (e.g. Tweedie 1954; Lim and Lee 1989). In conversations with harvesters and with the managers of processing facilities, we were told that harvest levels have been stable for decades, and that fluctuations in offtake are driven primarily by economic factors (e.g. the prices offered for snake skins and meat, compared with alternative products such as frog meat), rather than by fluctuating availability or capture rates (D. Natusch, unpubl. data. 2021).
Management implications
How might we further enhance our confidence in the sustainability of the commercial harvest of H. buccata in West Java without substantially reducing economic returns to harvesters and farmers? The problem is difficult because of the snake’s role as a pest (fish eater) as well as a commercial product (skins and meat). Nonetheless, two obvious possibilities are as follows: (1) restricting harvesting during January, a period when collectors obtain mostly gravid female snakes (Figs 2, 7a), and (2) imposing an upper size limit on snakes that are collected in January, because the largest snakes are females, and have exceptionally high fecundity (Figs 2, 8). Limits based on body size are easily enforced by measuring skins, and the method has been employed to manage impacts of harvesting other reptile species (Fitzgerald and Painter 2000; Colteaux and Johnson 2017; Natusch et al. 2020b). However, we note that collectors motivated by pest control (i.e. protecting farmed fish, rather than earning money from snake skins) might be reluctant to spare large specimens; the rationale for any such initiative would have to be carefully explained to local communities. Last, continued monitoring of the harvest would be worthwhile, because shifts in traits such as capture rates, body-size distributions and reproductive output can provide early warning of any unsustainable pressure enforced by commercial hunting (Natusch et al. 2020a).
Conflicts of interest
This research was partially funded by an initiative focused on the conservation of reptile species used for skins, which receives some of its funding from companies that use reptile skins. Donors had no influence at any stage of this research.
Declaration of funding
This work was undertaken with support from the Southeast Asian Reptile Conservation Alliance (SARCA) and an Indonesian Education Scholarship (LPDP) to Alamsyah Elang Nusa Herlambang.
Author contributions
Data collection: E. A., N. L. M., A. E. N. H., M., A. R., D. J. D. N.; design and analysis: E. A., A. H., R. S., D. J. D. N.; writing: E. A., R. S., D. J. D. N.; revision: all authors.
Acknowledgements
We thank the Indonesian CITES Management Authority (Ministry of Environment and Forestry; KKH), the Indonesian CITES Scientific Authority (National Research and Innovation Agency of Indonesia; BRIN), the Natural Resources Conservation Agency of West Java (BBKSDA), the Indonesian Reptile Skin Exporters Association (AIRAI), the Indonesian Reptile Meat Exporters Association (APEKLI), and the Indonesian Herpetological Association (PHI) for facilitating this research. Thanks go to Slamet Priambada, Supomo, Ade Kurniadi Karim, Rini Aini, Ramdani Manurung, Farits Alhadi, Fata Habiburrahman Faz, Taufan Sulaiman, Alfonsus Toribio, Eko Saputro, Elika Boscha, Frendi Irawan, Quraisy Zakky, and Rohmat Subandriyo for field assistance. Thanks to Asep, Mukti, Lilis, Tosin, and Tasrip for allowing us to collect data from their facilities.
References
Bergman RAM (1951) The anatomy of Homalopsis buccata. Proceeding of the Koninklijke Nederlandse Akademie van Wetenschappen. Series C 54, 511-524.
| Google Scholar |
Bernstein JM, Murphy JC, Voris HK, Brown RM, Ruane S (2021) Phylogenetics of mud snakes (Squamata: Serpentes: Homalopsidae): a paradox of both undescribed diversity and taxonomic inflation. Molecular Phylogenetics and Evolution 160, 107109.
| Crossref | Google Scholar | PubMed |
Berry PY, Lim GS (1967) The breeding pattern of the puff-faced water snake, Homalopsis buccata Boulenger. Copeia 1967, 307-313.
| Crossref | Google Scholar |
Brooks SE, Allison EH, Reynolds JD (2007b) Vulnerability of Cambodian water snakes: initial assessment of the impact of hunting at Tonle Sap Lake. Biological Conservation 139, 401-414.
| Crossref | Google Scholar |
Brooks SE, Allison EH, Gill JA, Reynolds JD (2009) Reproductive and trophic ecology of an assemblage of aquatic and semi-aquatic snakes in Tonle Sap, Cambodia. Copeia 2009, 7-20.
| Crossref | Google Scholar |
Brown GP, Shine R (2002) Reproductive ecology of a tropical natricine snake, Tropidonophis mairii (Colubridae). Journal of Zoology 258, 63-72.
| Crossref | Google Scholar |
Campos-Silva JV, Peres CA, Antunes AP, Valsecchi J, Pezzuti J (2017) Community-based population recovery of overexploited Amazonian wildlife. Perspectives in Ecology and Conservation 15, 266-270.
| Crossref | Google Scholar |
Colteaux BC, Johnson DM (2017) Commercial harvest and export of snapping turtles (Chelydra serpentina) in the United States: trends and the efficacy of size limits at reducing harvest. Journal for Nature Conservation 35, 13-19.
| Crossref | Google Scholar |
Dalerum F, Shults B, Kunkel K (2008) Estimating sustainable harvest in wolverine populations using logistic regression. The Journal of Wildlife Management 72, 1125-1132.
| Crossref | Google Scholar |
Dobson ADM, Milner-Gulland EJ, Ingram DJ, Keane A (2019) A framework for assessing impacts of wild meat hunting practices in the tropics. Human Ecology 47, 449-464.
| Crossref | Google Scholar |
Fitzgerald LA (1994) The interplay between life history and environmental stochasticity: implications for the management of exploited lizard populations. American Zoologist 34, 371-381.
| Crossref | Google Scholar |
Fitzgerald LA, Painter CW (2000) Rattlesnake commercialization: long-term trends, issues, and implications for conservation. Wildlife Society Bulletin 28, 235-253.
| Google Scholar |
Harlow PS, Taylor JE (2000) Reproductive ecology of the jacky dragon (Amphibolurus muricatus): an agamid lizard with temperature-dependent sex determination. Austral Ecology 25, 640-652.
| Crossref | Google Scholar |
Karns DR, Murphy JC, Voris HK (2010) Semi-aquatic snake communities of the central plain region of Thailand. Tropical Natural History 10, 1-25.
| Google Scholar |
Keogh JS, Barker DG, Shine R (2001) Heavily exploited but poorly known: systematics and biogeography of commercially harvested pythons (Python curtus group) in Southeast Asia. Biological Journal of the Linnean Society 73, 113-129.
| Crossref | Google Scholar |
Kusrini MD, Manurung R, Faz FH, Dwiputro A, Tajalli A, Prasetyo HN, Saputra PB, Kennedi UF, Parikesit DW, Shine R, Natusch D (2022) Abundance, demography, and harvesting of water snakes from agricultural landscapes in West Java, Indonesia. Wildlife Research 50(4), 272-282.
| Crossref | Google Scholar |
Leao TCC, Lobo D, Scotson L (2017) Economic and biological conditions influence the sustainability of harvest of wild animals and plants in developing countries. Ecological Economics 140, 14-21 10.1016/j.ecolecon.2017.04.030.
| Google Scholar |
Natusch DJD, Lyons JA, Mumpuni , Riyanto A, Shine R (2016) Jungle giants: assessing sustainable harvesting in a difficult-to-survey species (Python reticulatus). PLoS ONE 11, e0158397.
| Crossref | Google Scholar | PubMed |
Natusch DJD, Lyons JA, Riyanto A, Mumpuni , Khadiejah S, Shine R (2019a) Detailed biological data are informative, but robust trends are needed for informing sustainability of wildlife harvesting: a case study of reptile offtake in Southeast Asia. Biological Conservation 233, 83-92.
| Crossref | Google Scholar |
Natusch DJD, Lyons JA, Mumpuni , Riyanto A, Shine R (2020a) Harvest effects on blood pythons in North Sumatra. The Journal of Wildlife Management 84, 249-255.
| Crossref | Google Scholar |
Natusch DJD, Lyons JA, Mumpuni , Riyanto A, Khadiejah S (2020b) Applying skin-size limits for management of trade in Asian reptile skins. Wildlife Research 47, 89-98.
| Crossref | Google Scholar |
Saint Girons H, Pfeffer P (1972) Notes sur l’ecologie des serpents du Cambodge. Zoologische Mededelingen 47, 65-87.
| Google Scholar |
Shine R (1986) Predation upon filesnakes (Acrochordus arafurae) by aboriginal hunters: selectivity with respect to body size, sex and reproductive condition. Copeia 1986, 238-239.
| Crossref | Google Scholar |
Shine R, Ambariyanto , Harlow PS, Mumpuni (1999) Ecological attributes of two commercially-harvested python species in northern Sumatra. Journal of Herpetology 33, 249-257.
| Crossref | Google Scholar |
Stuart BL (2004) The harvest and trade of reptiles at U Minh Thuong National Park, southern Viet Nam. Traffic Bulletin 20, 25-34.
| Google Scholar |
Stuart BL, Smith J, Davey K, Din P, Platt SG (2000) Homalopsine watersnakes: the harvest and trade in Tonle Sap, Cambodia. Traffic Bulletin 18, 115-124.
| Google Scholar |
Webb GJ (2002) Conservation and sustainable use of wildlife – an evolving concept. Pacific Conservation Biology 8, 12-26.
| Crossref | Google Scholar |