Understanding the zoonotic pathogen, Coxiella burnetii in Australian fur seal breeding colonies through environmental DNA and genotyping
Brett R. Gardner A * , John P. Y. Arnould B , Jasmin Hufschmid A , Rebecca R. McIntosh C , Aymeric Fromant
A * , John P. Y. Arnould B , Jasmin Hufschmid A , Rebecca R. McIntosh C , Aymeric Fromant  B D , Mythili Tadepalli E and John Stenos E
B D , Mythili Tadepalli E and John Stenos E
A Melbourne Veterinary School, The University of Melbourne, Werribee Vic. 3030, Australia.
B School of Life and Environmental Sciences, Deakin University, Burwood, Vic. 3125, Australia.
C Conservation Department, Philip Island Nature Parks, PO Box 97, Cowes, Vic. 3922, Australia.
D Centre d’Etudes Biologiques de Chizé (CEBC), UMR 7372 CNRS-La Rochelle Université, Villiers-en-Bois 79360, France.
E Australian Rickettsial Reference Laboratory, University Hospital Geelong, Bellerine Street, Geelong, Vic. 3220, Australia.
Wildlife Research 50(10) 840-848 https://doi.org/10.1071/WR22136
Submitted: 6 August 2022 Accepted: 29 November 2022 Published: 20 December 2022
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Coxiella burnetii is suspected as a novel pathogen contributing to decreased pup production in Australian fur seals (Arctocephalus pusillus doriferus). It has recently been described from a single breeding colony in Bass Strait, has previously been associated with two decreasing populations of northern hemisphere pinnipeds and is a known reproductive pathogen. Data around its disease ecology in marine mammals are sparse.
Aims: To determine whether environmental DNA (eDNA) can be used to survey for C. burnetii in Australian fur seal breeding colonies. To determine whether C. burnetii in Australian fur seals is the same genotype as terrestrial Australian C. burnetii.
Methods: Soil samples were collected from Kanowna Island and Seal Rocks. Placental samples were collected from Kanowna Island. Soil was evaluated for eDNA using a quantitative polymerase chain reaction (qPCR) for com1 gene. Placental samples were evaluated with com1, htpAB and IS1111 markers. Multiple-locus variable number of tandem repeats analysis for three microsatellite loci (ms-24, ms-28 and ms-33) was used to determine relatedness to Australian C. burnetii genotypes.
Key results: eDNA results varied between pre-and post-pupping at Seal Rocks. When targeting the com1 gene, the post-pupping prevalence at Kanowna Island and Seal Rocks was 59.6% and 90%, respectively. eDNA PCR inhibition of samples was low at 1.9%. There was very poor, sporadic to absent IS1111 amplification in placental samples. The com1 and htpAB qPCRs had an overall prevalence across placental samples of 39.2% and 56.7% respectively. In 90.1% of placental samples (n = 11), the ms-28 locus amplified. Neither ms-24 nor ms-33 amplified.
Conclusions: eDNA is an effective tool to survey Australian fur seal breeding colonies in the post-pupping period for C. burnetii. The prevalence appears to be much higher in the Seal Rocks colony than in the Kanowna Island colony. It appears that this is not a terrestrial Australian genotype but rather closely related to genotypes detected in marine mammals in the northern hemisphere.
Implications: This research significantly expands our ability to survey for C. burnetii in Australian fur seals and other marine mammals. It highlights knowledge gaps in our understanding of the disease ecology and phylogeny of C. burnetii in marine mammals.
Keywords: breeding biology, conservation ecology, disease, ecosystem health, epidemiology, fertility, infectious disease, islands, microbiology, reproduction.
Introduction
Coxiella burnetii is a common infectious cause of reproductive failure in terrestrial mammals, including many species of wildlife (González-Barrio and Ruiz-Fons 2019). It is a known zoonotic pathogen presenting as Q-fever in human patients, with occasional human fatalities (Bond et al. 2018). Although a common differential diagnosis for reproductive failure in terrestrial mammals (Agerholm 2013), it has only in recent years been associated with declining populations of two species of pinnipeds in the northern hemisphere (Minor et al. 2013). It has only very recently been described in marine mammals in the southern hemisphere in Australian fur seals (Arctocephalus pusillus doriferus) (Gardner et al. 2022).
The Australian fur seal has had a slower than expected recovery after a cessation of commercial harvest and has shown decreased pup numbers in some key breeding colonies (McIntosh et al. 2022). A large percentage of Australian fur seal placentas from the third largest breeding colony for the species have both molecular and histopathological evidence of C. burnetii (Gardner et al. 2022). It is currently unknown whether this C. burnetii is related to a terrestrial spillover (Gardner et al. 2022) or a unique marine mammal-adapted genotype as previously postulated for northern fur seals (Callorhinus ursinus) (Duncan et al. 2013).
The remote offshore location and inaccessible nature of Australian fur seal colonies during their breeding season make it difficult to collect appropriate tissue samples to survey for the presence of C. burnetii in breeding colonies. The presence of large numbers of highly territorial males and densely concentrated pups makes sampling placental tissues risky to both researchers and animals. In ruminants, C. burnetii multiplies extensively to very high numbers within the trophoblast of the placenta. These bacterial organisms convert into a highly resistant form once expelled into the environment, producing significant environmental C. burnetii contamination (Roest et al. 2012). This form of the bacteria is known as the small-cell variant (SCV) and is the most infective, often being transmitted as an aerosol in dust from contaminated soil (Abeykoon Mudiyanselage et al. 2021a). It has, thus, been noted that soil samples as a source of environmental DNA (eDNA) can be used to determine the presence of C. burnetii in ruminant herds, especially goats, that have had a history of abortion (de Bruin et al. 2013).
Environmental DNA has also been used in declining populations of northern fur seals in Alaska to survey for C. burnetii (Duncan et al. 2013). Soil is the only eDNA sample for which an extraction process has been validated to determine the presence of C. burnetii (Abeykoon Mudiyanselage et al. 2021a). Extensive work is required to gain a better understanding of this organism in marine environments as data are considerably sparse in marine mammals (Duncan et al. 2013).
The aims of this study where to evaluate whether eDNA could be used to survey for C. burnetii in Australian fur seal breeding colonies. Additionally, whether C. burnetii in Australian fur seals is related to typical terrestrial Australian genotypes of the organism. Australian fur seals have a high breeding-site fidelity, with a short, concentrated pupping season (90% of births occurring over ~27 days; Geeson et al. 2022) and the peak of pupping occurring in late November. In the present study, it was hypothesised that this concentrated production of birthing materials could potentially result in high levels of environmental contamination and that it would therefore be possible to utilise eDNA to survey for the presence of C. burnetii in Australian fur seal breeding colonies. Further, it was expected that if this C. burnetii was related to the typical terrestrial Australian genotype, it would share the multiple-locus variable number of tandem repeats analysis (MVLA; Vincent et al. 2016). Additionally, it would have a readily identifiable IS1111, an insertion which is presumed absent or poorly represented in C. burnetii from northern hemisphere marine mammals (Duncan et al. 2012).
Materials and methods
Ethical treatment was ensured and complied with welfare requirements as per University of Melbourne Animal Ethics Committee approval for scavenged animal tissues, Approval number 19-009, Deakin University Animal Ethics Committee Approval B05-2020 under a Department of Environment, Land, Water and Planning wildlife research Permit 10009465, Phillip Island Nature Parks Animal Ethics Committee Approval 2-2019 under a Department of Environment, Land, Water and Planning wildlife research Permit 10009034.
Field sampling
The study was conducted at Seal Rocks (38°30′S, 145°10′E) and Kanowna Island (39°15′S, 146°30′E) in northern Bass Strait, south-eastern Australia (Fig. 1). A 50 mL conical Falcon tube was used to scrape through the surface substrate and filled with a mixture of gravel and sand, with occasional organic contaminants such as fur. Obvious fecal contamination was avoided. Samples were collected in known birthing areas from the outside of the colony inwards, to prevent accidental contamination during the sampling process. Several linear transects were collected with samples collected roughly once every 5–10 m, depending on the terrain and substrate. The location of each sample was logged via GPS. These are indicated in Figs 2–4. Soil samples were collected from Seal Rocks in October (n = 17) and December (n = 30) 2021. Soil samples were collected from Kanowna Island throughout November and December (n = 52) 2021. Australian fur seals are present at both breeding colonies throughout the year. The October samples were considered pre-pupping and samples collected in November and December considered post-pupping samples.
Full term placentas were collected during the peak of pupping from Kanowna Island throughout November and December, in both 2020 (n = 66) and 2021 (n = 54). Multiple small squares of placental tissue were collected with sterile instruments from the internal aspect of the opened placenta, avoiding any obvious gross contamination and then stored frozen at −18°C. These included samples previously analysed (Gardner et al. 2022) that were re-analysed to include additional polymerase chaing reaction (PCR) markers.
DNA extraction
Soil samples were pre-processed and extracted according to established, validated techniques (Kersh et al. 2012; Abeykoon Mudiyanselage et al. 2021b). Five grams of soil were mixed for each sample with 10 mL phosphate-buffered saline (PBS) and then incubated for 60 min at ambient room temperature on a rocking table. The sample was then centrifuged at 500g for 3 min at room temperature. All samples were centrifuged at room temperature. The supernatant was transferred to a fresh tube and centrifuged at 9500g for 30 min. The supernatant was discarded, and the pellet then resuspended in 0.5 mL PBS. A 200 µL aliquot of resuspended pellet was added to 1.4 mL of PBS and incubated at 95°C for 5 min. Subsequently, the sample was vortexed for 15 s and then centrifuged at 14 000g for 1 min. Of the supernatant, 200 µL was used with a HiYield Genomic DNA Mini Kit (Real Biotech Corporation, Banqiao City, Taiwan) as per the manufacturer’s details. Soil samples were extracted in duplicate, including a negative extraction control.
Genomic DNA was extracted and purified from frozen placental tissue by using a HiYield Genomic DNA Mini Kit (Real Biotech Corporation, Banqiao City, Taiwan) as per the manufacturer details from a total weight of 250–350 µg of placental tissue.
Quantitative polymerase chain reaction (qPCR)
For soil samples only the com1 target was used as the com1 qPCR assay has been validated for use on soil sample eDNA (Kersh et al. 2012; Abeykoon Mudiyanselage et al. 2021b). Additionally, it has been previously described that marine mammal strains of C. burnetii have a poorly identifiable or complete lack of the IS1111 insertion (Duncan et al. 2013). To determine whether environmental samples had the presence of PCR inhibitors, the samples were spiked with purified Listeria innocua DNA, targeting the lin02483 gene (Rodríguez-Lázaro et al. 2004). A duplex semi-quantitative PCR was run using the HEX and FAM fluorescent dye-tagged PCR primers for com1 and Listeria respectively. If there was failure to amplify using the Listeria primers or an increase of three times the Ct value of the controls, samples were considered to have had PCR inhibition. Samples were run in duplicate and both samples had to return similar Ct values for a sample to be considered positive for eDNA.
Placental samples were tested through three different qPCR techniques. The targets were the com1 and htpAB genes and the IS1111 insertion. The same qPCR technique was used as had been employed by researchers in the initial detection of C. burnetii in Australian fur seals (Gardner et al. 2022) other than the addition of the IS1111 insertion as a marker. IS1111 markers are based on previously described techniques (Schneeberger et al. 2010). All three markers were semi-quantified using real-time TaqMan PCR (qPCR) assays using the proprietary Invitrogen Platinum Quantitative PCR SuperMix-UDG (Thermo Fisher Scientific, Waltman, Massachusetts, United States). Details of all primers and assays used for placental tissue and eDNA C. burnetii detection are summarised in Table 1. All primers and probes were synthesised by Integrated DNA technologies. The positive control, Nine Mile Phase II, Clone 4 (RSA439) was obtained after repeated passage in vero cells was used as positive control.
Multiple-locus variable number of tandem-repeats analysis
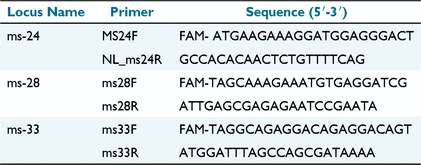
Multiple-locus variable number of tandem-repeats analysis (MLVA) for three microsatellite loci was evaluated. Microsatellite loci ms-24, ms-28 and ms-33 were used as these have previously been determined as specific to Australian C. burnetii genotypes (Vincent et al. 2016). Details of the locus and primers are summarised in Table 2. Eleven samples with good-quality pure DNA as determined using a Nanodrop Spectrophotometer (Thermo Fisher Scientific) with low Ct values were selected and processed according to a published validated technique (Arricau-Bouvery et al. 2006) with modifications according to (Tilburg et al. 2012; Vincent et al. 2016). All three primers were supplied by Invitrogen (Thermo Fisher Scientific).
|
Statistical analysis
Confidence intervals (95%) were calculated using Minitab statistical software (Minitab 21, Minitab LLC).
Results
Using a Fisher’s exact test, there was a significant (P < 0.001, CI 95%) seasonal difference in eDNA from the Seal Rocks colony, with only 5.9% (95% CI 0.1–28.7) of pre-pupping samples testing positive, compared with 90% (95% CI 73.5–97.9) of post-pupping samples. The post-pupping samples from Kanowna Island had a prevalence of 59.6% (95% CI 45.1–73.0). Comparing the post-pupping results between Kanowna Island and Seal Rocks with a two proportions test, there was an estimated 30.3% (P = 0.001, 95% CI 13.3–47.5) difference in the environmental prevalence. PCR inhibition was seen in 1.9% (95% CI 0.04–10.2) of all samples. Cycle thresholds (Ct) varied considerably across eDNA samples, with a range of 27.5–38.28. Pre-pupping sample sites from Seal Rocks are shown in Fig. 2. Locations of post-pupping sampling sites, including eDNA results, are shown in Figs 3 and 4 for Kanowna Island and Seal Rocks respectively.
All placental samples were tested for com1, htpAB and IS1111 amplification in both the 2020 and 2021 sample sets to determine placental prevalence of C. burnetii. The results are summarised in Table 3. Overall, IS1111 amplification was absent, with only very poor sporadic amplification, considered to be amplicon contamination. Both com1 and htpAB prevalence was higher in 2021 than in 2020 (P < 0.001). Overall, a total of 39.2% (95% CI 30.3–48.0) of placentas tested positive on com1 and 56.7% (95% CI 47.7–65.7) on htpAB. Across both sampling seasons, a total of 35% (95% CI 26.3–43.7) of placentas tested positive on both com1 and htpAB. On both the com1 and htpAB, qPCR Ct values were obtained, ranging from 21.17 to 38.45 and from 21.37 to 38.51 respectively.

|
Of the 11 samples evaluated for the three MLVA microsatellite loci, 10 samples amplified. In these 10 samples, only ms-28 amplified with no detectable amplification of ms-24 or ms-33.
Discussion
From this study it is evident that C. burnetii is present in more than one Australian fur seal breeding colony and that eDNA can be successfully employed to survey colonies for the presence of C. burnetii. The study has gathered additional data to indicate that this is a marine-adapted genotype that is molecularly quite different from the typical terrestrial genotypes present in Australia.
Environmental DNA: topography
The prevalence of positive eDNA samples varied between the two colonies. There was more extensive post-pupping environmental contamination with C. burnetii on Seal Rocks (90%) than Kanowna Island (59.6%). The reason for this could be linked to topography. On Kanowna Island, there are large areas that are not used as breeding sites by females. Contrary to this, the smaller size and much flatter topography of Seal Rocks means that females pup over much of the available terrain, potentially resulting in a wider spread of contamination. Two of the three negative post-pupping eDNA samples from Seal Rocks were obtained from inaccessible plateaux where pupping does not occur, immediately adjacent to high-density pupping areas immediately below (Fig. 4). Interestingly, the bachelor male haul-out on Kanowna Island, where no pupping occurs, the only sample site outside of the demarcated pupping areas on Fig. 2, had no detectable C. burnetii eDNA. It is currently unknown what role males play in the epidemiology of C. burnetii in Australian fur seals but most likely the absence of infected pupping material at this site has led to an absence of obvious environmental contamination. The prevalence of C. burnetii-positive samples in the present study was higher than what has been recorded from environmental samples in Australia more broadly. Such data are sparse, but rates of 2–7% have been recorded using soil and dust samples across wide geographic regions of Queensland (Tozer et al. 2014). However, those samples were collected without any bias, whereas the samples collected from Kanowna Island and Seal Rocks were selected predominantly from known pupping areas.
On Kanowna Island, samples collected towards the periphery of the colony had higher Ct values and a decreased prevalence of C. burnetii-positive samples. In contrast, there did not appear to be any difference among sample sites across the Seal Rocks colony. The Kanowna Island findings are consistent with a study describing C. burnetii outbreaks at a number of goat farms in Washington and Montana in the United States (Duncan et al. 2013). More than 70% of samples tested in that study had a positive qPCR from soil samples, but it was found that the samples had a rapid decline in positivity when moving from the birthing areas to the periphery. The breeding areas on Kanowna Island are funnel shaped, compared with the flatter terrain on Seal Rocks. This could potentially result in a lower C. burnetii exposure for females and pups occupying the periphery of the colony. The highest density of birthing products is produced in the central areas, where placentas also had lower Ct values. This may simply result in greater levels of contamination of other placentas. Alternatively, there could be cumulative seasonal environmental contamination leading to a build-up overtime, or potentially placentas from these areas have an increased C. burnetii load. Whether pups born into these areas are more likely to have been exposed to higher levels of C. burnetii in utero is unknown. This could perhaps result in an adverse outcome compared with pups born into or from areas of lower pup density.
Environmental DNA: seasonality
From the results of the present study, it appears that there is a seasonal variation of C. burnetii eDNA in Australian fur seal colonies. The pre-breeding season prevalence from Seal Rocks was low and the authors suspect that this might be due to extensive washout of pupping areas by strong storm action that had occurred in the preceding months. Environmental sampling has been shown to be affected by variations in meteorological conditions (de Bruin et al. 2013). The study is limited through by not having pre-pupping samples from Kanowna Island for comparison with Seal Rocks. There was increased positivity of samples collected in December compared with November, indicating that ideal sampling would be conducted at the close of the pupping season.
Environmental DNA: proximity to pupping areas
On both Kanowna Island and Seal Rocks, sampling was biased towards areas associated with pupping, resulting in high levels of C. burnetii eDNA being detected. Environmental shedding of C. burnetii in endemically infected goat herds within Australia has shown that despite the significant environmental contamination immediately post-kidding, the detectable eDNA appears to remain within close proximity to areas associated with the birthing process (Abeykoon Mudiyanselage et al. 2021a). It is likely though that environmental conditions on a goat farm are significantly less harsh than at Australian fur seal breeding colonies, potentially allowing for increased persistence of environmental contamination at terrestrial sites. The prevalence of positive com1 eDNA samples within northern fur seal colonies also appeared to be associated with known pupping areas (Duncan et al. 2013). This appears to be quite similar for pupping areas on both Kanowna Island and Seal Rocks, although a gradient between these and surrounding areas has not been determined in the present study. The more consistent nature of eDNA across the two colonies studied aligns with the presence of an endemic pathogen rather than a disease outbreak as eDNA has been found to be poorly and inconsistently detectable, both spatially and temporally in cases of the latter (Abeykoon Mudiyanselage et al. 2021a).
Comparing post-pupping samples, the present study found a higher environmental prevalence (59.6% Kanowna Island, 90% Seal Rocks) than the only other eDNA study in pinnipeds, which reported a 10.7% prevalence in post-pupping in a northern fur seal breeding colony (Duncan et al. 2013). It is possible that this difference is due to the sampling methodology of the present study. Sampling was targeted at known areas of pupping, using a consistently reliable sampling technique of collecting soil rather than swabs. Comparatively, Duncan et al. (2013) sampled pupping areas with swabs during the breeding season, which were found to be less effective. Additionally, the sampling of non-seal associated areas would decrease the prevalence despite sampling those areas with soil rather than swabs, making comparison of data difficult. The practice of collecting large volumes of soil (50 mL) rather than swabs, where possible avoiding contamination with feces and other obvious macroscopic biological contamination potentially contributed further to a very low level of PCR inhibition in the present study. However, the pre-pupping prevalence at Seal Rocks was much lower (5.9%) than the 67% detected prevalence for the northern fur seals (Duncan et al. 2013). Both study sites used soil for the pre-pupping sampling, so potentially the topography and meteorological conditions may have contributed to this variance. Unfortunately, no similar data are readily available for the declining Steller sea lion (Eumetopias jubatus) population to make comparisons with the environmental loads present in this declining species.
Placental PCRs
Of the placentas collected at Kanowna Island in 2020, only 10.6% were positive for C. burnetii on both com1 and htpAB compared with 64.8% for those in 2021. In the 2021 samples, the number of samples that returned a positive result on both markers was similar to the number of positive samples for each primer (Table 3). It is unknown why the results between the com1 and htpAB differ in the 2020 samples. The difference in prevalence between placentas sampled in 2021 and those in 2020 could be related to sampling location, rather than representing true temporal variation. During the 2020 collection season, a greater proportion of placentas were collected from the periphery of the colonies and prior to the peak of the pupping season, whereas, in 2021, a greater proportion of the placentas were collected from the main pupping areas during the peak of pupping. At present, with sampling being limited to 2 years, it is not possible to determine any temporal variation or whether the detected prevalence indicates a high level of normal-background C. burnetii environmental contamination or is related to a recent significant C. burnetii event in the population.
MLVA and IS1111
The limited amplification of the three microsatellites (only ms-28), and the poor to absent amplification of insertion sequence IS1111 do not match with the typical Australian terrestrial C. burnetii genotype (Vincent et al. 2016), suggesting that the Australian fur seal genotype is not directly related to it. It is necessary to determine the phylogeny of this C. burnetii genotype to evaluate its potential contribution to decreased reproductive success in Australian fur seals and other marine mammals. It has been shown that certain genotypes that have higher representation of the plasmid QpDV are associated with higher rates of fetal morbidity in humans (Angelakis et al. 2013) and it is currently unknown whether the C. burnetii in Australian fur seals encodes for this plasmid. However, the lack of IS1111 amplification is similar to that in northern fur seals, and appears to be a feature of C. burnetii in marine mammals (Duncan et al. 2012). Additional molecular work is required to fully understand C. burnetii in marine mammal disease ecology.
Conclusions
From the results of the present study, eDNA is a useful tool to survey for the presence of C. burnetii in Australian fur seal colonies in the period soon after the peak of pupping. The breeding behaviour of this species creates the ideal conditions for this approach. They congregate during the breeding season and have 90% of all pups born in a very narrow time span (Geeson et al. 2022). Collecting eDNA samples post-pupping is less of a risk to breeding females, their pups and researchers than is sampling during the breeding season and sampling for actual placental tissue. It is safer after the breeding season, once large territorial bulls have dispersed from the breeding colonies and pups become less vulnerable as they increase in age. This non-invasive technique can be utilised to survey additional breeding colonies of Australian fur seals and other pinniped species that congregate during pupping, which are more difficult to routinely access. Only a single visit to a colony post-breeding is required, and samples are easy to collect, store and process.
The exact significance of C. burnetii in Australian fur seal breeding colonies is not fully understood. It appears that the organism does not originate from terrestrial spillover but is rather closely related to genotypes of C. burnetii identified in northern hemisphere marine mammals. Additional molecular data are essential to compare typical known pathogenic and zoonotic genotypes to what is thought to be a marine mammal-adapted genotype. Further work is required to investigate the disease ecology and significance of C. burnetii in Australian fur seals and other marine mammals. It is currently associated with three marine mammals that have in certain breeding colonies shown population declines or decreases in pup production (Minor et al. 2013; McIntosh et al. 2022). Understanding this potential pathogen will allow for better management of the risks to vulnerable marine mammals and the zoonotic concerns for people working in proximity of these animals.
Data availability
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Conflicts of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflicts of interest.
Declaration of funding
This research was conducted without any specific reportable funding.
Acknowledgements
This work would not have been possible without the great piloting skills of Sean Best and the brilliant skippering of the Victorian Fisheries Authority officers who ensured that the field sites could be accessed despite difficult weather windows and ongoing COVID-19 restrictions. Sincere thanks go to Hasanthi Abeykoon who shared her environmental DNA knowledge. Also, a very big thank you goes to Hanna Geeson, Yonina Eizenberg, Brooke Johnston and Axel Dankwort for their assistance in collecting samples. Our sincere thanks go to Dr Steven Graves for his ongoing support of our research.
References
Abeykoon Mudiyanselage, HA, Clark, NJ, Soares Magalhaes, RJ, Vincent, GA, Stevenson, MA, Firestone, SM, and Wiethoelter, AK (2021a). Coxiella burnetii in the environment: a systematic review and critical appraisal of sampling methods. Zoonoses and Public Health 68, 165–181.| Coxiella burnetii in the environment: a systematic review and critical appraisal of sampling methods.Crossref | GoogleScholarGoogle Scholar |
Abeykoon Mudiyanselage, HA, Hou, K, Vincent, GA, Stevenson, MA, Firestone, SM, and Wiethoelter, A (2021b). Environmental contamination of Coxiella burnetii in and around an endemically infected goat farm. International Journal of Epidemiology 50, dyab168.007.
| Environmental contamination of Coxiella burnetii in and around an endemically infected goat farm.Crossref | GoogleScholarGoogle Scholar |
Agerholm, JS (2013). Coxiella burnetii associated reproductive disorders in domestic animals: a critical review. Acta Veterinaria Scandinavica 55, 13.
| Coxiella burnetii associated reproductive disorders in domestic animals: a critical review.Crossref | GoogleScholarGoogle Scholar |
Angelakis, E, Million, M, D’Amato, F, Rouli, L, Richet, H, Stein, A, Rolain, J-M, and Raoult, D (2013). Q fever and pregnancy: disease, prevention, and strain specificity. European Journal of Clinical Microbiology & Infectious Diseases 32, 361–368.
| Q fever and pregnancy: disease, prevention, and strain specificity.Crossref | GoogleScholarGoogle Scholar |
Arricau-Bouvery, N, Hauck, Y, Bejaoui, A, Frangoulidis, D, Bodier, CC, Souriau, A, Meyer, H, Neubauer, H, Rodolakis, A, and Vergnaud, G (2006). Molecular characterization of Coxiella burnetii isolates by infrequent restriction site-PCR and MLVA typing. BMC Microbiology 6, 38.
| Molecular characterization of Coxiella burnetii isolates by infrequent restriction site-PCR and MLVA typing.Crossref | GoogleScholarGoogle Scholar |
Bond, KA, Franklin, L, Sutton, B, Stevenson, MA, and Firestone, SM (2018). Review of 20 years of human acute Q fever notifications in Victoria, 1994–2013. Australian Veterinary Journal 96, 223–230.
| Review of 20 years of human acute Q fever notifications in Victoria, 1994–2013.Crossref | GoogleScholarGoogle Scholar |
de Bruin, A, Janse, I, Koning, M, de Heer, L, van der Plaats, RQJ, van Leuken, JPG, and van Rotterdam, BJ (2013). Detection of Coxiella burnetii DNA in the environment during and after a large Q fever epidemic in the Netherlands. Journal of Applied Microbiology 114, 1395–1404.
| Detection of Coxiella burnetii DNA in the environment during and after a large Q fever epidemic in the Netherlands.Crossref | GoogleScholarGoogle Scholar |
Duncan, C, Kersh, GJ, Spraker, T, Patyk, KA, Fitzpatrick, KA, Massung, RF, and Gelatt, T (2012). Coxiella burnetii in northern fur seal (Callorhinus ursinus) placentas from St. Paul Island, Alaska. Vector-Borne and Zoonotic Diseases 12, 192–195.
| Coxiella burnetii in northern fur seal (Callorhinus ursinus) placentas from St. Paul Island, Alaska.Crossref | GoogleScholarGoogle Scholar |
Duncan, C, Savage, K, Williams, M, Dickerson, B, Kondas, AV, Fitzpatrick, KA, Guerrero, JL, Spraker, T, and Kersh, GJ (2013). Multiple strains of Coxiella burnetii are present in the environment of St. Paul Island, Alaska. Transboundary and Emerging Diseases 60, 345–350.
| Multiple strains of Coxiella burnetii are present in the environment of St. Paul Island, Alaska.Crossref | GoogleScholarGoogle Scholar |
Gardner, BR, Stenos, J, Hufschmid, J, Arnould, JPY, McIntosh, RR, Tadepalli, M, Tolpinrud, A, Marenda, M, Lynch, M, and Stent, A (2022). An old pathogen in a new environment: implications of Coxiella burnetii in Australian fur seals (Arctocephalus pusillus doriferus). Frontiers in Marine Science 9, 809075.
| An old pathogen in a new environment: implications of Coxiella burnetii in Australian fur seals (Arctocephalus pusillus doriferus).Crossref | GoogleScholarGoogle Scholar |
Geeson, JJ, Hobday, AJ, Speakman, CN, and Arnould, JPY (2022). Environmental influences on breeding biology and pup production in Australian fur seals. Royal Society Open Science 9, 211399.
| Environmental influences on breeding biology and pup production in Australian fur seals.Crossref | GoogleScholarGoogle Scholar |
González-Barrio, D, and Ruiz-Fons, F (2019). Coxiella burnetii in wild mammals: a systematic review. Transboundary and Emerging Diseases 66, 662–671.
| Coxiella burnetii in wild mammals: a systematic review.Crossref | GoogleScholarGoogle Scholar |
Kersh, GJ, Lambourn, DM, Raverty, SA, Fitzpatrick, KA, Self, JS, Akmajian, AM, Jeffries, SJ, Huggins, J, Drew, CP, Zaki, SR, and Massung, RF (2012). Coxiella burnetii infection of marine mammals in the pacific northwest, 1997–2010. Journal of Wildlife Diseases 48, 201–206.
| Coxiella burnetii infection of marine mammals in the pacific northwest, 1997–2010.Crossref | GoogleScholarGoogle Scholar |
McIntosh, RR, Sorrell, KJ, Thalmann, S, Mitchell, A, Gray, R, Schinagl, H, Arnould, JP, Dann, P, and Kirkwood, R (2022). Sustained reduction in numbers of Australian fur seal pups: implications for future population monitoring. PLoS ONE 17, e0265610.
| Sustained reduction in numbers of Australian fur seal pups: implications for future population monitoring.Crossref | GoogleScholarGoogle Scholar |
Minor, C, Kersh, GJ, Gelatt, T, Kondas, AV, Pabilonia, KL, Weller, CB, Dickerson, BR, and Duncan, CG (2013). Coxiella burnetii in northern fur seals and Steller sea lions of Alaska. Journal of Wildlife Diseases 49, 441–446.
| Coxiella burnetii in northern fur seals and Steller sea lions of Alaska.Crossref | GoogleScholarGoogle Scholar |
Rodríguez-Lázaro, D, Hernández, M, Scortti, M, Esteve, T, Vázquez-Boland, JA, and Pla, M (2004). Quantitative detection of Listeria monocytogenes and Listeria innocua by real-time PCR: assessment of hly, iap, and lin02483 targets and amplifluor technology. Applied and Environmental Microbiology 70, 1366–1377.
| Quantitative detection of Listeria monocytogenes and Listeria innocua by real-time PCR: assessment of hly, iap, and lin02483 targets and amplifluor technology.Crossref | GoogleScholarGoogle Scholar |
Roest, H-J, van Gelderen, B, Dinkla, A, Frangoulidis, D, van Zijderveld, F, Rebel, J, and van Keulen, L (2012). Q fever in pregnant goats: pathogenesis and excretion of Coxiella burnetii. PLoS ONE 7, e48949.
| Q fever in pregnant goats: pathogenesis and excretion of Coxiella burnetii.Crossref | GoogleScholarGoogle Scholar |
Schneeberger, PM, Hermans, MHA, van Hannen, EJ, Schellekens, JJA, Leenders, ACAP, and Wever, PC (2010). Real-time PCR with serum samples is indispensable for early diagnosis of acute Q fever. Clinical and Vaccine Immunology 17, 286–290.
| Real-time PCR with serum samples is indispensable for early diagnosis of acute Q fever.Crossref | GoogleScholarGoogle Scholar |
Tilburg, JJHC, Rossen, JWA, van Hannen, EJ, Melchers, WJG, Hermans, MHA, van de Bovenkamp, J, Roest, HJIJ, de Bruin, A, Nabuurs-Franssen, MH, Horrevorts, AM, and Klaassen, CHW (2012). Genotypic diversity of Coxiella burnetii in the 2007-2010 Q fever outbreak episodes in the Netherlands. Journal of Clinical Microbiology 50, 1076–1078.
| Genotypic diversity of Coxiella burnetii in the 2007-2010 Q fever outbreak episodes in the Netherlands.Crossref | GoogleScholarGoogle Scholar |
Tozer, SJ, Lambert, SB, Strong, CL, Field, HE, Sloots, TP, and Nissen, MD (2014). Potential animal and environmental sources of Q fever infection for humans in Queensland. Zoonoses and Public Health 61, 105–112.
| Potential animal and environmental sources of Q fever infection for humans in Queensland.Crossref | GoogleScholarGoogle Scholar |
Vincent, G, Stenos, J, Latham, J, Fenwick, S, and Graves, S (2016). Novel genotypes of Coxiella burnetii identified in isolates from Australian Q fever patients. International Journal of Medical Microbiology 306, 463–470.
| Novel genotypes of Coxiella burnetii identified in isolates from Australian Q fever patients.Crossref | GoogleScholarGoogle Scholar |



