Rabbit warrens: an important resource for invasive alien species in semi-arid Australia
Abbey T. Dean A * , Robert Brandle B , Leon A. Barmuta A , Menna E. Jones A and Jeroen Jansen A
A * , Robert Brandle B , Leon A. Barmuta A , Menna E. Jones A and Jeroen Jansen A
A School of Biological Sciences, University of Tasmania, Private Bag 55, Hobart, Tas. 7001, Australia.
B Department for Environment and Water, Port Augusta, SA 5700, Australia.
Abstract
The decline and extinction of native burrowing mammals across much of Australia has resulted in a loss of underground refugia constructed by native fauna in the environment. The introduced European rabbit (Oryctolagus cuniculus) is now the most widespread mammalian burrower across Australia. Rabbits are an invasive species in Australia, and the destruction of rabbit warrens for pest management is common practice. This destruction of warrens removes a potential refuge for both rabbits and other species in the environment. In landscapes where critical weight range burrowing mammals have declined, the widespread destruction of rabbit warrens removes many underground refuges for several commensal animal species.
To identify the use of rabbit warrens by fauna in the seasonally hot, semi-arid Ikara-Flinders Ranges National Park (IFRNP) in South Australia.
We used camera traps placed at burrow entrances of warrens and nearby structure to identify the vertebrate species that interact with rabbit warrens in the IFRNP.
We recorded 11 bird, nine mammal and eight reptile species present at the entrances of rabbit warrens. Only four of the taxa recorded on cameras in our study showed a preference for warrens over adjacent above-ground structure, three of them introduced species. The alien commensal species recorded using the burrows were rabbits, house mice (Mus musculus) and feral cats (Felis catus).
Rabbit warrens in the IFRNP are an important resource for a range of native and alien commensal species. In our study, they seem to be of special importance for introduced species.
Warren removal in the IFRNP may negatively impact native commensal species in treated areas but is likely to be of long-term net benefit for a wider range of native animals.
Keywords: burrow, burrowing animals, camera survey, commensalism, European rabbit, impact of management, invasive alien species, multivariate abundance analysis, Oryctolagus cuniculus, refuge, thermal refuge.
Introduction
Burrowing mammals are important ecosystem engineers, particularly in harsh climatic environments such as in Australia, where both native and alien invasive mammals create underground refuges used by a wide range of other fauna (James and Eldridge 2007; Fleming et al. 2014; Dawson et al. 2019). Burrowing animals disturb and displace large amounts of soil to create and alter new subterranean habitats (Jones et al. 1994; James et al. 2011; Fleming et al. 2014). Burrowing actions provide refuges for animals from predators, shelter from the environment and breeding sites, and increase overall habitat heterogeneity (Davidson et al. 2012). Burrows created by mammals in arid and semi-arid Australia are important habitat for non-burrowing vertebrates living in these environments (Hofstede and Dziminski 2017; Dawson et al. 2019).
Commensalism is the interaction between two species where one species benefits and the other is not impacted (Schowalter 2016). Commensalism is common in burrows, the habitat and opportunities provided by a burrow are exploited by many species in addition to the species that created the burrow (Jackson and Milstrey 1989; Hofstede and Dziminski 2017; Thornett et al. 2017). For example, the gopher tortoise (Gopherus polyphemus), found across south-eastern USA, digs burrows that support at least 60 vertebrate species and 302 invertebrate species (Jackson and Milstrey 1989). The Indian crested porcupine (Hystrix indica) provides subterranean habitat for at least 22 species of vertebrates, including bats (Mukherjee et al. 2019). In Australia, the burrow systems of the greater bilby (Macrotis lagotis) support native mice and goannas, which reside inside the burrows in both arid and tropical climates (Hofstede and Dziminski 2017; Dawson et al. 2019). Large burrows of the southern hairy-nosed wombat (Lasiorhinus latifrons) are used by black-footed rock wallabies (Petrogale lateralis) and little penguins (Thornett et al. 2017).
The native burrowing mammals of Australia’s arid and semi-arid zones have experienced serious range decline. Since European colonisation, the lesser bilby (M. leucura) has become extinct and the greater bilby has declined to occupy less than 20% of its former range (Menkhorst and Knight 2011; Hofstede and Dziminski 2017). Boodies (Bettongia lesseur), which dig large warrens, are now only present within fenced feral-free enclosures on the Australian mainland (Finlayson 1958; Noble et al. 2007). Burrow creation by these important native mammals has ceased across large areas of Australia, although some relict burrows and warrens may remain (Burbidge et al. 2007). Both bilbies and boodies were once present in the Flinders Ranges, as evidenced by Aboriginal records (Tunbridge 1991; Brandle 2001). With these species now locally extinct, rabbits are the dominant burrowing mammal throughout the Flinders Ranges (Brandle 2001).
Vertebrate species introduced to Australia that dig burrows could potentially fill the niche originally provided by native burrowing species. European rabbits (Oryctolagus cuniculus) are prolific burrowers, often producing warrens which house large social groups; they are now Australia’s most widespread burrowing mammal (Eldridge and Myers 2001). Rabbits are the most widely distributed and abundant vertebrate pest species in Australia, impacting both natural and agricultural systems (Williams et al. 1995; Cooke 2012; Finlayson et al. 2022). Rabbits cause large, widespread damage across multiple trophic levels in the Australian environment, and exert immense grazing pressure on native vegetation, promote invasive weed species and cause extensive soil damage (Eldridge and Myers 2001; Eldridge and Simpson 2002; Eldridge et al. 2006). Rabbits also compete with native mammals, alter habitat and promote the presence of invasive predators in the environment (Cooke 2012; Woinarski et al. 2015; Pedler et al. 2016). In landscapes where bilbies and boodies were once creating burrows, rabbits and their warren systems now dominate.
Rabbit management in Australia is a multipronged approach encompassing biological, chemical and physical methods (Edwards and Dobbie 1999; Edwards et al. 2002). Methods of rabbit control that target warrens, such as destruction by ripping, are effective at suppressing the rabbit population (Sharp 2012). Control measures such as these would invariably impact any other species utilising the warren. This is the first study to place cameras on the burrow entrances of European rabbit warrens to understand commensal use of rabbit warrens and to inform management decisions.
We investigated the use of rabbit warrens by fauna in the Ikara-Flinders Ranges National Park (IFRNP) in semi-arid South Australia. Because rabbits are now the predominant burrowers in the region where native critical weight range (CWR) burrowing mammals were once common, understanding the importance of rabbit warrens to other species is important for assessing management options.
Materials and methods
Study area
The study was undertaken in the north of the IFRNP (−31.32, 138.61) in semi-arid South Australia (Fig. 1). The average annual rainfall, as recorded at Blinman, the closest meteorological station 25 km north-east of the study site, is 306.2 mm, although rainfall was below average for the 3 years prior to this study, with 2019 receiving only 93.8 mm (Bureau of Meteorology 2022).
Location of 25 rabbit warrens (red dots) where remote cameras were placed in the north of the Ikara-Flinders Ranges National Park, South Australia (Base Image: Esri Hillshade/QGIS).
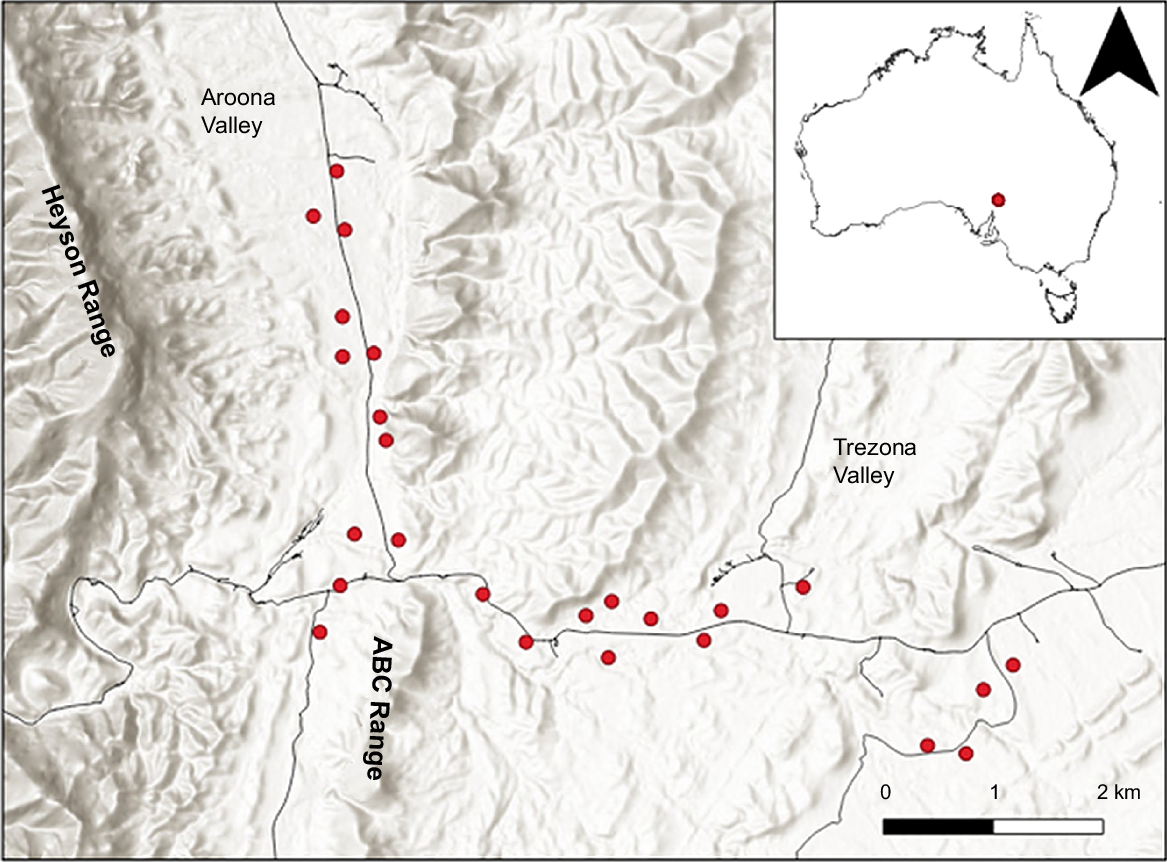
The study site is in the southern Aroona Valley between the Heyson and ABC Range, and east onto the flat plains around the Trezona valley (Fig. 1). Vegetation cover is relatively open, with some low shrubs and river red gums (Eucalyptus camaldulensis) in the riparian zone along the creek-lines. No grasses were present at the time of the study and undergrowth was dominated by introduced weed species.
The study site supports a large concentration of rabbits, and large warrens are a common occurrence. Twenty-five active rabbit warrens, with between 10 and 76 burrow entrances, were selected using a combination of satellite imagery, prior warren-mapping and on-ground inspection (Fig. 1, Supplementary Table S1).
Camera trapping
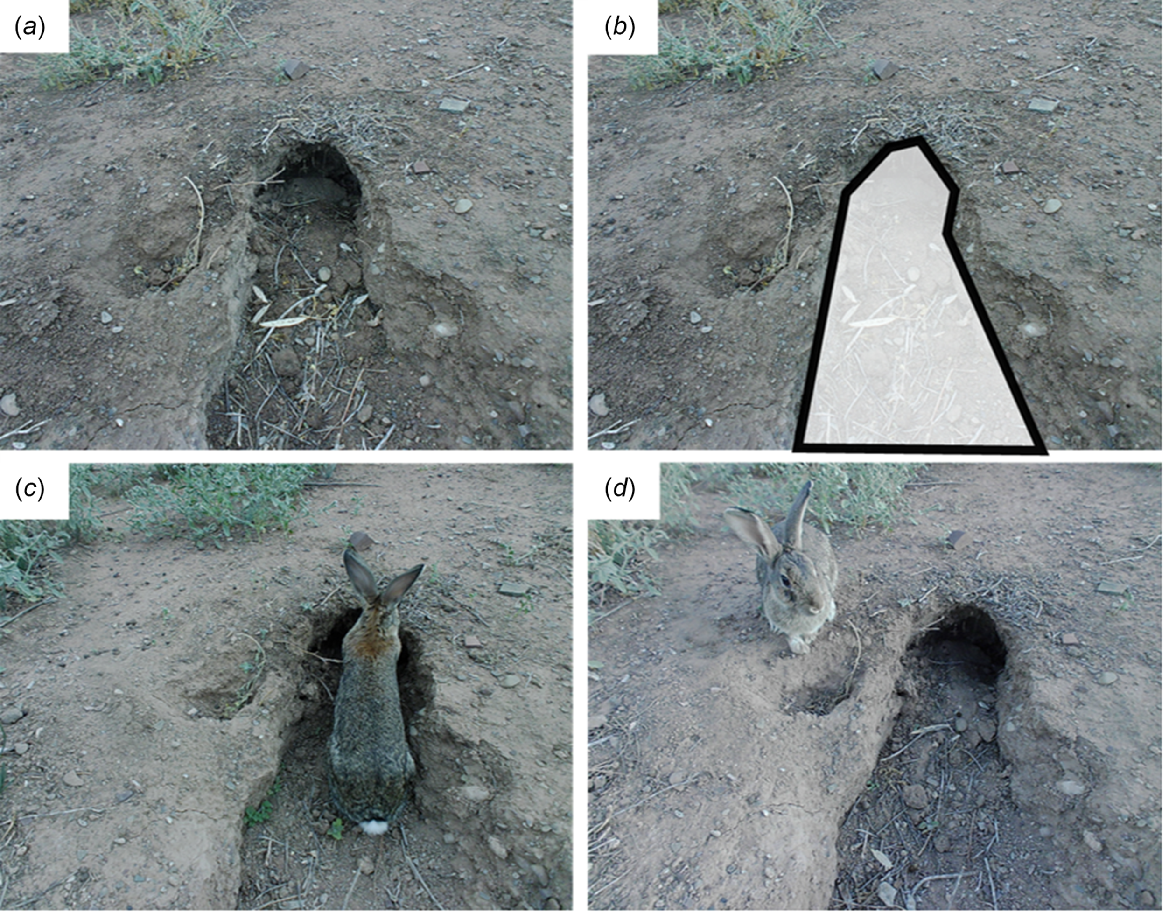
Paired remote Swift 3C cameras (Outdoor Cameras Australia, Toowoomba, Qld, Australia) were set up at each of the 25 chosen rabbit warrens. The 50 cameras in total were set from mid-March 2020 to mid-June 2020. These months represent autumn into early winter, when temperatures start to cool off from the summer high, and when ripping is most effective and therefore most likely to occur (Williams et al. 1995). March temperatures average 30.1°C and 15°C minimum, and these drop to an average maximum of 16.4°C and average minimum of 4.5°C in June, 60 km south in Hawker, the closest meteorological station with temperature records (Bureau of Meteorology 2022). One of the paired cameras was placed about 1–1.5 m from one or two active burrow entrances in the warren, at a height of 40 cm and angled down so that the field of view covered the entrance, the depression in front of the burrow entrance(s) and the surroundings of the burrow entrance(s) (Fig. 2a). This camera was designated the ‘warren’ camera. The second camera was set up identically 20 m behind the first camera focusing on the nearest above-ground environmental structure not part of the rabbit warren. This camera was designated the ‘structure’ camera, to compare animal usage of below-ground and above-ground refuges. This structure was typically shrubs but in very open areas, rock piles or fallen sticks were used. Cameras were checked for functionality and field of view by taking test images as they were set.
(a) Focal area of a camera positioned over a burrow entrance of a rabbit warren in the Ikara-Flinders Ranges National Park, South Australia. (b) Example of how location was tagged; translucent polygon = animal interacting with the burrow entrance; all else = animal passing the burrow entrance. (c) Example image of a European rabbit (Oryctolagus cuniculus) tagged as interacting with the burrow entrance of the warren. (d) Example image of a European rabbit tagged as passing the burrow entrance of the warren.
Cameras were set to take five images in rapid succession in response to any motion, with no time delay between image triggers, medium passive infrared sensitivity, and a balanced night mode setting. The cameras were set for 12 weeks, with some operational for less due to exhausted batteries or full SD cards caused by excessive triggering by vegetation (Table S1). Batteries in the cameras were recharged and images downloaded, and the camera focal area was cleaned of vegetation four times during the period they were set. A trap day in this study was defined as the 24-h period between midnight and 11:59 PM.
Images were tagged using the program DigiKam (https://www.digikam.org/). All images of vertebrates captured on camera were identified to species level where possible. Broader classifications were used when images were unclear, or when species separation was difficult using image alone (e.g. Sminthopsis species). We confidently identified the small mice as house mice (Mus musculus), despite their superficial similarity to native Pseudomys mouse species because pitfall trapping conducted concurrently to the study (and recent live trapping) only produced house mice (Brandle 2001; Lynch and Brandle 2018). For each image of an animal captured at a focal burrow entrance at a warren, the animal’s behaviour with respect to the burrow was recorded, following Dawson et al. (2019), as either:
Interacting: the animal was observed to enter or exit the burrow entrance or to walk on or across the depression in front of the burrow (Fig. 2b, c). Interacting was not separated by use of the burrow or just the burrow entrance because cameras may miss small, fast animals entering or exiting the burrow, or shadows may obscure the burrow at certain times of the day (Fig. 2c). Our definition, as used by (Dawson et al. 2019) for bilby burrow visitations, provides a standard method for counting animals whose behaviour puts them right at the burrow entrance or inside the burrow.
Passing: the animal moved past the burrow entrance without entering the depression in front of the burrow (Fig. 2d). This definition separates animals whose behaviour did not place them in close proximity to the burrow itself as they moved across the warren area.
Image metadata were extracted using the package ‘CamtrapR’ in R (Niedballa et al. 2016; R Core Team 2020). Consistent with previous study in the area (Moseby et al. 2021a), and similar study on burrows (Dawson et al. 2019), captures of animals on camera were counted as an independent capture event if they were separated by 10 min or more. Multiple images of the same species taken within a 10-min period were counted as one capture event or visitation of that species to the camera. Capture events were classified as interacting if the animal was observed interacting with the warren at least once.
Data analysis
To identify the differences among taxa on warren and paired above-ground structure cameras, we ran a multivariate multiple generalised linear model using the manyglm() function from the ‘mvabund’ package (Wang et al. 2012; R Core Team 2020). This function fits separate generalised linear models to each taxon with a common set of explanatory variables, and uses permutation methods to test hypotheses for each taxon while accounting for correlations among them (Wang et al. 2012). Only taxa with greater than 10 capture events across all cameras were included in the analysis. A negative binomial distribution was used because the data were too over-dispersed for reliable testing using a Poisson distribution. An offset (number of days a camera was active log-transformed) was used in the model to adjust for days when cameras were inactive. To account for cameras being in paired stations (one camera on the warren and the second on nearby structure), permutations were restricted to within stations. PIT (probability integral transform)-trap sampling with 999 iterations was used to determine the P-values; the community P-value as well as adjusted univariate P-values for each taxon were calculated. Species significantly more likely to be on the warren than on the structure camera were regarded as warren commensals.
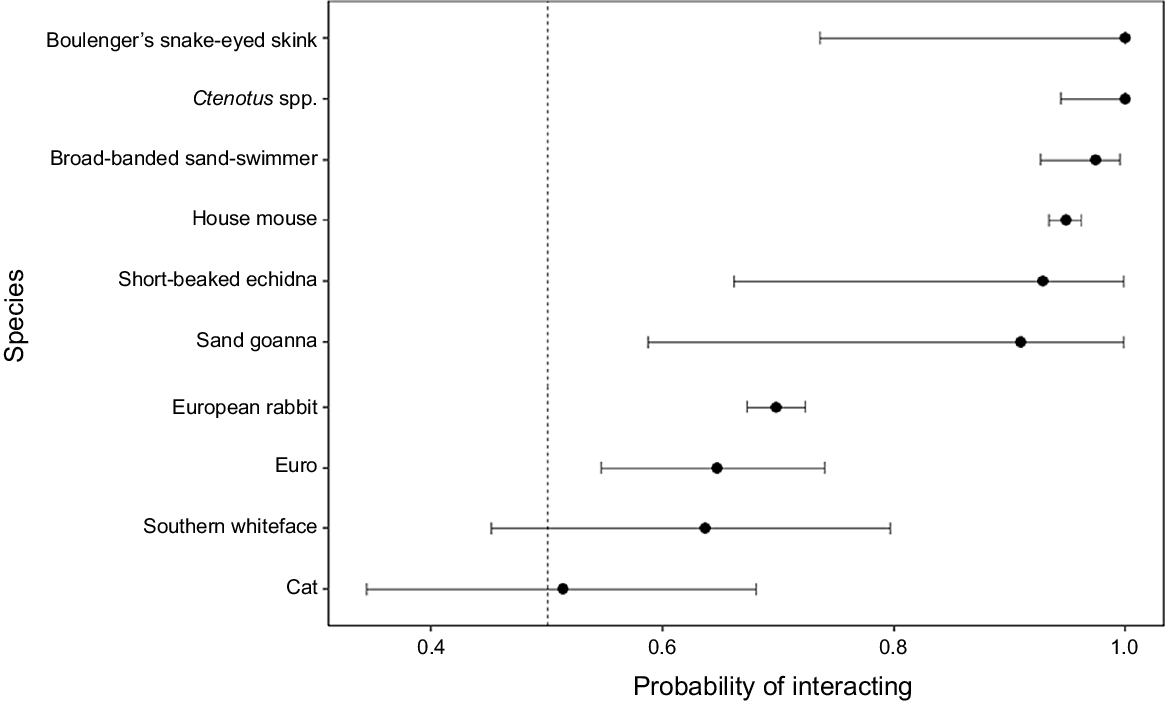
For species with more than 10 capture events on the warren cameras, an exact binomial test was used to determine the probability of a species interacting with the rabbit burrow entrance. The null expectation for this test was 0.5, i.e. an equal likelihood for an animal to be passing or interacting with the burrow entrance of a rabbit warren. Results closer to 1 mean a greater likelihood that an individual of that species will interact with the burrow entrance on the warren; results near 0 denote a low likelihood of interaction.
Results
Camera trapping
In total, 3974 trap days were recorded across all cameras, with the median number of days that a camera was active being 86 days. In over 38 000 images of vertebrates, 2946 separate capture events were recorded.
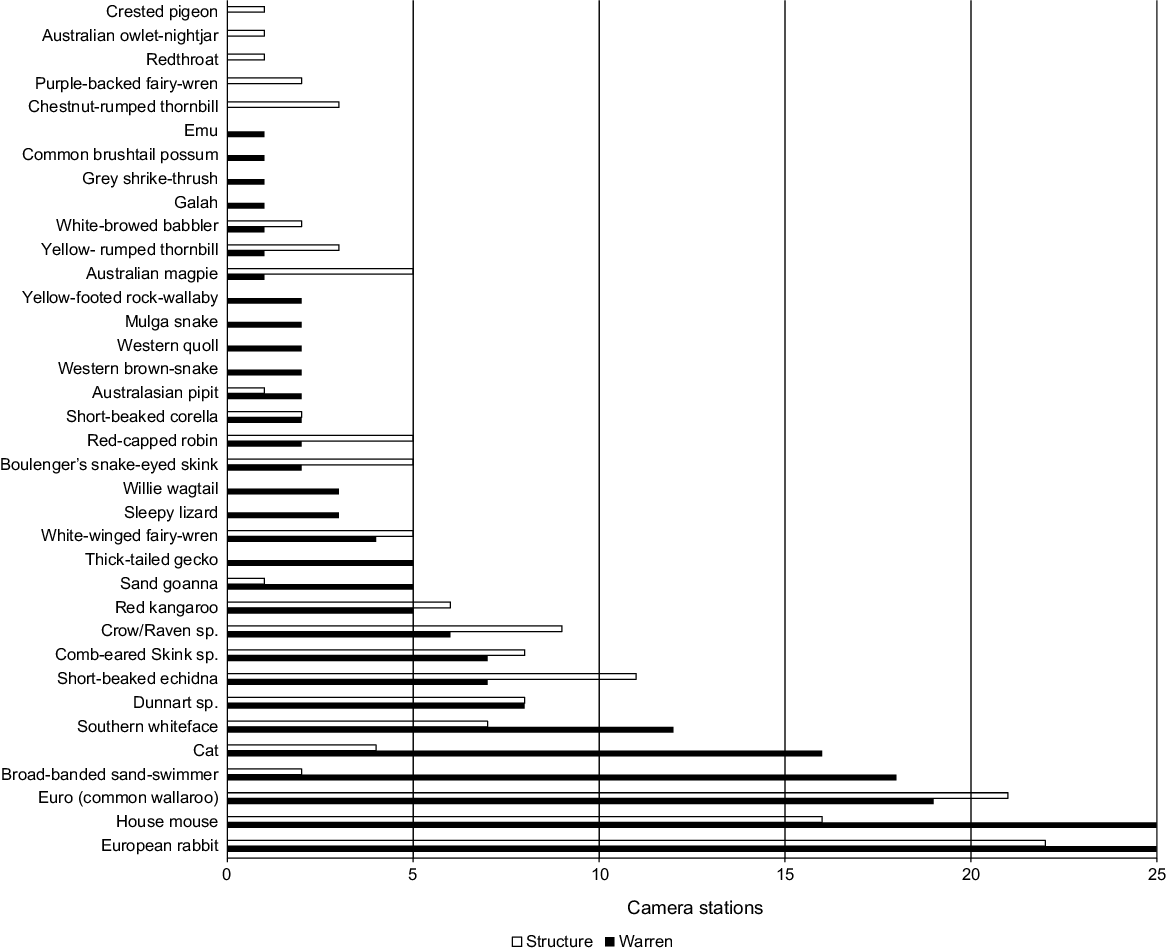
Overall, 36 vertebrate taxa were captured across all cameras, of which 27 taxa were seen to interact with the burrow entrance of the warren camera at least once (Table 1). All vertebrate taxa except three: Sminthopsis spp. (dunnarts); Ctenotus spp. (comb-eared skinks); and Corvus spp. (crows and ravens), could be identified to species level. Of all the capture events recorded of vertebrate species, 78% were of animals interacting with the burrow entrance (Table 1).
| Species | Common name | Warren | Structure | |||
|---|---|---|---|---|---|---|
| Interacting | Passing | |||||
| Mammal | Mus musculus | House mouse | 994 (88.1) | 54 (4.8) | 80 (7.1) | |
| Oryctolagus cuniculus | European rabbit | 932 (58.3) | 403 (25.2) | 264 (16.5) | ||
| Osphranter robustus | Euro (common wallaroo) | 66 (37.3) | 36 (20.3) | 75 (42.4) | ||
| Felis catus | Cat | 19 (46.3) | 18 (43.9) | 4 (9.8) | ||
| Tachyglossus aculeatus | Short-beaked echidna | 13 (35.1) | 1 (2.7) | 23 (62.2) | ||
| Sminthopsis spp. | Dunnart species | 5 (21.7) | 3 (13.0) | 15 (65.2) | ||
| Dasyurus geoffroii | Western quoll | 3 (60.0) | 2 (40.0) | 0 (0.0) | ||
| Petrogale xanthopus | Yellow-footed rock-wallaby | 3 (75.0) | 1 (25.0) | 0 (0.0) | ||
| Osphranter rufus | Red kangaroo | 2 (3.8) | 8 (15.4) | 42 (80.8) | ||
| Trichosurus vulpecula | Common brushtail possum | 0 (0.0) | 1 (100.0) | 0 (0.0) | ||
| Reptile | Eremiascincus richardsonii | Broad-banded sand-swimmer | 113 (95.8) | 3 (2.5) | 2 (1.7) | |
| Ctenotus spp | Comb-eared skink species | 64 (59.8) | 0 (0.0) | 43 (40.2) | ||
| Morethia boulengeri | Boulenger’s snake-eyed skink | 12 (42.9) | 0 (0.0) | 16 (57.1) | ||
| Varanus gouldii | Sand goanna | 10 (83.3) | 1 (8.3) | 1 (8.3) | ||
| Underwoodisaurus milii | Thick-tailed gecko | 7 (77.8) | 2 (22.2) | 0 (0.0) | ||
| Pseudonaja nuchalis | Western brown-snake | 4 (100.0) | 0 (0.0) | 0 (0.0) | ||
| Tiliqua rugosa | Sleepy lizard | 3 (100.0) | 0 (0.0) | 0 (0.0) | ||
| Pseudechis australis | Mulga snake | 1 (50.0) | 1 (50.0) | 0 (0.0) | ||
| Bird | Aphelocephala leucopsis | Southern whiteface | 21 (36.2) | 12 (20.7) | 25 (43.1) | |
| Pomatostomus superciliosus | White-browed babbler | 8 (47.1) | 0 (0.0) | 9 (52.9) | ||
| Acanthiza chrysorrhoa | Yellow-rumped thornbill | 4 (40.0) | 1 (10.0) | 5 (50.0) | ||
| Cacatua sanguinea | Short-beaked corella | 3 (18.8) | 7 (43.8) | 6 (37.5) | ||
| Corvus spp. | Crow/raven species | 3 (12.0) | 6 (24.0) | 16 (64.0) | ||
| Eolophus roseicapilla | Galah | 3 (100.0) | 0 (0.0) | 0 (0.0) | ||
| Anthus novaeseelandiae | Australasian pipit | 3 (33.3) | 0 (0.0) | 6 (66.7) | ||
| Malurus leucopterus | White-winged fairy-wren | 2 (13.3) | 3 (20.0) | 10 (66.7) | ||
| Rhipidura leucophrys | Willy wagtail | 2 (50.0) | 2 (50.0) | 0 (0.0) | ||
| Petroica goodenovii | Red-capped robin | 1 (6.7) | 2 (13.3) | 12 (80.0) | ||
| Colluricincla harmonica | Grey shrike-thrush | 1 (100.0) | 0 (0.0) | 0 (0.0) | ||
| Cracticus tibicen | Australian magpie | 0 (0.0) | 1 (6.3) | 15 (93.8) | ||
| Acanthiza uropygialis | Chestnut-rumped thornbill | 0 (0.0) | 0 (0.0) | 7 (100.0) | ||
| Ocyphaps lophotes | Crested pigeon | 0 (0.0) | 0 (0.0) | 2 (100.0) | ||
| Malurus assimilis | Purple-backed fairy-wren | 0 (0.0) | 0 (0.0) | 2 (100.0) | ||
| Pyrrholaemus brunneus | Redthroat | 0 (0.0) | 0 (0.0) | 2 (100.0) | ||
| Aegotheles cristatus | Australian owlet-nightjar | 0 (0.0) | 0 (0.0) | 1 (100.0) | ||
Numbers indicate the number of independent capture events (separated by at least 10 min) of each taxon detected on a warren camera recorded as interacting or passing, and the total number of capture events of each taxon on the structure cameras. Numbers in brackets correspond to percentage of total capture events for each species.
Bold = alien species.
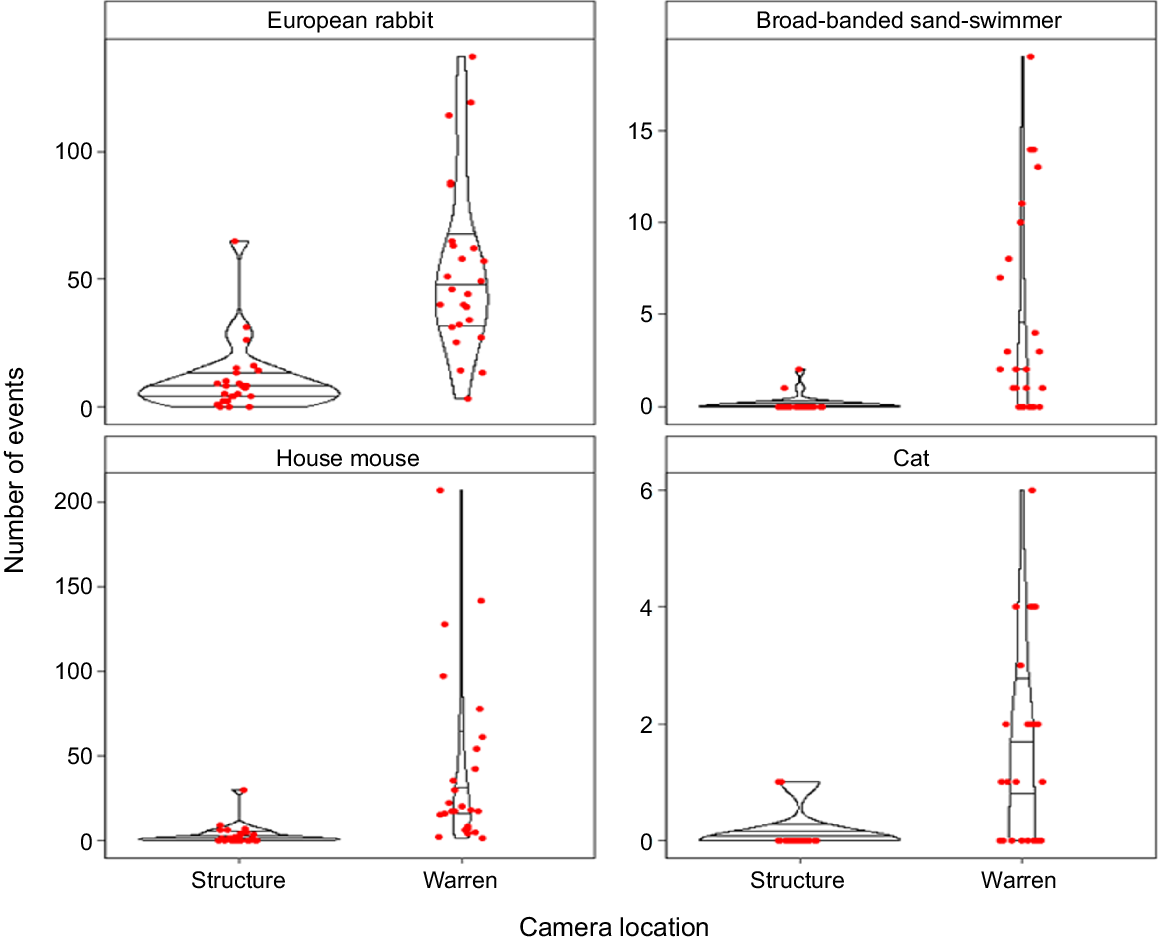
The warren creators, European rabbits, were the most frequently recorded species, with capture events on every warren camera and most structure cameras (Table 1, Fig. 3). Rabbits were more likely to be on warren cameras than structure cameras and more likely to be captured interacting with the burrow entrance than passing (Figs 4, 5).
Presence of each taxon observed on paired warren (n = 25) and structure (n = 25) camera stations within the Ikara-Flinders Ranges National Park. Species with only one capture event on warren cameras have been excluded.
Results from the multivariate abundance analysis showing the four taxa with a significant difference in detections between the structure and warren cameras.
Probability (P) of each taxon with >10 capture events interacting with the burrow entrance on cameras placed on rabbit warrens. Dotted vertical line: P = 0.5, i.e. an equal chance of interacting vs not interacting.
Ten mammal species were recorded, with nine interacting with the burrow at least once (Table 1). Of those mammals seen interacting with the burrow, all but the macropods were seen at least once entirely within the burrow entrance or in the process of entering the burrow; entering of the burrow was not quantified due to difficulty and variability of detection of animals within the burrow itself. The second-most frequently recorded species was the alien invasive species house mouse (Table 1, Fig. 6a). Feral cats (Felis catus) were also recorded, and all but one of the capture events of feral cats interacting with the burrow were of cats investigating but not physically entering the burrow (Table 1, Fig. 6b). All three macropod species recorded on camera were seen to interact with the burrow entrance, with euros (Osphranter robustsus) the most frequently recorded macropod species (Table 1, Fig. 6c). The reintroduced western quoll (Dasyurus geoffroi) was detected interacting with rabbit warrens (Table 1, Fig. 6d). Both short-beaked echidna (Tachyglossus aculeata) and dunnart species were recorded more frequently on the cameras placed on structure adjacent to the warrens. When they were recorded on the cameras on the burrow entrances on the warrens themselves, these species were more often seen interacting with than passing the burrow (Table 1, Fig. 6e).
Animals interacting with the burrow entrance of rabbit warrens in the Ikara-Flinders Ranges National Park, South Australia. (a) House mouse (Mus musculus), (b) Cat (Felis catus), (c) Euro (Osphranter robustus), (d) Western quoll (Dasyurus geofroii), (e) Short-beaked echidna (Tachyglossus aculatus), (f) Mulga snake (Pseudechis australis), (g) Broad-banded sand-swimmer (Eremiascincus richardsonii), (h) Sand goanna (Varanus gouldii) and (i) Southern whiteface (Aphelocephala leucopsis).
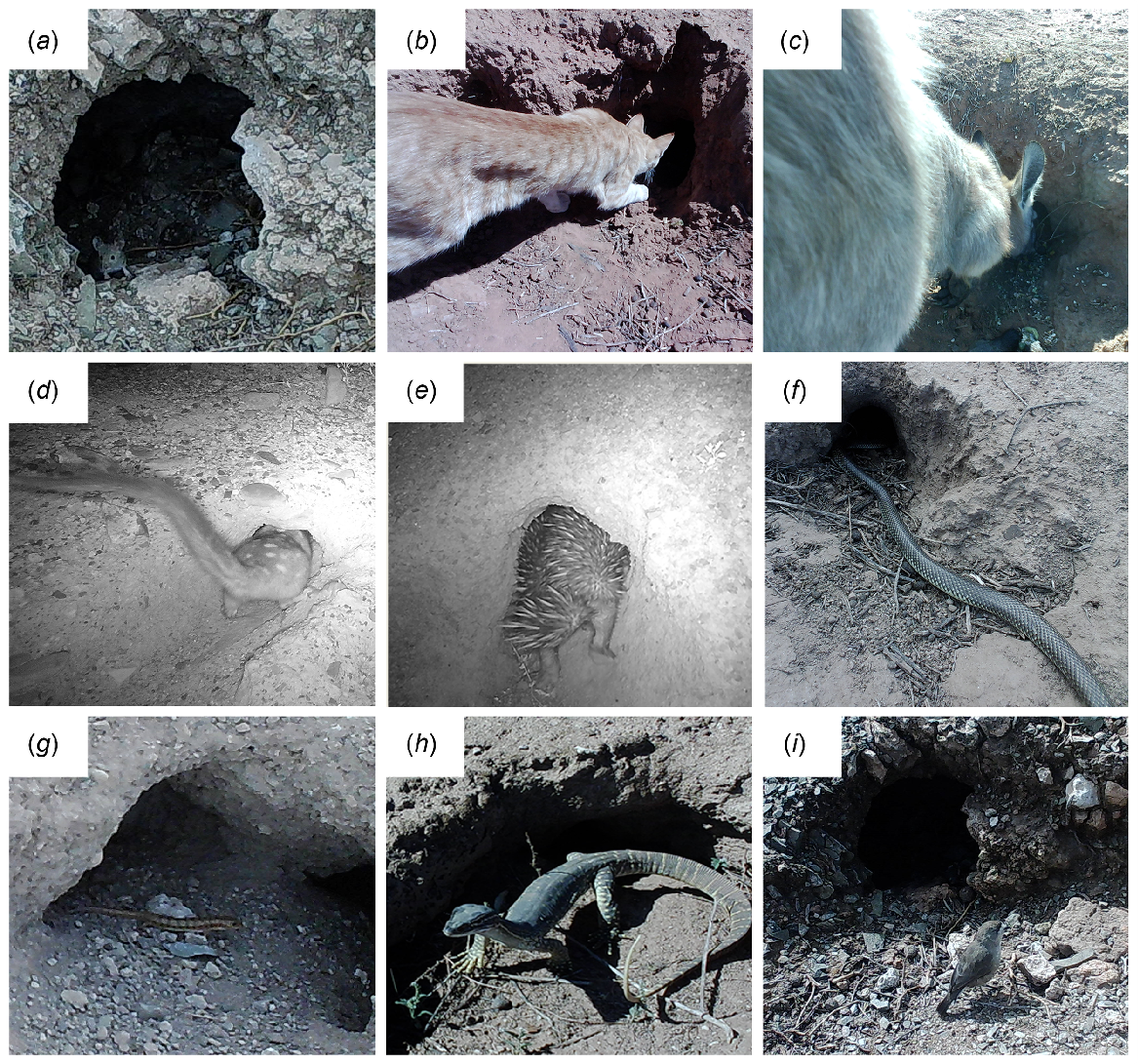
For all eight species of reptiles captured on camera, each had at least one capture event where they were interacting with the burrow (Table 1, Fig. 6f–h). Only two taxa, the broad-banded sand-swimmer (Eremiascincus richardsonii) and comb-eared skink spp., were recorded frequently, with more than 30 capture events (Table 1, Fig. 6g). Several reptiles with low capture rates on cameras, including the sand goanna and thick tailed gecko, had a greater number of records where they were interacting with the burrow than records showing the species to be passing the burrow or present on the structure camera (Table 1, Fig. 6h).
Of the 17 bird species recorded on cameras, 11 species were seen to interact with the burrow entrance (Table 1). The southern whiteface (Aphelocephala leucopsis) was the most common species of bird seen, with 41 more capture events recorded than the next-most observed species, white-browed babbler (Pomatostomus superciliosus) (Table 1, Fig. 6i).
There was wide variation in species presence across both warren and structure cameras. There was a small number of taxa recorded across most cameras and a long tail of species only recorded at five or fewer warren cameras. House mice were present at each of the 25 warrens (Fig. 3). Comb-eared skink species, though having a high number of capture events (64), were present only at seven warrens (Table 1, Fig. 3). Only seven species were recorded at greater than 10 warrens. Seven bird species were recorded at three or fewer rabbit warrens. The threatened western quoll and yellow-footed rock-wallaby (Petrogale xanthopus) were only recorded at two warrens each. Each capture of a dunnart on a warren camera was at a unique warren (Table 1, Fig. 3).
Structure vs warren cameras
Of the 19 taxa with greater than 10 capture events recorded across all cameras, only four showed differences (P < 0.05) in the detection rates between warren and structure cameras (Fig. 4). All three taxa, apart from the warren-creating rabbits (house mouse, feral cat, and broad-banded sand-swimmer), were more likely to be present on warren cameras than structure cameras, indicating these species are commensals of rabbit warrens (Fig. 4, Table S2).
Interacting vs passing the warren camera
For the 10 species with greater than 10 capture events recorded on the warren cameras, all were equally or more likely to be interacting with the warren burrows than passing the warren (Fig. 5). Cats showed an almost equal probability of interacting or passing the burrow entrance. For five of these species, while they were not more likely to be on the warren than on the structure cameras, they were more likely to be interacting with the burrow entrance than passing it when they were recorded on the warren camera (Table 1, Fig. 5). The large confidence intervals for some taxa are likely a result of the limited number of captures; however, they could also be indicative of highly variable responses.
Discussion
Rabbit warrens in the seasonally hot semi-arid zone of South Australia are visited by a wide range of animal taxa, but only a small proportion of these species prefer to use the refuge offered by the burrows (and their entrances) over refuge or structural cover provided in the adjacent vegetation. Of the four species that showed a preference for the warrens, three were invasive alien species: rabbits, house mice and feral cats. The low reliance on warrens for most of the native animals recorded may reflect the ecology of the native species or the timing of the study, in late summer and during a drought. Animal use of rabbit burrows might be different during the height of summer or the middle of winter, or indeed when the study area is not in drought. A number of animal species were recorded interacting with the warren burrow entrances, and several mammals other than rabbits were captured entering the warren, suggesting that the warrens provide an underground refuge for them. The high detection rate on the warren cameras of alien species demonstrates the importance of these subterranean refuges for these animals, including as an important source of food for feral cats in abundant rabbits and house mice.
Burrows are especially important to animals in hot environments because they provide a buffered refuge for thermoregulation (Kearney et al. 2009). Three invasive mammal species were seen to use and potentially benefit from this thermal refuge in the IFRNP: rabbits, cats and house mice. As the constructors of the warren, it is unsurprising to see such high detection on warrens of rabbits. In large parts of inland Australia, it is thought that cats would be unable to survive without access to burrows, especially those of rabbits, for at least part of the year (Briscoe et al. 2022). In this study, a cat was seen to enter the warren only on one occasion. This is possibly a result of cooler temperatures during the study period – when cats might rely less on the warren as a thermal refuge. The thermal refuge value of rabbit burrows extends to reptiles, with the nocturnal broad-banded sand-swimmer being more likely to be recorded on burrow than structure cameras. As a nocturnal species with a mean preferred body temperature in the mid-20°C, the relatively constant temperature of an underground refuge would allow this species to avoid the temperature extremes experienced above-ground (Bennett and John-Alder 1986). Any animal that enters a rabbit warren undoubtedly benefits from the thermal stability of underground burrows.
Rabbit warrens are a valuable resource for both native and introduced vertebrates in the Flinders Ranges, as indicated by the number of animals seen interacting with them. The depression in the ground at the burrow entrance accumulates organic matter and moisture, providing a food source for a range of fauna, including herbivores, but also insectivores through promoting invertebrate presence (Read et al. 2008; James et al. 2010; Chapman 2013; Desbiez and Kluyber 2013; Dawson et al. 2019). Several species of macropods, birds and lizards were observed interacting with the depression at the burrow entrance but rarely or never entering the burrow itself. This type of commensalism has been noted also at burrows of the native bilby in Western Australia (Dawson et al. 2019). Unlike the omnivorous native burrowers (bilbies and boodies), however, rabbits are grazing and browsing herbivores – and at high densities denude the low vegetation, including grasses, forbs and woody plants, in the vicinity of warrens. This will limit food for native herbivores such as euros, and may explain the focus of euros on burrow entrances. These will provide food in the form of dead wind-blown vegetation during drought conditions and germinating seedlings. Additionally, rabbit warrens are typically covered in introduced weed species, even during periods of drought when the surrounding landscape is bare, because the weeds are not grazed by most herbivores (Eldridge and Myers 2001; Cooke 2012). This may further add to both the accumulation of vegetation and moisture in burrow entrances while providing further habitat and resources. The organic matter, moisture and invertebrates in burrow entrances provide food for insectivorous birds (e.g. southern whiteface) and reptiles (e.g. broad-banded sand-swimmer; Ctenotus sp.), whether these are rabbit warrens (this study) or bilby burrows (Dawson et al. 2019). Broad-banded sand-swimmers, and other species that live within the soil or dig their own burrows, could also benefit by the vegetation collection and looser soil present at a burrow entrance allowing for easier digging and burying into the soil (Wilson and Swan 2017). The food resources accumulated by rabbit warren entrances are likely to be important to both native and introduced animals. Other structure in the landscape, though, might provide the same ecological function as warren entrances in accumulating food, as seen by the number of animals detected on our structure cameras.
Rabbit warrens provide a concentrated food source to predators. Native predators were documented using rabbit burrows in the IFRNP, albeit in low numbers, including western quolls, sand goannas (Varanus gouldii) and mulga snakes (Pseudechis australis). Quolls in the IFRNP use rabbit warrens as refuge during the day, and rabbits are a common component of their diet (Moseby et al. 2021b, 2022). The high populations of rabbits and mice within the warren similarly would also provide a centralised prey resource for snakes and goannas interacting with rabbit warrens. Alien predators like cats make use of rabbit warrens as hunting grounds. Rabbits typically comprise a large proportion of the diet of cats when they are available (Doherty et al. 2015; Murphy et al. 2019; Moseby et al. 2022). This study confirms and strengthens this knowledge, with cats more likely to be on warren than structure cameras. Warrens will provide both food and shelter to this invasive alien predator. The equal likelihood of cats passing or interacting with the burrow entrance provides further insight into cat behaviour. Cats prefer hunting in more open areas, and individual cats may show preference for certain prey species (Dickman and Newsome 2015; McGregor et al. 2015). This selectiveness of cats could extend to individual cats showing a preference for particular entrances within a rabbit warren, which provide better hunting opportunities; a topic worthy of further study. Foxes (Vulpes vulpes) have strong associations with rabbit populations elsewhere in Australia, but were not recorded on rabbit warrens in our study, being in low abundance in the IFRNP due to a successful long-term baiting program (Robley et al. 2004; Woinarski et al. 2018; Stobo-Wilson et al. 2020). Predator presence at rabbit warrens leads to increased predation pressure for animals in the local environs. This is the first study to demonstrate widespread and extensive use of rabbit warrens by house mice. House mice are a pest species in Australia that damages commercial crops, pastures and native vegetation (Witmer and Jojola 2006). House mice are an extremely resilient and adaptive invasive species that can dig their own burrows and seek shelter elsewhere in the environment (Dickman 1992; Witmer and Jojola 2006; Menkhorst and Knight 2011). House mice have similarly been observed using the burrows of boodies and southern hairy-nosed wombats (Read et al. 2008; Thornett et al. 2017). House mice probably use a wide range of refuge types in the IFRNP, and extensive destruction of warrens would have some – but probably not a definitive impact – on this pest species. No native rodents were recorded on any cameras in this study, indicative of the degraded faunal assemblage of the Flinders Ranges, particularly in drought conditions.
Warren ripping as a method of rabbit control in the IFRNP could thus impact three introduced pest species: rabbits, cats and house mice, along with those native animals present. Destruction of rabbit warrens by ripping rapidly reduces rabbit numbers, subsequently reducing feral predator presence (Edwards et al. 2002; Holden and Mutze 2002; McPhee and Butler 2010; Sharp 2012). The impact on feral predators, including foxes in other areas, could be extended if ripping happens simultaneously with predator control methods such as poison baiting. In low prey densities and when animals are starved, they are more likely to uptake bait (Christensen et al. 2013). This would help to reduce the potential for prey-switching, which could temporarily increase predation pressure on other prey individuals following warren destruction. This control method would reduce the rabbit population in the IFRNP, because it would physically destroy the burrow systems that rabbits depend on for survival (Mutze 1991). If warren destruction is coordinated over large areas, and rabbits are prevented from reopening warrens, the extended time-frame until rabbit recovery would have positive effects across many trophic levels of this semi-arid environment, through reduced rabbit grazing pressure and cat abundance (Pedler et al. 2016; Finlayson et al. 2022). Many native animals seeking refuge in a rabbit warren would undoubtedly be impacted in the destruction process (Sharp 2012). The native species observed regularly interacting with rabbit warrens in IFRNP are not threatened, are widely distributed, and utilise a variety of habitats (Menkhorst and Knight 2011; Menkhorst et al. 2017; Wilson and Swan 2017). Additionally, some native species while using warrens when they are in the environment do not necessarily rely on them, and are present at similar or higher abundance post warren destruction (Elsworth et al. 2019; Finlayson et al. 2022). There is probably a net benefit trade-off of warren destruction as a conservation tool. Widespread destruction of rabbit warrens could be detrimental for burrow-using vertebrates in the ecosystem (Read et al. 2008). In the long term, however, the removal of rabbit warrens and cascading trophic effects would be beneficial to many of the native vertebrate species. For those native species not directly using warrens for shelter, warren destruction is largely beneficial because of the associated reduction of both grazing pressure and introduced predator populations (Elsworth et al. 2019). Vegetation recovers following rabbit population reduction, providing more food resources and above-ground shelter. Native herbivores, such as euros in the IFRNP, benefit from this greater food availability, and previously have been shown to increase in population size following rabbit reduction (Edwards and Dobbie 1999). Small mammals may exhibit a decrease in population for some time following rabbit removal as feral predators switch to new prey sources, but predators eventually die or leave the area, resulting in a net increase in small mammal populations (Lurgi et al. 2018). Warren destruction is most effective when all warrens in an area are destroyed, but if rabbit warrens in certain locations were shown to be vital habitat for important native species, other methods of control such as poisoning or biocontrol could be explored (Parer and Milkovits 1994; Henzell et al. 2008). For native species only interacting with warrens, and not dependent on them for refuge, warren destruction will result in increased populations due to the net benefit of rabbit removal.
Rabbit warrens are important sites of commensalism for burrow-using species in the semi-arid Australian environment. Commensalism may arise from species directly using the warren as a refuge or by species foraging or hunting at the entrance of the warren. In an environment that has lost its native burrowing mammals, rabbits are an important ecosystem engineer in creating below-ground refuge for native vertebrates. Destruction of rabbit warrens is an effective long-term means of rabbit control but removes thermal refugia for fauna from an ecosystem. In the IFRNP, however, the predominant usage of rabbit burrows was by alien species. The reduction of these introduced species in the semi-arid IFRNP will be beneficial for native fauna and for improving ecosystem functioning.
Data availability
The data that support this study will be shared on request to the corresponding author.
Acknowledgements
We acknowledge the Adnyamathanha people, the traditional custodians of the land in this study. We also thank Alice Allington and volunteers Alex Neilson and Franziska Knappe for their help in conducting fieldwork and National Parks and Wildlife Service for ongoing support and access to park facilities.
References
Bennett AF, John-Alder H (1986) Thermal relations of some Australian skinks (Sauria: Scincidae). Copeia 1986, 57-64.
| Crossref | Google Scholar |
Briscoe NJ, McGregor H, Roshier D, Carter A, Wintle BA, Kearney MR (2022) Too hot to hunt: mechanistic predictions of thermal refuge from cat predation risk. Conservation Letters 15, e12906.
| Crossref | Google Scholar |
Burbidge A, Short J, Fuller P (2007) Relict Bettongia lesueur warrens in Western Australian deserts. Australian Zoologist 34, 97-103.
| Crossref | Google Scholar |
Bureau of Meteorology (2022) Climate Data Online. Available at http://www.bom.gov.au/climate/data/ [Accessed 1 September 2022]
Chapman TF (2013) Relic bilby (Macrotis lagotis) refuge burrows: assessment of potential contribution to a rangeland restoration program. The Rangeland Journal 35, 167-180.
| Crossref | Google Scholar |
Christensen PES, Ward BG, Sims C (2013) Predicting bait uptake by feral cats, Felis catus, in semi-arid environments. Ecological Management & Restoration 14, 47-53.
| Crossref | Google Scholar |
Cooke BD (2012) Rabbits: manageable environmental pests or participants in new Australian ecosystems? Wildlife Research 39, 279-289.
| Crossref | Google Scholar |
Davidson AD, Detling JK, Brown JH (2012) Ecological roles and conservation challenges of social, burrowing, herbivorous mammals in the world’s grasslands. Frontiers in Ecology and the Environment 10, 477-486.
| Crossref | Google Scholar |
Dawson SJ, Broussard L, Adams PJ, Moseby KE, Waddington KI, Kobryn HT, Bateman PW, Fleming PA (2019) An outback oasis: the ecological importance of bilby burrows. Journal of Zoology 308, 149-163.
| Crossref | Google Scholar |
Desbiez ALJ, Kluyber D (2013) The role of giant armadillos (Priodontes maximus) as physical ecosystem engineers. Biotropica 45, 537-540.
| Crossref | Google Scholar |
Dickman CR (1992) Predation and habitat shift in the house mouse, Mus domesticus. Ecology 73, 313-322.
| Crossref | Google Scholar |
Dickman CR, Newsome TM (2015) Individual hunting behaviour and prey specialisation in the house cat Felis catus: implications for conservation and management. Applied Animal Behaviour Science 173, 76-87.
| Crossref | Google Scholar |
Doherty TS, Davis RA, van Etten EJB, Algar D, Collier N, Dickman CR, Edwards G, Masters P, Palmer R, Robinson S, McGeoch M (2015) A continental-scale analysis of feral cat diet in Australia. Journal of Biogeography 42, 964-975.
| Crossref | Google Scholar |
Edwards GP, Dobbie W, Berman DM (2002) Warren ripping: its impacts on European rabbits and other wildlife of central Australia amid the establishment of rabbit haemorrhagic disease. Wildlife Research 29, 567-575.
| Crossref | Google Scholar |
Eldridge DJ, Myers CA (2001) The impact of warrens of the European rabbit (Oryctolagus cuniculus L.) on soil and ecological processes in a semi-arid Australian woodland. Journal of Arid Environments 47, 325-337.
| Crossref | Google Scholar |
Eldridge DJ, Simpson R (2002) Rabbit (Oryctolagus cuniculus L.) impacts on vegetation and soils, and implications for management of wooded rangelands. Basic and Applied Ecology 3, 19-29.
| Crossref | Google Scholar |
Eldridge DJ, Costantinides C, Vine A (2006) Short-term vegetation and soil responses to mechanical destruction of rabbit (Oryctolagus cuniculus L.) warrens in an Australian box woodland. Restoration Ecology 14, 50-59.
| Crossref | Google Scholar |
Elsworth P, Berman D, Brennan M (2019) Changes in small native animal populations following control of European rabbits (Oryctolagus cuniculus) by warren ripping in the Australian arid zone. Wildlife Research 46, 343-354.
| Crossref | Google Scholar |
Finlayson HH (1958) On Central Australian mammals (with notice of related species from adjacent tracts): part III the potoroinae. Records of the South Australian Museum 13, 235-302.
| Google Scholar |
Finlayson G, Taggart P, Cooke B (2022) Recovering Australia’s arid-zone ecosystems: learning from continental-scale rabbit control experiments. Restoration Ecology 30, e13552.
| Crossref | Google Scholar |
Fleming PA, Anderson H, Prendergast AS, Bretz MR, Valentine LE, Hardy GES (2014) Is the loss of Australian digging mammals contributing to a deterioration in ecosystem function? Mammal Review 44, 94-108.
| Crossref | Google Scholar |
Henzell RP, Cooke BD, Mutze GJ (2008) The future biological control of pest populations of European rabbits, Oryctolagus cuniculus. Wildlife Research 35, 633-650.
| Crossref | Google Scholar |
Hofstede L, Dziminski MA (2017) Greater bilby burrows: important structures for a range of species in an arid environment. Australian Mammalogy 39, 227-237.
| Crossref | Google Scholar |
Holden C, Mutze G (2002) Impact of rabbit haemorrhagic disease on introduced predators in the Flinders Ranges, South Australia. Wildlife Research 29, 615-626.
| Crossref | Google Scholar |
Jackson D, Milstrey E (1989) The fauna of gopher tortoise burrows. In ‘Proceedings of the Gopher Tortoise Relocation Symposium’. (Eds JE Diemer, DR Jackson, JL Landers, JN Layne, DA Wood) pp. 86–98. (Florida Game and Fresh Water Fish Commission, Nongame Wildlife Program, Technical Report No. 5, Tallahassee, FL, USA)
James AI, Eldridge DJ (2007) Reintroduction of fossorial native mammals and potential impacts on ecosystem processes in an Australian desert landscape. Biological Conservation 138, 351-359.
| Crossref | Google Scholar |
James AI, Eldridge DJ, Moseby KE (2010) Foraging pits, litter and plant germination in an arid shrubland. Journal of Arid Environments 74, 516-520.
| Crossref | Google Scholar |
James AI, Eldridge DJ, Koen TB, Moseby KE (2011) Can the invasive European rabbit (Oryctolagus cuniculus) assume the soil engineering role of locally-extinct natives? Biological Invasions 13, 3027-3038.
| Crossref | Google Scholar |
Jones CG, Lawton JH, Shachak M (1994) Organisms as ecosystem engineers. Oikos 69, 373-376.
| Crossref | Google Scholar |
Kearney M, Shine R, Porter WP (2009) The potential for behavioral thermoregulation to buffer “cold-blooded” animals against climate warming. Proceedings of the National Academy of Sciences 106, 3835-3840.
| Crossref | Google Scholar |
Lurgi M, Ritchie EG, Fordham DA, Frair J (2018) Eradicating abundant invasive prey could cause unexpected and varied biodiversity outcomes: the importance of multispecies interactions. Journal of Applied Ecology 55, 2396-2407.
| Crossref | Google Scholar |
Lynch C, Brandle R (2018) What impact do cats have on small vertebrates in IFRNP. Journal of the Scientific Expedition Group Inc. 34, 9-10.
| Google Scholar |
McGregor H, Legge S, Jones ME, Johnson CN (2015) Feral cats are better killers in open habitats, revealed by animal-borne video. PLoS ONE 10, e0133915.
| Crossref | Google Scholar |
McPhee SR, Butler KL (2010) Long-term impact of coordinated warren ripping programmes on rabbit populations. Wildlife Research 37, 68-75.
| Crossref | Google Scholar |
Moseby K, Hodgens P, Bannister H, Mooney P, Brandle R, Lynch C, Young C, Jansen J, Jensen M (2021a) The ecological costs and benefits of a a feral cat poison-baiting programme for protection of reintroduced populations of the western quoll and brushtail possum. Austral Ecology 46, 1366-1382.
| Crossref | Google Scholar |
Moseby KE, Hodgens P, Peacock D, Mooney P, Brandle R, Lynch C, West R, Young CM, Bannister H, Copley P, Jensen MA (2021b) Intensive monitoring, the key to identifying cat predation as a major threat to native carnivore (Dasyurus geoffroii) reintroduction. Biodiversity and Conservation 30, 1547-1571.
| Crossref | Google Scholar |
Moseby KE, Jensen MA, Tatler J (2022) Dietary flexibility and high predator efficacy facilitate coexistence in a novel predator interaction. Journal of Mammalogy 103, 124-135.
| Crossref | Google Scholar |
Mukherjee A, Pal A, Velankar AD, Kumara HN, Bhupathy S (2019) Stay awhile in my burrow! Interspecific associations of vertebrates to Indian crested porcupine burrows. Ethology Ecology & Evolution 31, 313-328.
| Crossref | Google Scholar |
Murphy BP, Woolley L-A, Geyle HM, Legge SM, Palmer R, Dickman CR, Augusteyn J, Brown SC, Comer S, Doherty TS, Eager C, Edwards G, Fordham DA, Harley D, McDonald PJ, McGregor H, Moseby KE, Myers C, Read J, Riley J, Stokeld D, Trewella GJ, Turpin JM, Woinarski JCZ (2019) Introduced cats (Felis catus) eating a continental fauna: the number of mammals killed in Australia. Biological Conservation 237, 28-40.
| Crossref | Google Scholar |
Mutze GJ (1991) Long-term effects of warren ripping for rabbit control in semi-arid South Australia. The Rangeland Journal 13, 96-106.
| Crossref | Google Scholar |
Niedballa J, Sollmann R, Courtiol A, Wilting A (2016) camtrapR: an R package for efficient camera trap data management. Methods in Ecology and Evolution 7, 1457-1462.
| Crossref | Google Scholar |
Noble JC, Müller WJ, Detling JK, Pfitzner GH (2007) Landscape ecology of the burrowing bettong: Warren distribution and patch dynamics in semiarid eastern Australia. Austral Ecology 32, 326-337.
| Crossref | Google Scholar |
Parer I, Milkovits G (1994) Recolonisation by rabbits (Oryctolagus cuniculus) after warren ripping or warren fumigation. The Rangeland Journal 16, 51-63.
| Crossref | Google Scholar |
Pedler RD, Brandle R, Read JL, Southgate R, Bird P, Moseby KE (2016) Rabbit biocontrol and landscape-scale recovery of threatened desert mammals. Conservation Biology 30, 774-782.
| Crossref | Google Scholar |
Read JL, Carter J, Moseby KM, Greenville A (2008) Ecological roles of rabbit, bettong and bilby warrens in arid Australia. Journal of Arid Environments 72, 2124-2130.
| Crossref | Google Scholar |
Sharp T (2012) Rabbit warren destruction by ripping. Standard Operating Procedure. Available at https://pestsmart.org.au/toolkit-resource/rabbit-warren-destruction-by-ripping/ [Accessed 4 January 2022]
Stobo-Wilson AM, Brandle R, Johnson CN, Jones ME (2020) Management of invasive mesopredators in the Flinders Ranges, South Australia: effectiveness and implications. Wildlife Research 47, 720-730.
| Crossref | Google Scholar |
Thornett E, Ostendorf B, Taggart DA (2017) Interspecies co-use of southern hairy-nosed wombat (Lasiorhinus latifrons) burrows. Australian Mammalogy 39, 205-212.
| Crossref | Google Scholar |
Wang Y, Naumann U, Wright ST, Warton DI (2012) mvabund – an R package for model-based analysis of multivariate abundance data. Methods in Ecology and Evolution 3, 471-474.
| Crossref | Google Scholar |
Woinarski JCZ, Burbidge AA, Harrison PL (2015) Ongoing unraveling of a continental fauna: decline and extinction of Australian mammals since European settlement. Proceedings of the National Academy of Sciences 112, 4531-4540.
| Crossref | Google Scholar |
Woinarski JCZ, South SL, Drummond P, Johnston GR, Nankivell A (2018) The diet of the feral cat (Felis catus), red fox (Vulpes vulpes) and dog (Canis familiaris) over a three-year period at Witchelina Reserve, in arid South Australia. Australian Mammalogy 40, 204-213.
| Crossref | Google Scholar |


