Decline in semi-arid reptile occurrence following habitat loss and fragmentation
R. E. L. Simpson A B * , D. G. Nimmo A B , L. J. Wright A B , S. Wassens A B and D. R. Michael
A B * , D. G. Nimmo A B , L. J. Wright A B , S. Wassens A B and D. R. Michael  B
B
A School for Agricultural, Environmental and Veterinary Sciences, Charles Sturt University, Wagga Wagga, NSW 2650, Australia.
B Gulbali Institute for Agriculture, Water and Environment, Charles Sturt University, Albury, NSW 2640, Australia.
Abstract
Habitat loss and fragmentation are leading causes of biodiversity decline worldwide. In Australia, woodland habitat has been extensively cleared and fragmented yet there has been limited research on the effects of habitat loss and fragmentation on semi-arid reptiles, impeding conservation planning and recovery efforts.
We aimed to investigate factors influencing the distribution and occurrence of habitat specialist and generalist reptile species on a large agricultural holding in south-eastern Australia that has experienced habitat loss and fragmentation.
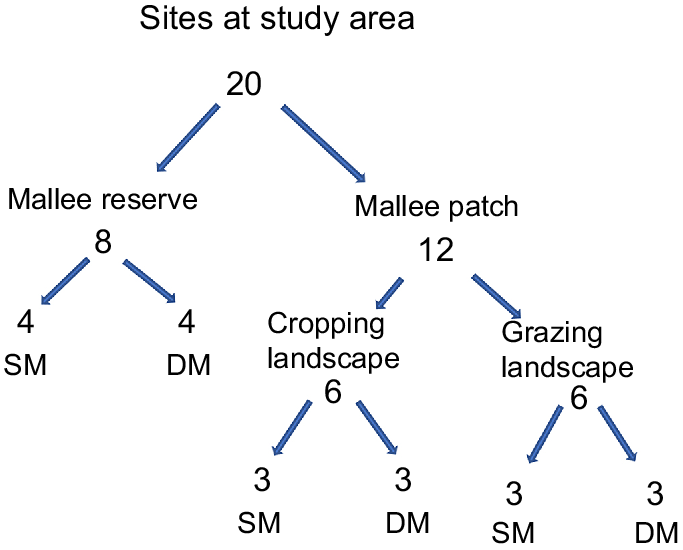
Reptiles were surveyed using pitfall and funnel traps and active searches across 20 sites stratified by land use and vegetation type. Twelve sites were established in remnant woodland patches embedded within an agricultural matrix and eight sites were established in a private conservation reserve on the same property. Generalised linear models were used to explore relationships between the occurrence of eight reptile species and predictor variables describing site, landscape and vegetation variables.
Of the 31 reptile species that were detected, eight were modelled. The results revealed that four specialist species, the eastern mallee dragon (Ctenophorus spinodomus), nobbi dragon (Diporiphora nobbi), barred wedge-snouted ctenotus (Ctenotus schomburgkii) and shrubland pale-flecked morethia (Morethia obscura), were closely associated with the conservation reserve, and that the southern spinifex ctenotus (Ctenotus atlas) had a strong association with spinifex (Triodia scariosa) dominated vegetation community.
Reptile habitat specialists are particularly sensitive to habitat loss and fragmentation and are at a higher risk of local extinction compared with habitat generalists. Reptile occurrence was reduced in remnant woodland patches, but remnant patches also supported a suite of habitat generalists.
A suite of semi-arid reptile species are sensitive to the effects of habitat loss and fragmentation and are susceptible to localised extinction. However, the presence of habitat generalists within woodland remnants highlights the value of retaining representative habitat patches in agricultural landscapes. Conservation of semi-arid woodland reptiles will depend on the retention of large tracts of protected vegetation across a broad range of soil types to maintain habitat heterogeneity and reptile diversity.
Keywords: agricultural intensification, generalist, habitat fragmentation, habitat relationships, landscape modification, mallee, reptile occurrence, semi-arid woodland, specialist.
Introduction
Human activities are causing extinction rates to increase rapidly (Haddad et al. 2015; Maxwell et al. 2016; Garnett et al. 2022). Approximately 28% of all species assessed by the International Union for Conservation of Nature are currently threated with extinction (IUCN 2022). Habitat loss and fragmentation due to agricultural development is a major threat to biodiversity globally (Levy et al. 2010; Youngentob et al. 2013; Maxwell et al. 2016; Cox et al. 2022). Habitat fragmentation occurs as a direct result of habitat loss, where continuous native vegetation is divided into multiple, smaller, isolated patches (Fahrig 2003; Haddad et al. 2015; Keinath et al. 2017).
Following habitat loss and fragmentation, ecological communities within remnant patches are faced with isolation and disturbances that place some species at greater risk of local extinction (Haddad et al. 2015). Loss of connectivity between patches can limit species dispersal, resulting in reduced gene flow and genetic diversity (Gibbs 2001; Jules and Shahani 2003). Elevated levels of disturbance by livestock and stochastic events (e.g. fire) can result in further changes in species composition (Doherty et al. 2020). A species’ ability to survive in a fragmented landscape can be influenced by ecological traits, habitat requirements, and factors such as the composition, size, and type of remnant vegetation (Haddad et al. 2015; Keinath et al. 2017).
Australia has one of the highest levels of reptile diversity worldwide, accounting for approximately 10% of reptile species globally (Uetz et al. 2022; Tan et al. 2023). Habitat loss and fragmentation are two of the main causes of reptile decline in Australia (Tingley et al. 2019). However, reptiles remain one of the most poorly studied taxonomic groups among vertebrates in Australia (Slatyer et al. 2007; Thompson et al. 2016; Triska et al. 2017; Tingley et al. 2019). Unlike other terrestrial vertebrates, basic knowledge on species distributions and habitat requirements, for even common and widespread reptile species, are limited (Meiri and Chapple 2016; Tingley et al. 2016). In particular, the order Squamata, which includes lizards and snakes, has received relatively little scientific attention (Tingley et al. 2019), impeding conservation efforts.
Habitat specialists are often more susceptible to habitat loss and fragmentation than habitat generalists (Devictor et al. 2008; Keinath et al. 2017; Yan et al. 2022). The loss of habitat specialists that often accompanies habitat fragmentation (Driscoll 2004) can result in an influx of generalist species, potentially masking the decline of specialists (Matthews et al. 2014). Keinath et al. (2017) found specialist reptile species were highly sensitive to habitat fragmentation and although data on reptile species are limited, Doherty et al. (2020) suggest that reduced mobility may limit some reptile species ability to move between patches of remnant vegetation. Small-bodied habitat specialists such as arboreal geckos are often restricted to patches and are sensitive to habitat fragmentation (Hansen et al. 2020) compared with terrestrial habitat generalists, such as many widespread skink species, which often persist in modified agricultural landscapes (Jellinek et al. 2014; Michael et al. 2016; Pulsford et al. 2017).
The impact of habitat fragmentation on less vagile species can be exacerbated by matrix type (the dominant land use surrounding habitat patches) (Franklin and Lindenmayer 2009; Pulsford et al. 2017). Disturbance incurred from cropping and grazing practices can negatively affect reptile abundance and occurrence patterns (Doherty et al. 2020). Agricultural matrices can limit animal movement and therefore reduce dispersal by acting as a barrier (Driscoll et al. 2013; Hansen et al. 2020), while also increasing species vulnerability to predation (Hansen et al. 2019). Other properties of the matrix may also influence movements patterns. For example, Kay et al. (2016) found homing ability and movement patterns in the southern marbled gecko (Christinus marmoratus) to be influenced by crop orientation. Remnant woodland patches embedded within grazing matrices may be impacted by grazing pressure and disturbance, resulting in reduced habitat quality (Driscoll 2004). Understanding how matrix conditions (e.g. disturbance regimes, habitat structure) affect the distribution and abundance of reptiles within patches is therefore important for understanding community composition in fragmented landscapes (Mulhall et al. 2022).
In south-eastern Australia, semi-arid woodland vegetation communities, including mallee woodland, support relatively high levels of reptile diversity compared with temperate woodland or riparian vegetation communities (Menkhorst and Bennett 1990). High-diversity patterns may be maintained by the presence of specific plant species, coupled with habitat heterogeneity (Clarke et al. 2021). Spinifex (Triodia sp.) is a dominant hummock-forming grass species found throughout arid Australia, particularly mallee woodland vegetation communities on sandy soils, and is considered a foundation species (Verdon et al. 2020). A number of studies have found strong association between Triodia cover and lizard distribution patterns (Nimmo et al. 2013; Sadlier et al. 2019; Bell et al. 2021a). However, Triodia structure and abundance can be negatively affected by soil compaction and elevated soil nutrients caused by agricultural activities (Bell et al. 2021b).
In this study, we aimed to investigate factors influencing the distribution and abundance of mallee woodland reptiles in a fragmented agricultural landscape that was cleared recently (~20 years ago) in western New South Wales (NSW), Australia. We posed three specific questions:
Which mallee woodland reptile species are sensitive to habitat fragmentation? We predicted that habitat specialists will be more sensitive to habitat fragmentation than habitat generalists, and therefore, will be absent or in lower abundance in remnant patches compared with a conservation reserve (hereafter referred to as reserve) (Michael et al. 2015; Keinath et al. 2017).
How important is the matrix (land use type) in explaining species distribution patterns? We predicted that in comparison with sites within patches surrounded by a grazing matrix, patches surrounded by a cropping matrix will support fewer species, especially fossorial species, due to the barrier effects of cropping activities on soil-dwelling species (Ricketts 2001; Fischer et al. 2005; Franklin and Lindenmayer 2009).
How important is vegetation type in explaining species distribution patterns? Given variation in vegetation communities across small spatial scales can influence reptile community composition, we predicted that species dependant on Triodia scariosa (a foundation plant species) will be restricted in distribution, whereas habitat generalists would be widespread across the study area (Verdon et al. 2020; Bell et al. 2021a).
Material and methods
Study area
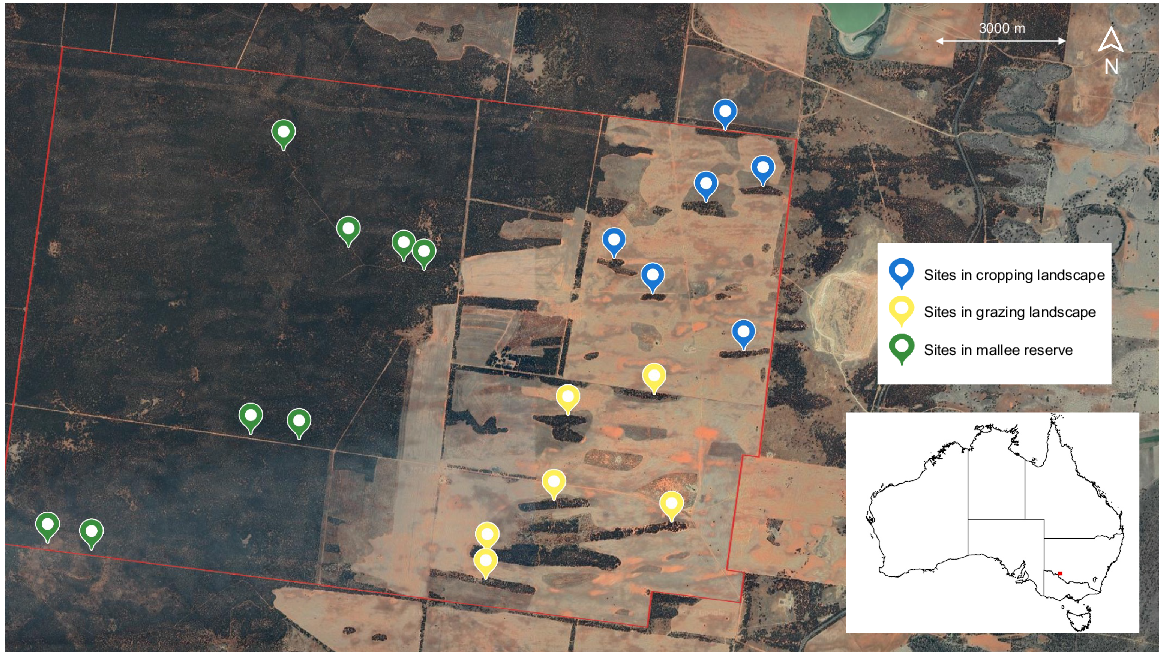
The study was conducted on a mixed-agricultural property 12 km north of Balranald in south-eastern Australia (34°32′13″S, 143°31′11″E). The study area was selected due to the spatial configuration of remnant habitat in two agricultural land use types (cropping and grazing) coupled with a private reserve. Historically, the entire property was grazed by sheep (Ovis aries). In 1975, a large fire burnt through the entire property leaving only a small number of unburnt trees and resulting in relatively even-aged stands of regrowth. In 1998, a 4000-ha reserve was established on the property to offset a 3-year phase of land clearing for agricultural purposes, whereby all livestock grazing in the reserve ceased. Between 1998 and 2001, a network of remnant mallee patches was retained. Grazing occurs across the eastern section of the property and in 2003, crops were established on approximately half of the cleared land and sown biennially. During the study period, the cropping areas remained fallow and stocking levels were 1000 head of sheep per 1500 ha. Pest control, including 1080 baiting and lethal destruction via shooting, was applied throughout the cropping and grazing areas, targeting European red foxes (Vulpes vulpes), feral cats (Felis catus) and European rabbits (Oryctolagus cuniculus). No pest control was conducted in the reserve during the study period.
The main vegetation communities in the study area include sandplain mallee woodland (hereafter referred to as sandplain mallee) and dune mallee woodland (hereafter referred to as dune mallee). Mallee woodlands are widespread throughout the arid zone and have experienced significant habitat loss and extensive clearing due to the expansion of agricultural enterprises (Clarke et al. 2021). Sandplain mallee is dominated by yorrell (Eucalyptus gracilis) and oil mallee (E. oleosa) overstorey, on fertile red-brown soils with a shrubby understorey of various chenopod and Acacia sp. species (OEH 2022). Dune mallee is dominated by white mallee (E. dumosa) and slender-leaved red mallee (E. leptophylla) overstorey, typically containing a low soil clay content with deep red sand dunes supporting T. scariosa hummocks (OEH 2022). The climate is classified as semi-arid, the average annual rainfall is 323 mm and the mean minimum and maximum temperature ranges from 16.6 to 33.1°C in summer and 3.5 to 15.7°C in winter (BOM 2022).
Study design
In total, 20 sites were selected in this study (Fig. 1), classified as either reserve or patch. Eight sites were established within the reserve to serve as reference sites and 12 sites were established within patches of remnant vegetation and ranged in size from three to 21 ha (mean = 14 ha). Each site was further stratified by vegetation type (sandplain mallee or dune mallee) and dominant land use (continuous vegetation, cropland or grazing land) (Fig. 2). The distance between sites ranged from 900 m to 1510 m in the cropping landscape, 500 m to 1700 m in the grazing landscape and 400 m to 2400 m in the reserve, thus ensuring independence between sites and survey periods. Because we were primarily interested in sampling a wide variety of patches, area-controlled survey effort was not employed; instead, a single site was established within each patch. Time and budget constraints also prevented additional sites being established within the larger remnant patches. All sites were constrained to the same property to avoid confounding effects associated with potential differences in land management practices.
Survey protocol
At each site, reptiles were surveyed on three repeat occasions between November 2021 and February 2022 using pitfall and funnel traps (Baumgardt et al. 2021), with active searches of natural habitat constrained by time (1 h) and area (1 ha). Repeated surveying was conducted to minimise bias in species detection levels and account for potential issues associated with imperfect detection (MacKenzie et al. 2009). Active searches involved scanning the area for reptile activity (visual encounters), turning over and inspecting logs or debris, sifting through leaf litter and lifting loose bark on trees. Reptiles were captured by hand where possible, identified to species level using Wilson and Swan (2021) and released at their point of capture.
Trap arrays consisted of 3 × 20-L buckets spaced 15 m apart, connected by a 30-m drift fence (damp coarse), with two funnel traps (dimensions: 75 cm long × 18 cm wide × 18 cm high) placed either side at the 10-m and 20-m points along the fence. Funnel traps are a complementary method to pitfall traps because they often capture more snake species and larger-sized lizards (Thompson and Thompson 2007). In total, 25 trap nights were applied to each site during each of the three survey periods, amounting to 1500 trap nights (i.e. three buckets + two funnels × five nights × three surveys × 20 sites). Traps were checked each morning and before sundown. In each bucket, a paper plate, sand and leaf litter were placed in the bottom to provide shelter to captured animals. Branches and leaf litter were placed on top of funnel traps to provide shade. A single trapping array was established at each site and positioned at least 50 m from the edge of the patches to avoid potential confounding associated with edge effects. Each individual reptile was weighed to the nearest g using a handheld spring scale (Persola) and calico bag, measured (total length and snout–vent–length) to the nearest mm using a ruler and sexed from external features when possible. Individuals were marked using a permanent marker pen to identify recaptures and released 5 m from their point of detection. Richgro© ant sand was applied around the pits and funnels, and all funnel traps were closed when temperatures were forecasted to exceed 33°C to minimise animal welfare issues.
Statistical analysis
Prior to modelling species occurrence, we investigated which species could be reliably detected at a site by using single season occupancy-detection models (MacKenzie et al. 2002), fit using the ‘unmarked’ package (Fiske and Chandler 2011). Species were considered at this stage if they occurred at >25% of sites and had a minimum of 10 detections. Occupancy–detectability models were fit to the repeated surveys (n = 3) with a single predictor variable for detectability indicating the survey method type: either pitfall/funnel trapping or active search. This generated a nightly detection probability for each of the two survey methods. Next, we calculated the cumulative probability of detecting each species, if present, across the 15 nights of pitfall/funnel trapping and the three active searches following Kéry (2002). To reduce the probability of false absences, we used a threshold adapted from Nimmo et al. (2014) where only species with >80% cumulative probability of being detected (if present) were included in subsequent modelling of presence/absence or abundance. Eight of the 10 species meeting the selection criterion met the cumulative detection probability threshold. All species were classified as either habitat generalists or specialists, specifically within the Murray–Darling Depression bioregion. Justification for the designation of species as habitat specialists or habitat generalists was based on the primary literature cited in Greer (2022), and macro and microhabitat accounts reported in Wilson and Swan (2021) and the Atlas of Living Australia (ALA) (2023) (see Supplementary Table S1). Thus, habitat specialists were species restricted to a specific type of vegetation community or microhabitat type within the specified bioregion.
Because we were interested in the influence of habitat loss and fragmentation on the presence/absence of reptile species, we fit a series of Generalised Linear Models (GLM) using logistic regression. The robust slider (Lerista punctatovittata) was present at 95% of sites, so models of presence/absence for this species would not be informative. Instead, this species was modelled using abundance (count data) as the response variable, thus specifying a Poisson distribution. Patch size can influence species occurrence patterns; therefore, we first explored the relationship between patch area (ha) (n = 12) and species presence/absence. We then considered six additional GLMs for each species. The first model concatenated all categorical variables considered during the experimental design (site type × vegetation type × matrix type) to create a categorical variable with six levels. This model was supported if a species was sensitive to all three predictor variables. The second model included a four-level categorical variable that concatenated site type and vegetation type, the third model included a three-level categorical variable that captured the landscape matrix surrounding the site (i.e. continuous vegetation, cropland or grazing land), the fourth model included only vegetation type and the fifth model included only site type. The sixth model was a ‘null’ model and included only an intercept term and would be supported if the species did not respond significantly to any of the other predictor variables. Some models failed to converge due to complete separation and were therefore refit within a Bayesian framework using the brglm package (Kosmidis 2021).
Akaike’s Information Criterion (AIC) was used to identify the best-performing model(s) for each species using the package ‘AICmcodavg’ (Mazerolle 2020). The AICc value was used to determine models with a considerable level of support. The AICc of candidate models were compared against the best model (lowest AICc) (Burnham and Anderson 2002). Models with AICc values <2 were considered to have the greatest support in explaining species presence (and abundance for L. punctatovittata) (Burnham and Anderson 2002). The coefficient of determination (R2) was calculated for each best fit model(s) to determine ‘goodness of fit’. Model diagnostics were examined (QQ plots, residual vs predicted values) using DHARMAa package (Hartig 2022). Models with significant effects (P-value ≤ 0.05) were plotted using packages ‘ggeffects’ (Lüdecke 2018) and ‘ggplot2’ (Wickham 2016). All analysis was performed in R ver. 4.2.2 (R Core Team 2022).
Results
Summary statistics
In total, 480 individuals from 31 species and nine families were detected (Table 1). Acknowledging the uneven sampling effort between reserve and patch sites, we recorded a total of 332 individuals (69%) from 24 species in the eight reserve sites and 148 individuals from 12 sites in the remnant patches (17 species accounting for 73 individuals detected in the cropping matrix and 16 species with 75 individuals in the grazing matrix). The most abundant species was the eastern mallee dragon (Ctenophorus spinodomus), accounting for 114 observations (24% of total detections), followed by the eastern robust slider (L. punctatovittata; 70 observations) and dwarf three-toed slider (Lerista timida; 46 observations).
| Common name | Scientific name | Agricultural patches | Conservation reserve | Total count | |
|---|---|---|---|---|---|
| Agamidae | |||||
| Eastern Mallee Dragon | Ctenophorus spinodomus | 0 | 113 | 113 | |
| Nobbi Dragon | Diporiphora nobbi | 0 | 24 | 24 | |
| Eastern Bearded Dragon | Pogona barbata | 3 | 2 | 5 | |
| Carphodactylidae | |||||
| Common Thick-tailed Gecko | Underwoodisaurus milii | 3 | 0 | 3 | |
| Diplodactylidae | |||||
| Eastern Stone Gecko | Diplodactylus vittatus | 0 | 6 | 6 | |
| Beaded Gecko | Lucasium damaeum | 3 | 7 | 10 | |
| Eastern Beaked Gecko | Rhynchoedura ormsbyi | 0 | 2 | 2 | |
| Southern Spiny-tailed Gecko | Strophurus intermedius | 1 | 4 | 5 | |
| Elapidae | |||||
| Eastern Brown Snake | Pseudonaja textilis | 3 | 0 | 3 | |
| Mitchell’s Short-tailed Snake | Suta nigriceps | 0 | 1 | 1 | |
| Common Bandy Bandy | Vermicella annulata | 1 | 0 | 1 | |
| Gekkonidae | |||||
| Variegated Dtella | Gehyra versicolor | 5 | 0 | 5 | |
| Bynoe’s Gecko | Heteronotia binoei | 2 | 4 | 6 | |
| Pygopodidae | |||||
| Red-tailed Worm-lizard | Aprasia inaurita | 0 | 2 | 2 | |
| Spinifex Delma | Delma butleri | 1 | 1 | 2 | |
| Burton’s Legless Lizard | Lialis burtonis | 3 | 1 | 4 | |
| Scincidae | |||||
| Southern Spinifex Ctenotus | Ctenotus atlas | 14 | 12 | 26 | |
| Short-clawed Ctenotus | Ctenotus brachyonyx | 1 | 10 | 11 | |
| Royal Ctenotus | Ctenotus regius | 0 | 1 | 1 | |
| Barred Wedge-snouted Ctenotus | Ctenotus schomburgkii | 1 | 12 | 13 | |
| Bougainville’s Slider | Lerista bougainvillii | 0 | 1 | 1 | |
| Eastern Robust Slider | Lerista punctatovittata | 46 | 24 | 70 | |
| Dwarf Three-toed Slider | Lerista timida | 25 | 21 | 46 | |
| Desert Skink | Liopholis inornata | 0 | 1 | 1 | |
| Common Dwarf Skink | Menetia greyii | 6 | 11 | 17 | |
| Boulenger’s Morethia | Morethia boulengeri | 3 | 10 | 13 | |
| Shrubland Pale-flecked Morethia | Morethia obscura | 1 | 29 | 30 | |
| Shingleback | Tiliqua rugosa | 1 | 0 | 1 | |
| Typhlopidae | |||||
| Dark-spined Blind Snake | Anilios bicolour | 1 | 0 | 1 | |
| Prong-snouted Blind Snake | Anilios bituberculatus | 3 | 0 | 3 | |
| Varanidae | |||||
| Sand Goanna | Varanus gouldii | 16 | 4 | 20 | |
| Total Records | 148 | 332 | 480 | ||
Counts exclude recaptured animals and include data from both survey methods (active searches and pitfall/funnel trapping).
Nine species (29%) were recorded only in the reserve and accounted for 152 detections, whereas seven species (23%) were only found in patches and accounted for 18 detections. Species restricted to the reserve included C. spinodomus, nobbi dragon (Diporiphora nobbi), eastern stone gecko (Diplodactylus vittatus), eastern beaked gecko (Rhynchoedura ormsbyi), red-tailed worm-lizard (Aprasia inaurita), Mitchell’s short-tailed snake (Suta nigriceps), royal ctenotus (Ctenotus regius), Bougainville’s slider (Lerista bougainvillii) and the desert skink (Liopholis inornata). Species found exclusively in patches included the eastern thick-tailed gecko (Underwoodisaurus milii), eastern brown snake (Pseudonaja textilis), bandy bandy (Vermicella annulata), Bynoe’s gecko (Heteronotia binoei), shingleback (Tiliqua rugosa), dark-spined blind snake (Anilios bicolor) and prong-snouted blind snake (Anilios bituberculatus). L. punctatovittata and L. timida were the only species detected across all treatments (see Fig. S1).
Detection probability by survey method
In total, 10 species met the criteria to be modelled, occurring at >25% of sites with a minimum of 10 detections (Table 2). There was no significant difference in the probability of detection across survey methods for six species: barred wedge-snouted skink (Ctenotus schomburgkii); common dwarf skink (Menetia greyii); L. punctatovittata; sand goanna (Varanus gouldii); shrubland pale-flecked morethia (Morethia obscura); and the southern spinifex ctenotus (C. atlas). Four species, D. nobbi, M. boulengeri, L. timida and C. spinodomus, were significantly less likely to be detected using pitfall/funnel traps compared with active searches (see Table S2). The cumulative detection probability for the pitfall/funnel trap nights and three active searches revealed that eight of the 10 species satisfied the requirements of having a >80% chance of being detected given their presence at a site (Table 2).
| Species | Cumulative detection probability (%) | Trapping detection probability (%) | Active search detection probability (%) | |
|---|---|---|---|---|
| Ctenotus schomburgkii | 92.9 | 15.5 | 3.9 | |
| Morethia boulengeri | 59.4 | 2.3 | 17.0 | |
| Menetia greyii | 53.4 | 4.3 | 3.6 | |
| Lerista timida | 86.3 | 8.0 | 21.7 | |
| Ctenophorus spinodomus | 100 | 31.1 | 72.2 | |
| Lerista punctatovittata | 94.9 | 18.0 | 0.0 | |
| Diporiphora nobbi | 96.0 | 12.8 | 32.0 | |
| Varanus gouldii | 92.9 | 15.5 | 3.9 | |
| Morethia obscura | 96.5 | 14.7 | 27.6 | |
| Ctenotus atlas | 97.1 | 20.4 | 4.6 |
Model selection
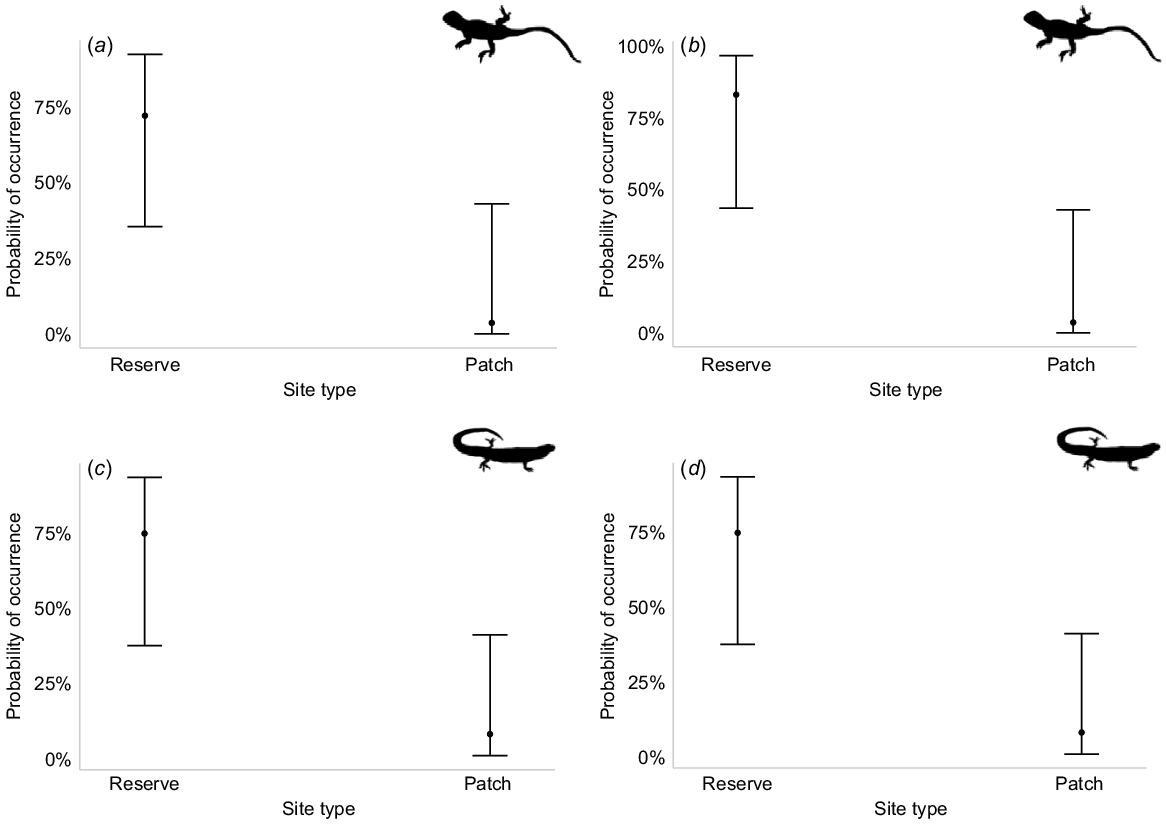
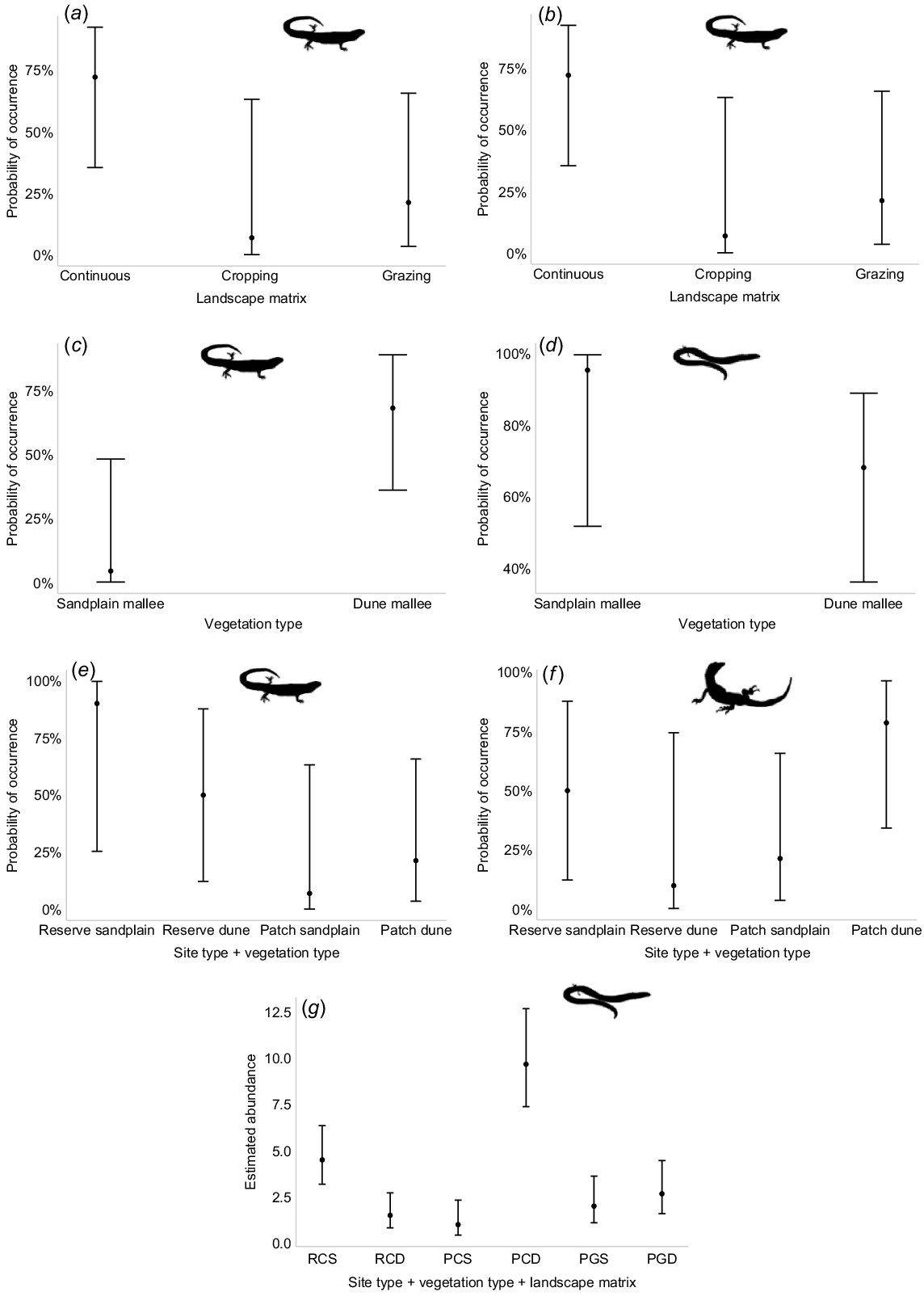
We found no significant relationships between patch size (n = 12) and occurrence patterns for any species. Therefore, patch size was not included in subsequent model building. The model with site type (reserve or patch) was the best-fitting model for C. spinodomus, D. nobbi, C. schomburgkii and M. obscura (Table 3). All four species were more likely to occur in the reserve (probability of occurrence 75%+) compared with patches (Table 4, Fig. 3a–d). The landscape matrix model (continuous, cropping or grazing) was supported by C. schomburgkii and M. obscura (Table 3). Both species were more likely to be detected in continuous vegetation followed by patches in the grazing matrix (Table 4, Fig. 4a, b). The only model with substantial support for C. atlas and L. timida was the vegetation-type model (sandplain mallee or dune mallee) (Table 3). Ctenotus atlas was significantly more likely to occur in dune mallee (Table 4, Fig. 4c), whereas L. timida was more likely to occur in sandplain mallee, although the difference was not significant (Table 4, Fig. 4d). The site and vegetation-type model had substantial support from M. obscura and V. gouldii (Table 3). M. obscura was most likely to occur in sandplain mallee reserve sites and was least likely to occur in sandplain mallee patches (Table 4, Fig. 4e). Varanus gouldii was most likely to occur in dune mallee vegetation patches (Table 4, Fig. 4f). The best-performing model that explained L. punctatovittata abundance included site type × vegetation type × matrix type (Table 3). Lerista punctatovittata was significantly more abundant in dune mallee patches surrounded by cropland and sandplain mallee in the reserve (Table 4, Fig. 4g). Confidence intervals around these predictions are wide in many instances.
| Species | Model | K | AICc | Δi | ModelLik | wi | LL | Cumulative wi | R2 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Ctenophorus spinodomus | Site type | 2 | 13.703 | 0.000 | 1.000 | 0.648 | −4.499 | 0.648 | 0.632 | |
| Diporiphora nobbi | Site type | 2 | 10.734 | 0.000 | 1.000 | 0.727 | −3.014 | 0.727 | 0.767 | |
| Ctenotus schomburgkii | Site type | 2 | 20.587 | 0.000 | 1.000 | 0.601 | −7.941 | 0.601 | 0.387 | |
| Ctenotus schomburgkii | Matrix type | 3 | 21.904 | 1.317 | 0.518 | 0.311 | −7.202 | 0.912 | 0.444 | |
| Morethia obscura | Site type | 2 | 20.587 | 0.000 | 1.000 | 0.419 | −7.941 | 0.419 | 0.387 | |
| Morethia obscura | Matrix type | 3 | 21.904 | 1.317 | 0.518 | 0.217 | −7.202 | 0.887 | 0.444 | |
| Morethia obscura | Site type × veg type | 4 | 21.619 | 1.031 | 0.597 | 0.250 | −5.476 | 0.670 | 0.577 | |
| Lerista timida | Veg type | 2 | 16.923 | 0.000 | 1.000 | 0.655 | −6.109 | 0.655 | 0.277 | |
| Ctenotus atlas | Veg type | 2 | 16.923 | 0.000 | 1.000 | 0.937 | −6.109 | 0.937 | 0.528 | |
| Varanus gouldii | Site type × veg type | 4 | 27.025 | 0.000 | 1.000 | 0.516 | −8.179 | 0.516 | 0.392 | |
| Lerista punctatovittata | Site type × veg type × matrix type | 6 | 83.000 | 0.000 | 1.000 | 0.946 | −32.297 | 0.946 | 0.812 |
The AICc and Δi outputs identify best-performing model(s) and models with substantial support (Δi < 2). The coefficient of determination is also represented as R2.
| Species | Model | Variable | Estimate | s.e. | z-value | Pr(>|z|) | |
|---|---|---|---|---|---|---|---|
| Ctenophorus spinodomus | Site type | (Intercept) | 0.956 | 0.789 | 1.211 | 0.226 | |
| Patch | −4.174 | 1.696 | −2.461 | 0.014 | |||
| Diporiphora nobbi | Site type | (Intercept) | 1.609 | 0.949 | 1.696 | 0.090 | |
| Patch | −4.828 | 1.776 | −2.719 | 0.007 | |||
| Ctenotus schomburgkii | Site type | (Intercept) | 1.099 | 0.817 | 1.346 | 0.178 | |
| Patch | −3.497 | 1.326 | −2.637 | 0.008 | |||
| Ctenotus schomburgkii | Landscape matrix | (Intercept) | 0.956 | 0.789 | 1.211 | 0.226 | |
| Cropping | −3.521 | 1.771 | −1.988 | 0.047 | |||
| Grazing | −2.255 | 1.270 | −1.775 | 0.076 | |||
| Morethia obscura | Site type | (Intercept) | 1.099 | 0.817 | 1.346 | 0.178 | |
| Patch | −3.497 | 1.326 | −2.637 | 0.008 | |||
| Morethia obscura | Landscape matrix | (Intercept) | 0.956 | 0.789 | 1.211 | 0.226 | |
| Cropping | −3.521 | 1.771 | −1.988 | 0.047 | |||
| Grazing | −2.255 | 1.270 | −1.775 | 0.076 | |||
| Morethia obscura | Site type × vegetation type | (Intercept) | 2.197 | 1.667 | 1.318 | 0.187 | |
| Reserve dune mallee | −2.197 | 1.944 | −1.130 | 0.258 | |||
| Patch sandplain mallee | −4.762 | 2.300 | −2.070 | 0.038 | |||
| Patch dune mallee | −3.497 | 1.941 | −1.801 | 0.072 | |||
| Lerista timida | Vegetation type | (Intercept) | 3.045 | 1.518 | 2.005 | 0.045 | |
| Dune mallee | −2.282 | 1.663 | −1.372 | 0.170 | |||
| Ctenotus atlas | Vegetation type | (Intercept) | −3.045 | 1.518 | −2.005 | 0.045 | |
| Dune mallee | 3.807 | 1.663 | 2.289 | 0.022 | |||
| Varanus gouldii | Site type × vegetation type | (Intercept) | 0.000 | 1.000 | 0.000 | 1.000 | |
| Reserve dune mallee | −2.197 | 1.944 | −1.130 | 0.258 | |||
| Patch sandplain mallee | −1.299 | 1.411 | −0.921 | 0.357 | |||
| Patch dune mallee | 1.299 | 1.411 | 0.921 | 0.357 | |||
| Lerista punctatovittata | Site type × vegetation type × landscape matrix | (Intercept) | 1.504 | 0.175 | 8.578 | 0.000 | |
| Reserve dune mallee | −1.099 | 0.351 | −3.133 | 0.007 | |||
| Cropping dune mallee | 0.765 | 0.223 | 3.425 | 0.004 | |||
| Grazing dune mallee | −0.523 | 0.316 | −1.655 | 0.120 | |||
| Cropping sandplain mallee | −1.504 | 0.464 | −3.242 | 0.006 | |||
| Grazing sandplain mallee | −0.811 | 0.351 | −2.312 | 0.036 |
Bold values are significant at P < 0.05.
The probability of occurrence of (a) Ctenophorus spinodomus, (b) Diporiphora nobbi, (c) Ctenotus schomburgkii and (d) Morethia obscura between reserve and patch sites. Error bars represent 95% confidence intervals.
The probability of occurrence of: (a) C. schomburgkii and (b) M. obscura on sites surrounded by continuous, cropping or grazing land use types; (c) C. atlas and (d) L. timida in sandplain mallee or dune mallee vegetation type; (e) M. obscura and (f) V. gouldii in site and vegetation type combinations; and (g) L. punctatovittata estimated abundance across treatments (RC, reserve continuous; PC, patch cropping; PG, patch grazing; S, sandplain; D, dune). Error bars represent 95% confidence intervals.
Discussion
Our study provides insight into which reptile species are potentially vulnerable to habitat loss and fragmentation in an arid zone landscape, with two species, M. obscura and C. schomburgkii, being rarely detected in remnant patches and C. spinodomus and D. nobbi being absent from remnant patches. We found support for our prediction that habitat loss and fragmentation would have greater impacts on habitat specialists, but less support for matrix or vegetation-type effects. Although habitat fragmentation can lead to a decline in habitat specialists, small remnant patches of mallee woodland provide important habitat for generalist reptile species.
Which species are sensitive to habitat fragmentation?
Reptiles are often highly sensitive to habitat loss and fragmentation (Keinath et al. 2017). Of the eight species we modelled, five species were classified as habitat specialists (C. spinodomus, D. nobbi, C. schomburgkii, C. atlas and M. obscura). Of these species, C. spinodomus, D. nobbi, C. schomburgkii and M. obscura were significantly associated with the reserve, with between 73% and 83% probability of occurring in the reserve and only 3–10% probability of occurring in patches. These results are consistent with previous studies on mallee reptiles in south-central NSW, where D. nobbi (syn. Amphibolurus nobbi), M. obscura and C. schomburgkii were found to be biased towards a mallee woodland reserve and C. spinodomus (syn. Ctenophorus fordi) was detected exclusively within a reserve (Driscoll 2004). Driscoll (2004) concluded that D. nobbi and C. spinodomus are extremely sensitive to fragmentation, occurring in high abundances in the reserve while being absent from patches. By contrast, Driscoll and Hardy (2005) found genetic evidence indicating high dispersal and migration in D. nobbi from a reserve within a fragmented agricultural landscape to ungrazed linear remnants. It is possible that the patches in our study were too widely spaced (averaging 1000 m) between neighbouring patches, or the reserve, to enable sufficient dispersal and maintain viable populations within remnant patches, or that disturbance by livestock grazing (and trampling) influenced shelter and breeding site suitability. The habitat specialist, C. atlas, was the only species that was not significantly associated with the reserve. This may be due to C. atlas having the ability to disperse through fragmented landscapes or because its requirements of mid to late post-fire successional stage Triodia cover were met within patches dominated by dune mallee (Smith et al. 2011; Verdon et al. 2020).
Three of the eight species modelled were classified as habitat generalists (V. gouldii, L. timida and L. punctatovittata). These three species were widespread across the study area. L. punctatovittata occurred at 100% of patches and L. timida and V. gouldi occurred at 83% and 50% respectively, suggesting these species are less vulnerable to processes associated with habitat loss and fragmentation. Furthermore, we found L. punctatovittata was significantly more abundant in dune mallee patches in the cropping landscape compared with any other site type. L. punctatovittata is a nocturnal, fossorial species associated with extensive leaf litter (Henle 1989). Driscoll (2004) found this species attained extremely high numbers along linear roadsides, conceivably due to increased nutrients and food resources. Cultivation around patches may act as a barrier to dispersal and reduced competition for resources with specialists could also explain the higher numbers observed. Lerista punctatovitta also responds positively to destocking (Neilly et al. 2021), so another plausible explanation is that this species has increased in response to the reduced level of grazing pressure across the property.
How important is the matrix?
The matrix can have a substantial influence on ecological communities and species distribution patterns (Michael et al. 2008; Munguia-Vega et al. 2013). Cropland in particular can act as a barrier to reptiles and restrict movement among remnant vegetation (Kay et al. 2016; Hansen et al. 2020). We predicted that patches in the cropping matrix would have lower occurrence rates than continuous vegetation (reserve) or patches in the grazing matrix. However, we found little difference in species occurrence patterns in patches among matrix types. Matrix type only featured in the models for C. schomburgkii and M. obscura, where both species were more likely to occur in sites surrounded by continuous vegetation and therefore were more likely to occur in the reserve. The limited support for our prediction could be attributed to the relatively small sample size, lack of replication, relatively recent land clearing and potentially confounding influence of unmeasured disturbances. Structural similarities and the same time since clearing between cropping and grazing matrices may indicate similar ecological impacts on reptiles. Pulsford et al. (2017) found that the intensity of matrix use for agricultural purposes was more important than matrix type itself in predicting reptile distribution patterns in temperate woodland landscapes. Therefore, improving matrix quality and appropriately designed matrix improvements can help increase conservation outcomes in modified landscapes (Franklin and Lindenmayer 2009; Driscoll et al. 2013).
How important is vegetation type in explaining species distribution patterns?
At large spatial scales, reptile communities are strongly influenced by vegetation communities. Sass (2006) found that mallee sites supporting Triodia maintained higher abundance and diversity of reptile species than areas without Triodia. We predicted that habitat specialists would be restricted to dune mallee, due to the presence of Triodia, whereas habitat generalists would be distributed across vegetation types. We found support for this prediction for C. atlas, which was associated with dune mallee. This pattern is consistent with previous studies that found Triodia cover to be an important predictor of C. atlas occurrence (Verdon et al. 2020; Bell et al. 2021a). We found limited support for a vegetation type effect for C. spinodomus, a species reported to have affiliations with early–mid stage Triodia cover (Nimmo et al. 2012; Sadlier et al. 2019; Verdon et al. 2020). However, the lack of a vegetation type relationship in our study may be due to the species’ complete absence from patches, including those with Triodia, hence the overriding effect of fragmentation. The only other species that exhibited a vegetation type effect was M. obscura, which was most likely to occur in sandplain mallee within the reserve. Preference for chenopod-dominated vegetation communities with open canopies and abundant leaf litter are consistent with documented habitat relationships for this species (Triska et al. 2016; Dundas et al. 2021).
Management implications
There are several broader management implications that stem from this study. First, offsetting habitat loss by establishing in-perpetuity conservation areas representative of the vegetation communities affected by agricultural intensification is an important factor in preserving reptile diversity on private land. Second, remnant patches within cleared areas could be better managed to improve habitat suitability for reptile specialists. Although we were unable to explain the mechanisms behind the decline in habitat specialists in this study, strong habitat affiliations with foundation species such as Triodia suggest that declining habitat condition and/or extent could be responsible. Triodia cover can be substantially altered due to agricultural activities, especially soil compaction and increased soil nutrients (Bell et al. 2021b). Fencing remnants to control livestock grazing pressure immediately following habitat fragmentation may help to preserve ground cover condition (Pulsford et al. 2017). Small-scale ecological burns could also be trialled to promote Triodia growth and post-fire seral stages. Third, improved matrix management through managing stocking levels and strategically orientating crops (Kay et al. 2016) could reduce barrier effects between remnants. Lastly, future research could trial small-scale translocations (Watson and Watson 2015) to investigate the feasibility of recovering locally extinct species.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Acknowledgements
We pay respects to the Mutthi Mutthi people, who are the traditional owners of the land on which this research was based. We thank Peter and Sue Morton for access to their property and the NSW Parks and Wildlife Services for providing accommodation. Kylee Imlach provided administrative assistance and Mikayla Green and Hannah Wells assisted with fieldwork. This study was approved by Charles Sturt University Animal Care and Ethics Committee (Protocol No. A21058) under the NSW NPWS scientific licence (OEH: SL102236).
References
Atlas of Living Australia (ALA) (2023) Atlas of Living Australia. Available at https://www.ala.org.au/ [Accessed 15 February 2023]
Baumgardt JA, Morrison ML, Brennan LA, Thornley M, Campbell TA (2021) Variation in herpetofauna detection probabilities: implications for study design. Environmental Monitoring and Assessment 193, 658.
| Crossref | Google Scholar | PubMed |
Bell KJ, Doherty TS, Driscoll DA (2021a) Predators, prey or temperature? Mechanisms driving niche use of a foundation plant species by specialist lizards. Proceedings of the Royal Society B: Biological Sciences 288, 20202633.
| Crossref | Google Scholar |
Bell K, Driscoll DA, Patykowski J, Doherty TS (2021b) Abundance, condition and size of a foundation species vary with altered soil conditions, remnant type and potential competitors. Ecosystems 24, 1516-1530.
| Crossref | Google Scholar |
BOM (2022) Climate statistics for Australian locations. Available at http://www.bom.gov.au/ [Accessed 9 June 2022]
Clarke MF, Kelly LT, Avitabile SC, Benshemesh J, Callister KE, Driscoll DA, Ewin P, Giljohann K, Haslem A, Kenny SA, Leonard S, Ritchie EG, Nimmo DG, Schedvin N, Schneider K, Watson SJ, Westbrooke M, White M, Wouters MA, Bennett AF (2021) Fire and its interactions with other drivers shape a distinctive, semi-arid ‘mallee’ ecosystem. Frontiers in Ecology and Evolution 9, 647557.
| Crossref | Google Scholar |
Cox N, Young BE, Bowles P, Fernandez M, Marin J, Rapacciuolo G, Böhm M, Brooks TM, Hedges SB, Hilton-Taylor C, Hoffmann M, Jenkins RKB, Tognelli MF, Alexander GJ, Allison A, Ananjeva NB, Auliya M, Avila LJ, Chapple DG, Cisneros-Heredia DF, Cogger HG, Colli GR, de Silva A, Eisemberg CC, Els J, Fong G. A, Grant TD, Hitchmough RA, Iskandar DT, Kidera N, Martins M, Meiri S, Mitchell NJ, Molur S, Nogueira CdC, Ortiz JC, Penner J, Rhodin AGJ, Rivas GA, Rödel M-O, Roll U, Sanders KL, Santos-Barrera G, Shea GM, Spawls S, Stuart BL, Tolley KA, Trape J-F, Vidal MA, Wagner P, Wallace BP, Xie Y (2022) A global reptile assessment highlights shared conservation needs of tetrapods. Nature 605, 285-290.
| Crossref | Google Scholar |
Devictor V, Julliard R, Jiguet F (2008) Distribution of specialist and generalist species along spatial gradients of habitat disturbance and fragmentation. Oikos 117, 507-514.
| Crossref | Google Scholar |
Doherty TS, Balouch S, Bell K, Burns TJ, Feldman A, Fist C, Garvey TF, Jessop TS, Meiri S, Driscoll DA (2020) Reptile responses to anthropogenic habitat modification: a global meta-analysis. Global Ecology and Biogeography 29, 1265-1279.
| Crossref | Google Scholar |
Driscoll DA (2004) Extinction and outbreaks accompany fragmentation of a reptile community. Ecological Applications 14, 220-240.
| Crossref | Google Scholar |
Driscoll DA, Hardy CM (2005) Dispersal and phylogeography of the agamid lizard Amphibolurus nobbi in fragmented and continuous habitat. Molecular Ecology 14, 1613-1629.
| Crossref | Google Scholar | PubMed |
Driscoll DA, Banks SC, Barton PS, Lindenmayer DB, Smith AL (2013) Conceptual domain of the matrix in fragmented landscapes. Trends in Ecology & Evolution 28, 605-613.
| Crossref | Google Scholar | PubMed |
Dundas SJ, Ruthrof KX, Hardy GESJ, Fleming PA (2021) Some like it hot: drought-induced forest die-off influences reptile assemblages. Acta Oecologica 111, 103714.
| Crossref | Google Scholar |
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Annual Review of Ecology, Evolution, and Systematics 34, 487-515.
| Crossref | Google Scholar |
Fischer J, Fazey I, Briese R, Lindenmayer DB (2005) Making the matrix matter: challenges in Australian grazing landscapes. Biodiversity & Conservation 14, 561-578.
| Crossref | Google Scholar |
Fiske I, Chandler R (2011) unmarked: an R package for fitting hierarchical models of wildlife occurrence and abundance. Journal of Statistical Software 43, 1-23.
| Crossref | Google Scholar |
Franklin JF, Lindenmayer DB (2009) Importance of matrix habitats in maintaining biological diversity. Proceedings of the National Academy of Sciences of the United States of America 106, 349-350.
| Crossref | Google Scholar |
Garnett ST, Hayward-Brown BK, Kopf RK, Woinarski JCZ, Cameron KA, Chapple DG, Copley P, Fisher A, Gillespie G, Latch P, Legge S, Lintermans M, Moorrees A, Page M, Renwick J, Birrell J, Kelly D, Geyle HM (2022) Australia’s most imperilled vertebrates. Biological Conservation 270, 109561.
| Crossref | Google Scholar |
Gibbs JP (2001) Demography versus habitat fragmentation as determinants of genetic variation in wild populations. Biological Conservation 100, 15-20.
| Crossref | Google Scholar |
Greer A (2022) Encyclopedia of Australian reptiles. Version 1, April 2022. Available at https://www.researchgate.net [Accessed 21 July 2022]
Haddad NM, Brudvig LA, Clobert J, Davies KF, Gonzalez A, Holt RD, Lovejoy TE, Sexton JO, Austin MP, Collins CD, Cook WM, Damschen EI, Ewers RM, Foster BL, Jenkins CN, King AJ, Laurance WF, Levey DJ, Margules CR, Melbourne BA, Nicholls AO, Orrock JL, Song D-X, Townshend JR (2015) Habitat fragmentation and its lasting impact on Earth’s ecosystems. Science Advances 1, e1500052.
| Crossref | Google Scholar | PubMed |
Hansen NA, Sato CF, Michael DR, Lindenmayer DB, Driscoll DA (2019) Predation risk for reptiles is highest at remnant edges in agricultural landscapes. Journal of Applied Ecology 56, 31-43.
| Crossref | Google Scholar |
Hansen NA, Driscoll DA, Michael DR, Lindenmayer DB (2020) Movement patterns of an arboreal gecko in fragmented agricultural landscapes reveal matrix avoidance. Animal Conservation 23, 48-59.
| Crossref | Google Scholar |
Hartig F (2022) DHARMa: residual diagnostics for hierarchical (multi-level/mixed) regression models. R package version 0.4.6. Available at https://CRAN.R-project.org/package=DHARMa
Henle K (1989) Ecological segregation in a subterranean reptile assemblage in arid Australia. Amphibia-Reptilia 10, 277-295.
| Crossref | Google Scholar |
IUCN (2022) The IUCN red list of threatened species. Available at https://www.iucnredlist.org [Accessed 28 June 2022]
Jellinek S, Parris KM, McCarthy MA, Wintle BA, Driscoll DA (2014) Reptiles in restored agricultural landscapes: the value of linear strips, patches and habitat condition. Animal Conservation 17, 544-554.
| Crossref | Google Scholar |
Jules ES, Shahani P (2003) A broader ecological context to habitat fragmentation: why matrix habitat is more important than we thought. Journal of Vegetation Science 14, 459-464.
| Crossref | Google Scholar |
Kay GM, Driscoll DA, Lindenmayer DB, Pulsford SA, Mortelliti A (2016) Pasture height and crop direction influence reptile movement in an agricultural matrix. Agriculture, Ecosystems & Environment 235, 164-171.
| Crossref | Google Scholar |
Keinath DA, Doak DF, Hodges KE, Prugh LR, Fagan W, Sekercioglu CH, Buchart SHM, Kauffman M (2017) A global analysis of traits predicting species sensitivity to habitat fragmentation. Global Ecology and Biogeography 26, 115-127.
| Crossref | Google Scholar |
Kéry M (2002) Inferring the absence of a species: a case study of snakes. The Journal of Wildlife Management 66, 330-338.
| Crossref | Google Scholar |
Kosmidis I (2021) brglm: bias reduction in binomial-rsponse generalized linear models. R package version 0.7.2. Available at https://CRAN.R-project.org/package=brglm
Levy E, Kennington JW, Tomkins JL, Lebas NR (2010) Land clearing reduces gene flow in the granite outcrop-dwelling lizard, Ctenophorus ornatus. Molecular Ecology 19, 4192-4203.
| Crossref | Google Scholar | PubMed |
Lüdecke D (2018) ggeffects: tidy data frames of marginal effects from regression models. Journal of Open Source Software 3, 772.
| Crossref | Google Scholar |
MacKenzie DI, Nichols JD, Lachman GB, Droege S, Andrew Royle J, Langtimm CA (2002) Estimating site occupancy rates when detection probabilities are less than one. Ecology 83, 2248-2255.
| Crossref | Google Scholar |
MacKenzie DI, Nichols JD, Seamans ME, Gutiérrez RJ (2009) Modeling species occurrence dynamics with multiple states and imperfect detection. Ecology 90, 823-835.
| Crossref | Google Scholar | PubMed |
Matthews TJ, Cottee-Jones HE, Whittaker RJ (2014) Habitat fragmentation and the species–area relationship: a focus on total species richness obscures the impact of habitat loss on habitat specialists. Diversity and Distributions 20, 1136-1146.
| Crossref | Google Scholar |
Maxwell SL, Fuller RA, Brooks TM, Watson JEM (2016) Biodiversity: the ravages of guns, nets and bulldozers. Nature 536, 143-145.
| Crossref | Google Scholar | PubMed |
Mazerolle MJ (2020) AICcmodavg: model selection and multimodel inference based on (Q)AIC(c) (version 2.3.1). Available at https://cran.r-project.org/package=AICcmodavg [Accessed 27 June 2022]
Meiri S, Chapple DG (2016) Biases in the current knowledge of threat status in lizards, and bridging the ‘assessment gap’. Biological Conservation 204, 6-15.
| Crossref | Google Scholar |
Menkhorst PW, Bennett AF (1990) Vertebrate fauna of mallee vegetation in southern Australia. In ‘The mallee lands: a conservation perspective: proceedings of the National Mallee Conference, Adelaide, April, 1989’. (Eds JC Noble, PJ Joss, GK Jones) pp. 39–53. (CSIRO Publishing: East Melbourne, Victoria)
Michael DR, Cunningham RB, Lindenmayer DB (2008) A forgotten habitat? Granite inselbergs conserve reptile diversity in fragmented agricultural landscapes. Journal of Applied Ecology 45, 1742-1752.
| Crossref | Google Scholar |
Michael DR, Kay GM, Crane M, Florance D, MacGregor C, Okada S, McBurney L, Blair D, Lindenmayer DB (2015) Ecological niche breadth and microhabitat guild structure in temperate Australian reptiles: implications for natural resource management in endangered grassy woodland ecosystems. Austral Ecology 40, 651-660.
| Crossref | Google Scholar |
Michael DR, Wood JT, O’Loughlin T, Lindenmayer DB (2016) Influence of land sharing and land sparing strategies on patterns of vegetation and terrestrial vertebrate richness and occurrence in Australian endangered eucalypt woodlands. Agriculture, Ecosystems & Environment 227, 24-32.
| Crossref | Google Scholar |
Mulhall SJ, Sitters H, Di Stefano J (2022) Vegetation cover and configuration drive reptile species distributions in a fragmented landscape. Wildlife Research
| Crossref | Google Scholar |
Munguia-Vega A, Rodriguez-Estrella R, Shaw WW, Culver M (2013) Localized extinction of an arboreal desert lizard caused by habitat fragmentation. Biological Conservation 157, 11-20.
| Crossref | Google Scholar |
Neilly H, Ward M, Cale P (2021) Converting rangelands to reserves: small mammal and reptile responses 24 years after domestic livestock grazing removal. Austral Ecology 46, 1112-1124.
| Crossref | Google Scholar |
Nimmo DG, Kelly LT, Spence-Bailey LM, Watson SJ, Haslem A, White JG, Clarke MF, Bennett AF (2012) Predicting the century-long post-fire responses of reptiles. Global Ecology and Biogeography 21, 1062-1073.
| Crossref | Google Scholar |
Nimmo DG, Kelly LT, Spence-Bailey LM, Watson SJ, Taylor RS, Clarke MF, Bennett AF (2013) Fire mosaics and reptile conservation in a fire-prone region. Conservation Biology 27, 345-353.
| Crossref | Google Scholar | PubMed |
Nimmo DG, Kelly LT, Farnsworth LM, Watson SJ, Bennett AF (2014) Why do some species have geographically varying responses to fire history? Ecography 37, 805-813.
| Crossref | Google Scholar |
OEH (2022) Vegetation Keith class NSW Office of Environment and Heritage. Available at https://www.environment.nsw.gov.au [Accessed 25 July 2022]
Pulsford SA, Lindenmayer DB, Driscoll DA (2017) Reptiles and frogs conform to multiple conceptual landscape models in an agricultural landscape. Diversity and Distributions 23, 1408-1422.
| Crossref | Google Scholar |
R Core Team (2022) R Studio: integrated development environment for R. Available at http://www.rstudio.com/ [Accessed 18 July 2022]
Ricketts TH (2001) The matrix matters: effective isolation in fragmented landscapes. The American Naturalist 158, 87-99.
| Crossref | Google Scholar | PubMed |
Sadlier RA, Colgan DJ, Beatson CA, Cogger HG (2019) Ctenophorus spinodomus sp. nov., a new species of dragon lizard (Squamata: Agamidae) from Triodia Mallee habitat of southeast Australia. Records of the Australian Museum 71, 199-215.
| Crossref | Google Scholar |
Sass S (2006) The reptile fauna of Nombinnie Nature Reserve and State Conservation Area, western New South Wales. Australian Zoologist 33, 511-518.
| Crossref | Google Scholar |
Slatyer C, Rosauer D, Lemckert F (2007) An assessment of endemism and species richness patterns in the Australian Anura. Journal of Biogeography 34, 583-596.
| Crossref | Google Scholar |
Smith AL, Gardner MG, Bull CM, Driscoll DA (2011) Primers for novel microsatellite markers in “fire-specialist” lizards (Amphibolurus norrisi, Ctenotus atlas and Nephrurus stellatus) and their performance across multiple populations. Conservation Genetics Resources 3, 345-350.
| Crossref | Google Scholar |
Tan WC, Herrel A, Rödder D (2023) A global analysis of habitat fragmentation research in reptiles and amphibians: what have we done so far? Biodiversity and Conservation 32, 439-468.
| Crossref | Google Scholar |
Thompson GG, Thompson SA (2007) Usefulness of funnel traps in catching small reptiles and mammals, with comments on the effectiveness of the alternatives. Wildlife Research 34, 491-497.
| Crossref | Google Scholar |
Thompson ME, Nowakowski AJ, Donnelly MA (2016) The importance of defining focal assemblages when evaluating amphibian and reptile responses to land use. Conservation Biology 30, 249-258.
| Crossref | Google Scholar | PubMed |
Tingley R, Meiri S, Chapple DG (2016) Addressing knowledge gaps in reptile conservation. Biological Conservation 204, 1-5.
| Crossref | Google Scholar |
Tingley R, Macdonald SL, Mitchell NJ, Woinarski JCZ, Meiri S, Bowles P, Cox NA, Shea GM, Böhm M, Chanson J, Tognelli MF, Harris J, Walke C, Harrison N, Victor S, Woods C, Amey AP, Bamford M, Catt G, Clemann N, Couper PJ, Cogger H, Cowan M, Craig MD, Dickman CR, Doughty P, Ellis R, Fenner A, Ford S, Gaikhorst G, Gillespie GR, Greenlees MJ, Hobson R, Hoskin CJ, How R, Hutchinson MN, Lloyd R, McDonald P, Melville J, Michael DR, Moritz C, Oliver PM, Peterson G, Robertson P, Sanderson C, Somaweera R, Teale R, Valentine L, Vanderduys E, Venz M, Wapstra E, Wilson S, Chapple DG (2019) Geographic and taxonomic patterns of extinction risk in Australian squamates. Biological Conservation 238, 108203.
| Crossref | Google Scholar |
Triska MD, Craig MD, Stokes VL, Pech RP, Hobbs RJ (2016) The relative influence of in situ and neighborhood factors on reptile recolonization in post-mining restoration sites. Restoration Ecology 24, 517-527.
| Crossref | Google Scholar |
Triska MD, Craig MD, Stokes VL, Pech RP, Hobbs RJ (2017) Conserving reptiles within a multiple-use landscape: determining habitat affiliations of reptile communities in the northern jarrah forest of south-western Australia. Australian Journal of Zoology 65, 21.
| Crossref | Google Scholar |
Uetz P, Freed P, Aguilar R, Hošek J (2022) The reptile database. Available at http://www.reptile-database.org [Accessed 11 August 2022]
Verdon SJ, Watson SJ, Nimmo DG, Clarke MF (2020) Are all fauna associated with the same structural features of the foundation species Triodia scariosa? Austral Ecology 45, 773-787.
| Crossref | Google Scholar |
Watson DM, Watson MJ (2015) Wildlife restoration: mainstreaming translocations to keep common species common. Biological Conservation 191, 830-838.
| Crossref | Google Scholar |
Yan Y, Jarvie S, Liu Q, Zhang Q (2022) Effects of fragmentation on grassland plant diversity depend on the habitat specialization of species. Biological Conservation 275, 109773.
| Crossref | Google Scholar |
Youngentob KN, Wood JT, Lindenmayer DB (2013) The response of arboreal marsupials to landscape context over time: a large-scale fragmentation study revisited. Journal of Biogeography 40, 2082-2093.
| Crossref | Google Scholar |