Evaluation of oral baits and distribution methods for Tasmanian devils (Sarcophilus harrisii)†
Sean Dempsey A B , Ruth J. Pye A , Amy T. Gilbert C , Nicholas M. Fountain-Jones B , Jennifer M. Moffat
A , Amy T. Gilbert C , Nicholas M. Fountain-Jones B , Jennifer M. Moffat  A , Sarah Benson-Amram D , Timothy J. Smyser C and Andrew S. Flies
A , Sarah Benson-Amram D , Timothy J. Smyser C and Andrew S. Flies  A *
A *
A Menzies Institute for Medical Research, College of Health and Medicine, University of Tasmania, Private Bag 23, Hobart, Tas. 7000, Australia.
B School of Natural Sciences, College of Science and Engineering, University of Tasmania, Hobart, Tas. 7001, Australia.
C National Wildlife Research Center, US Department of Agriculture, Animal and Plant Health Inspection Service, Wildlife Services, Fort Collins, CO 80521, USA.
D University of British Columbia, Vancouver, BC V6T 1Z4, Canada.
Wildlife Research 50(10) 807-819 https://doi.org/10.1071/WR22070
Submitted: 14 April 2022 Accepted: 22 October 2022 Published: 22 November 2022
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Diseases are increasingly contributing to wildlife population declines. Tasmanian devil (Sarcophilus harrisii) populations have locally declined by 82%, largely owing to the morbidity and mortality associated with two independent transmissible devil facial tumours (DFT1 and DFT2). Toxic baits are often used as a management tool for controlling vertebrate pest populations in Australia, but in other areas of the world, oral baits are also used to deliver vaccines or pharmaceuticals to wildlife.
Aim: Our goal was to evaluate the potential use of edible baits as vehicles for vaccine delivery to Tasmanian devils.
Method: We first tested bait palatability with captive devils. Bait interactions were recorded, and consumption and bait interaction behaviours were quantified. We next trialled baits containing inert capsules as potential vaccine containers in captivity. After confirming bait palatability in captivity, ground baiting was trialled at six field sites and monitored using camera traps. Finally, an automated bait dispenser was trialled at field sites to attempt to limit bait consumption by non-target species.
Key results: Captive devils consumed all types of placebo baits, but consumed a higher percentage of ruminant- and fish-based baits than cereal-based baits. Captive devils also consumed inert capsules inserted into placebo baits. Ground-baiting trials in the field showed that 53% of baits were removed from bait stations, with 76% of the removals occurring on the first night. Devils were suspected or confirmed to remove about 7% of baits compared with 93% by non-target species. We also evaluated an automated bait dispenser, which reduced bait removal by non-target species and resulted in over 50% of the baits being removed by devils.
Conclusions: This study demonstrated that captive and wild devils will accept and consume placebo versions of commercial baits. Bait dispensers or modified baits or baiting strategies are needed to increase bait uptake by devils.
Implications: Bait dispensers can be used at a regional scale to deliver baits to devils. These could potentially be used as vaccine-delivery vehicles to mitigate the impacts of disease on devil populations.
Keywords: bait dispenser, captive trials, devil facial tumour disease, enteric-coated capsule, feeding behaviour, landscape vaccine distribution, oral vaccine, pest control, transmissible cancer, wildlife disease.
Introduction
Disease is increasingly a driver of wildlife declines in Australia (Woinarski et al. 2019). Disease-induced population declines are most common when new pathogens emerge or non-endemic pathogens are introduced to wildlife populations that have no prior history with the pathogen. Two completely new pathogens were discovered in Tasmanian devils (Sarcophilus harrisii) in 1996 and 2014 (Jones et al. 2004; Pye et al. 2016a). Devil facial tumour 1 (DFT1) is a transmissible cancer that was first observed in 1996 (Pearse and Swift 2006) and has been the primary driver of an estimated 82% decline in regional devil abundance during the past 25 years (Lazenby et al. 2018; Cunningham et al. 2021). DFT1 infection is nearly always fatal to devils and has spread across more than 90% of Tasmania (Cunningham et al. 2021). A second independent devil facial tumour (DFT2) was discovered in 2014 (Pye et al. 2016a) and it has been detected only on devils in southern Tasmania.
The island state of Tasmania is the sole place where devils exist in the wild, making this area a critical conservation priority for this species. In addition to disease, other threats to long-term persistence of devil populations include habitat loss, roadkill (Jones 2000; Hobday and Minstrell 2008), domestic animal predation (Holderness-Roddam and McQuillan 2014), and population genetic inbreeding (Lawrence and Wiersma 2019). The decline of the devil population has been associated with other conservation issues, such as a greater abundance of invasive feral cats (Felis catus) and a reduced abundance of native southern brown bandicoots (Isoodon obesulus; Cunningham et al. 2020).
A key management strategy for the persistence of devils has involved breeding of disease-free insurance populations both in captivity, and wild-living on Maria Island (Thalmann et al. 2016). These insurance populations have been used to supplement genetic diversity of wild populations. However, indefinite maintenance of a captive insurance population is costly, and the devils introduced to Maria Island may have negative consequences for native island fauna (e.g. birds) there (Scoleri et al. 2020). Alternative management strategies must be considered and may be necessary to recover wild devil populations.
Development of a vaccine to protect devils or improve resistance from lethal DFT1 or DFT2 infection would be a valuable management tool. Recent work has shown that the devil immune system can recognise DFT1, and that tumour regression can occur (Pye et al. 2016b; Hamede et al. 2021). Furthermore, priming the immune system of healthy devils with a vaccine followed by immunotherapy after tumours developed was able to induce complete tumour regressions in captive trials (Tovar et al. 2017). Previous field vaccine trials were not efficacious in protecting devils from developing tumours, but did increase devil immune recognition of tumours (Pye et al. 2018, 2021). These findings demonstrated that the devil immune system can be stimulated to protect devils against DFT1. However, delivering vaccines efficiently to wild devils throughout the rugged Tasmanian landscape presents additional challenges.
Vaccines have been delivered in baits to control wildlife rabies since the late 1970s. An estimated 665 000 000 oral baits were used in Europe alone during 1978–2014 and resulted in the elimination of rabies in red foxes (Vulpes vulpes) from western and central Europe (Müller et al. 2015). Following the success of the rabies oral bait vaccine (OBV) programs, OBVs have been tested for other wildlife diseases including tuberculosis vaccines for wild boars (Sus scrofa; Ballesteros et al. 2011) and European badgers (Palphramand et al. 2017), and sylvatic plague vaccines for prairie dogs (Cynomys ludovicianus; Rocke et al. 2017). Rabies OBV campaigns have demonstrated success despite the challenges of working with a multi-host pathogen and transboundary zoonosis.
Because Tasmania is an island state, controlling devil facial tumour disease through OBVs presents a potentially more tractable problem than does controlling rabies across national boundaries. This is supported by two independent analyses that recently suggested an OBV could potentially eliminate DFTD from Tasmania (Lamp 2021; Drawert et al. 2022). An OBV platform for DFT1 and DFT2 is in development (Flies et al. 2020; Kayigwe et al. 2022); however, the success of an OBV will depend not only on the effectiveness of the vaccine but also the bait distribution system. OBV strategies will depend on the ecology of the target species, characteristics of the bait matrix, production costs, method of bait delivery, bait distribution system, and the environment where baits are distributed. Unfortunately, little is known about bait palatability and bait uptake among Tasmanian wildlife.
The objective of this study was to evaluate the potential use of edible baits as vaccine delivery vehicles for Tasmanian devils. We tested placebo versions of commercially available toxic baits used for control of vertebrate pests on mainland Australia. We first offered baits to captive devils to assess bait palatability, quantify feeding behaviours, and determine suitability and acceptance of inert capsules in baits for downstream vaccine encapsulation. We then performed bait fate trials in select habitats in southern Tasmania to assess bait uptake by wild devils and non-target species. Finally, we evaluated an automated bait dispenser to potentially increase specificity of bait uptake to devils. This study advances proof of concept for an OBV distribution strategy for devils and information about bait uptake, which may be relevant to vertebrate-pest control programs.
Materials and methods
Study sites
Trowunna Wildlife Sanctuary (Mole Creek) is a private property located within a eucalypt forest in north-western Tasmania (Fig. 1). Data collection at this site occurred during late autumn (May) through mid-winter (July) in 2021. Trowunna was visited three times for a total of nine trial days to test bait palatability and feeding behaviours. Studies with captive devils were also performed at the Department of Natural Resources and Environment Tasmania Cressy Research and Development Station in north-eastern Tasmania (Fig. 1). Data collection at this site occurred during May through August. The Cressy facility was visited three times for a total of 11 trial days.
All captive devils used for bait trials in this study were individually housed and provided water ad libitum. At Trowunna, we offered baits without capsules to 10 devils and baits containing capsules to five devils. Three of the five devils at Trowunna offered baits without capsules were involved in the initial study without capsules. A minimum period of 60 days elapsed between bait-only and bait-containing capsule trials. At Cressy, we offered baits without capsules to eight devils and baits containing capsules to seven devils. Seven devils were included in both trials with a minimum of 90 days between the two types of trial.
Captive devils’ pen size and setup were variable, but each had a minimum area of 100 m2 with a minimum fence height of 1.2 m and access to natural light and photo period (Supplementay material Figs S1, S2). Substrates were primarily soil and vegetation, with concrete making up less than 10% of the floor space. Each pen also contained shelter, such as hollow logs or wooden nest boxes. Trowunna devils are typically fed a daily ration consisting primarily of meat and bone from Bennett’s wallaby (Notamacropus rufogriseus), Tasmanian pademelon (Thylogale billardierii), brushtail possum (Trichosurus vulpeca), rabbit (Oryctolagus cuniculus) and a variety of domestic poultry. Devils at Trowunna were fasted 24 h prior to the first day of each bait trial and were then fed the daily ration at the conclusion of each trial day. Cressy devils were typically fed a diet consisting primarily of brushtail possum, Tasmanian pademelon and Bennett’s wallaby three times per week as well as weekly enrichment feeding such as chicken (Gallus gallus) eggs. Cressy devils were fasted 24 h prior to the first day of each trial and were then offered their food ration according to their usual weekly schedule at the conclusion of each trial day.
Bait uptake by wild devils was tested at six private sites in southern Tasmania (Fig. 1). Three sites were located on a 150-ha property near Sandfly that contains three permanent dams, open grassland, and an olive tree grove surrounded by eucalypt forest and at least 500 m from the nearest residence. Individual sites in Lenah Valley, Middleton, and Ridgeway were within 100 m of a residence and were either bordered or surrounded by eucalypt forest. One site in Lenah Valley had equine and sheep paddocks within 100 m and eucalypt forest within 500 m. All sites were selected on the basis of owner-reported devil sightings on the property prior to this study. Property owners volunteered to place the non-toxic baits and retrieve images from remote cameras at least once per week. One site in Sandfly had a trail camera but was not baited.
Baits
Animal Control Technologies Australia (Melbourne, Australia) supplied four types of placebo baits for this study (Fig. 2). These baits have been used in mainland Australia as toxic baits containing sodium monofluoroacetate (known as 1080) for reducing populations of introduced vertebrate pest species (e.g. wild canids, feral pigs). The ruminant-based bait was a rectangular bait weighing approximately 35 g. The fish-based bait was cylindrical and weighed approximately 35 g. The 35-g ruminant and fish baits used for these trials contained indigestible green plastic beads that could be detected in scat after consumption. The cereal-based bait was a fish-flavoured cylindrical bait that weighed 70 g. The cereal baits were cut approximately in half for this study and did not contain scat markers. We also used dried kangaroo baits weighing approximately 40 g. Kangaroo baits were treated as a positive control given that macropod meat is a common prey item in the diet of wild devils. We used only fish-based baits (hereafter referred to as fish baits) for the inert capsule trials. Custom fish baits without scat markers (20-mm height by 30-mm diameter; approximately 17 g) were produced to fit the dimensions of the bait magazine and receptacle of an automated bait dispenser.
|
|
Video recording in captivity
Cameras were positioned on the boundary of devil pens, which were approximately 1.5 m high, facing the area of bait placement and programmed to record for 30 min post-bait offering. Footage was recorded with GoPro8, GoPro9, and DC-FT7 Lumix cameras, and Samsung Note 2, and Samsung S7 phone cameras. Multiple cameras were used to record independent tests simultaneously.
Bait testing in captivity
Bait trials were conducted between 10:00 hours and 15:00 hours. Baits were weighed prior to each trial and disposable gloves were worn to avoid adding human scent to the baits. Each captive devil was used in only one trial each day. Handling tools (Nifty Nabber, Unger #92134) were used to simultaneously lower two different bait types into a pen. Devils had full access to the pens at all times and thus could approach the baits immediately, rather than being let in from a separate pen. Separate tools were used for each bait type to minimise scent transfer. Baits were placed approximately 0.5 m apart. Human presence was minimised during filming but could not be eliminated in every instance (e.g. trials occurring at Trowunna Wildlife Sanctuary occurred during visitation by the public). The behaviours of each devil post-bait offering were filmed for approximately 30 min.
At the conclusion of filming, pens were checked for presence of baits. Bait remains were weighed to the nearest gram to estimate the percentage consumed. Intact baits and/or bait remains were then returned to the pen to evaluate whether these would be consumed overnight. A bait was recorded as ‘consumed’ only if an individual was observed eating at least part of the bait. The proportion of the bait consumed could not be estimated when the baits were taken off camera and could not be located. These ‘removed’ baits were not included in the results because brushtail possums and rodents were observed to enter some of the devil enclosures overnight. No possums or rodents were observed consuming a bait, but this possibility cannot be excluded.
In a second set of captive bait trials, we tested baits containing an inert white-opaque Capsugel DRcaps® capsules made of hypromellose, titanium dioxide and gellan gum (Lonza Australia, #G69CS000753). The enteric coating prevented bait moisture from dissolving the capsules, which otherwise readily dissolve in most liquids. Capsules were filled with a sucrose solution (golden syrup). Fish oil has been reported to be an effective lure, so the capsules were covered in fish oil prior to being inserted into the baits (Andersen et al. 2016). A single encapsulated fish bait was offered in each trial and the fish baits in this trial did not contain scat markers; otherwise the methods were as per the two bait trials without capsules. We attempted to retrieve the bait and capsule following the trials to weigh baits and determine whether the capsule was consumed, punctured, or crushed.
Video analysis of devil behaviour
Behavioural data were quantified from videos of captive trials using Behavioural Observation Research Interaction Software (BORIS; Friard and Gamba 2016). All video recordings were imported to BORIS as MP4 files at 30 frames per second. The frequency of ‘point events’ (non-continuous) and the duration (seconds) of ‘state events’ (continuous) were calculated in BORIS. Behaviours were defined on the basis of an ethogram (Supplementary material Table S1) developed by Marissa Parrott and staff at the Healesville Sanctuary (Pollock et al. 2022). Behaviours of primary interest were associated with how devils interacted with the baits, consumed the baits, and groomed themselves after interacting with the bait because all these behaviours could affect the dose and route of vaccine exposure in future vaccine trials. Behaviours not associated with feeding (drinking, bait taken out of frame, devil sniffing the air) were not included in the results. The occurrence of behaviours was not mutually exclusive, and up to three events could be logged at the same time.
Bait testing on private properties
Baits were placed by a gloved hand approximately 3 m away from cameras by volunteer landowners on private property. Bait stations were left without baits for a 1-week period to ensure that cameras were operating adequately. Baits were then placed on the ground with 7-day bait-replacement intervals during the first 2 weeks of the study. These early trials showed that baits were mostly consumed or removed by non-target species on the first night, so we modified the trials. For the remaining 6 weeks, volunteers covered the baits with two cups of soil to limit visual detection of baits as in the prior study that tested non-toxic bait uptake by Tasmanian devils (Hughes et al. 2011). We also reduced the interval between bait replenishment from 7 days to a minimum of 48 h after disappearance of the prior bait.
In total, 289 nights of footage from six sites were checked for presence of animals. Of these 289 nights, 85 nights had baits present and are referred to as bait-exposure nights (BENs). A ‘visit’ was defined as an animal observed on camera at a bait station. This included animals that showed interest in the bait as well as animals that were passing through the site of bait placement. To account for repeat visits, if an individual of the same species was seen multiple times at a bait station within 10 min and qualitatively appeared to be of similar size and appearance, it was counted as one visit. Camera-traps recorded either 20-s, 1-min, or 2-min videos. Visits by species were taxonomically grouped for analysis as the ‘macropod’ group (Tasmanian pademelon, Bennett’s wallaby, and Tasmanian bettong (Bettongia gaimardi)), the ‘other marsupials’ group (southern brown bandicoot (I. obesulus) and eastern-barred bandicoot (Perameles gunnii)), the ‘rodents’ group (presumable Rattus species and Mus species), and the ‘bird’ group (Tasmanian native hen (Tribonyx mortierii), tawny frogmouth (Podargus strigoides) and blackbird (Turdus merula)). Brushtail possums and eastern quolls (Dasyurus viverrinus) were recorded at the species level because these species were clearly distinguishable and they were among the most common species recorded interacting with baits.
An animal was listed as ‘No ID’ when it could not be identified. A bait was marked as ‘suspected taken’ by a particular animal when bait removal could not be confirmed on camera-trap footage (e.g. obstructed view), but we had reason to believe that the animal was responsible for the removal (e.g. evidence of small tunnel to the bait, suggesting a rodent had removed a bait). When a bait had disappeared from a bait station but there was no footage of the removal, the bait was listed as ‘confirmed removal’ by an unidentifiable animal (No ID). Cameras used to capture footage include one Flag Power camera, four Keep Guard cameras, four Browning BTC-8E cameras, and a set of Arlo Pro 2 cameras.
Automated bait dispensers
Automated bait dispensers (Fig. S3) developed for delivering fishmeal polymer baits (e.g. containing oral rabies vaccines) to raccoons (Procyon lotor; Smyser et al. 2015) were modified for the delivery of baits to devils. Specifically, the square magazine used for the delivery of cubical baits to raccoons was replaced with 32-mm Vinidex Rural Plus® polyethylene pipe to accommodate the cylindrical 30-mm-diameter fish baits used in this study. Ten baits were loaded vertically into each magazine. One of the baits was immediately pushed through to the receptacle platform (and was, therefore, available for consumption) by a motorised piston. A sensor in the receptacle detects when a bait has been removed from the platform and dispenses a new bait 40 min after removal of the previous bait. On presentation by the dispenser, the bait was available to devils within a polyvinyl chloride (PVC) cylinder (77 mm in diameter × 155 mm in length) to restrict access to non-target species that cannot physically reach the baits with their paw and/or mouth.
Automated bait dispensers were tested at the two Lenah Valley sites and one Sandfly site. The Sandfly site and one Lenah Valley site had confirmed devil and eastern quoll sightings during the ground-baiting trials; the other Lenah Valley site had no devil sightings during the initial trials, but had baits that were regularly consumed by possums. Dispensers were secured to posts with rubber straps at either 200 mm or 350 mm above the ground. A camera-trap was set up approximately 2–3 m away from the dispenser. Approximately 5 mL of fish oil was dispersed around the base of the dispenser to attract devils. Ten custom-made fish baits (20 mm × 30 mm, approximately 17.5 g) were loaded into the dispenser magazine. We monitored bait dispensers with trail cameras for 33 nights. A dispenser interaction was defined as ‘an animal showing interest in and/or contacting the dispenser, including sniffing, making physical contact, or scent marking’.
Statistical analyses
Data collected from the captive-trial data sheets were analysed using R v4.1.1 (R Core Team 2021). A mixed-effects logistic regression model (i.e. a generalised linear mixed model, GLMM) was used to analyse bait palatability among single devils. The binary response variable was whether a bait was eaten (1) or uneaten (0). In most trials, the bait was either not consumed or was completely consumed (Fig. 3). In trials with partially consumed baits, less than 50% of the baits were generally consumed, suggesting that the animal tasted the bait but at that time was not motivated to consume the entire bait. Thus, baits were categorised as eaten in captive trials if more than 50% of the pre-trial mass was consumed.
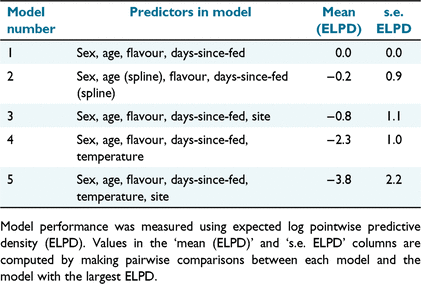
The data were modelled for the response variable using a Bernoulli distribution (log-link). Five models were specified with different combinations of fixed effects (Table 1). Fixed effects included age, sex, days since the devil’s most recent food ration, bait flavour, site, and temperature. The random effect was the individual animal ID. Bait flavour, sex, age, and days-since-last fed were the primary variables of interest and thus were included in the base model. Model 2 tested whether adding splines to incorporate non-linearities associated with age and days-since-fed could improve model performance compared with our base model (Model 1). Because splines did not improve model fit, we did not include them in subsequent models, which added site (Model 3), temperature (Model 4), or site and temperature (Model 5) to the base model. We used kangaroo baits as a positive control in only a few trials and all kangaroo baits were consumed; the data from kangaroo baits were not included in models because it was considered impractical to deliver a vaccine within dried meat baits.
|
We fitted our models using Bayesian inference with Hamiltonian Monte Carlo (HMC) ‘no U-turn sampling’ (NUTS) by using the brms package (Bürkner 2017) and plotted by using bayesplot package (Gabry and Mahr 2022). Four Markov chains of 8000 iterations were run using non-informative priors in model specification and every 16th draw was thinned to reduce autocorrelation. Effective sample size (ESS) and R-hat convergence diagnostics showed no evidence of divergence or autocorrelation in any model (Figs S4–S6).
Model selection was performed using approximate leave-one-out (LOO) cross-validation of the posterior predictive log density with the loo package (Vehtari et al. 2018). The observed values were compared to the modelled predictions by using the 95% credibility intervals of the posterior predictive distribution to determine which fixed effects were affecting whether a bait would be eaten in a trial. Model evaluation was validated using area-under-the-curve (AUC; Delong et al. 1988) to measure the ability of the model to correctly distinguish an eaten bait from an uneaten one. Missing data were removed before graphing/analysis. Scatterplots showing the percentage of the bait consumed in captive trials and stacked bar charts showing bait removals by species in field trials were made using ggplot2 package (Wickham 2016). Results from behavioural analysis and field trials are presented directly in tables and figures without statistical testing.
Ethical approval
The research study was approved by the University of Tasmania Animal Ethics Committee (#23220) and the Department of Natural Resources and Environment Tasmania Save the Tasmanian Devil Program’s Captive Research Advisory Group (‘Bait preference testing in Tasmanian devils’).
Results
Bait consumption by captive devils
In total 19 of 153 offered baits, including 12 fish, six ruminant, and one kangaroo bait, disappeared from the trial pens, but the animal taking or eating the bait could not be confirmed (Table S2). Three cereal baits and one ruminant bait (four total) were partially eaten during trials but crumbled into small pieces that were unable to be weighed and checked for consumption proportions. Circumstantial evidence suggests the baits taken out of view or crumbled were at least partially consumed, but because the baits could not be recovered and weighed they were recorded as ‘removed’ and not included in the analysis. For the baits included in the analysis, complete consumption of baits was observed for 59% (27/46) of ruminant and 53% (20/38) of fish baits, compared with 33% (13/40) of the cereal-based baits (Table S2). All six kangaroo meat baits were completely consumed. Approximately 15% of the ruminant, fish, and cereal baits were partially consumed (Table S2).
The average mass of baits consumed by captive male devils (n = 9) was about 25% more than the mass of bait consumed by captive female devils (n = 9) for all bait types (Fig. 3). Among male devils, the percentage of the bait mass consumed was highest for the fish (73%) and ruminant (71%) baits. Ruminant (46%) and fish (39%) baits also had the highest percentage consumption by female devils. Interestingly, 4-year-old devils (n = 3) completely consumed 24/26 baits, whereas 6-year-old devils (n = 3) consumed only 3/23 baits.
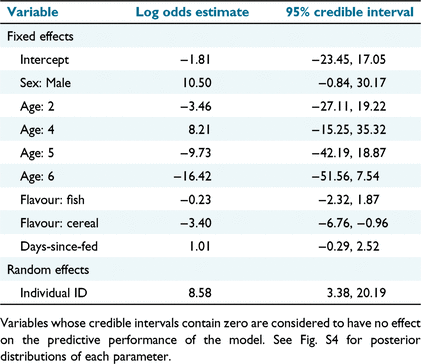
The results of the leave-one-out cross-validation approach showed that the simplest linear model (Model 1) had the highest expected log pointwise predictive density (ELPD); Model 1 included sex, age, bait flavour and days since fed as fixed effects, along with individual as a random effect. The area-under-the-curve (AUC = 0.985) and coefficient of determination (Bayesian R2 = 0.76) for Model 1 suggested that the model accurately described whether baits were eaten or uneaten. In agreement with the visual interpretation of Fig. 3, captive devils were more likely to eat the ruminant and fish baits than the cereal baits (Table 2). There was a moderate indicator for greater percentage consumption by male devils (95% CI −0.84, 30.17) and 4-year-old devils (95% credible interval (CI) −15.25, 35.32; Table 2).
|
Bait and capsule consumption by captive devils
We performed trials with fish baits loaded with an enteric-coated capsule containing golden syrup by using 12 singly housed devils across two sites. We used fish baits for these trials because they performed similarly to ruminant baits in the initial trials, fish oil could be coated on the capsule itself, and the fish baits could be used in subsequent bait-dispenser trials more readily than would ruminant baits. Fourteen baits were taken out of view and recorded as ‘removed’. In three trials with removed baits, the capsule had separated from the bait and was not consumed. These ‘removed’ baits could not be logged as ‘eaten’, ‘uneaten’, or ‘partially eaten’ and were thus not included in capsule interaction results.
In the 21 capsule trials included in the final analysis, 67% (14/21) of capsule-loaded fish baits were completely eaten, 29% (6/21) were uneaten, and 5% (1/21) were partially eaten. The percentage of fish baits completely consumed in these single-bait trials (67%) was higher than that in the two-bait trials (53%). Among the 14 baits consumed, 50% (7/14) of capsules were completely consumed, and a further 21% (3/14) were punctured. Capsules separated from the baits in 48% (10/21) of these trials. On the occasions where capsules became separated, 60% (6/10) of capsules were still consumed or punctured.
Captive-devil feeding behaviour
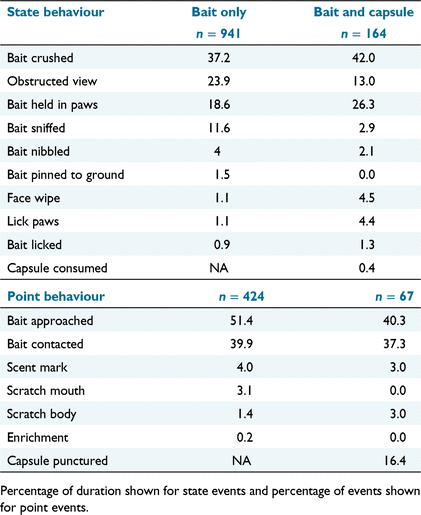
In total, 941 state events were logged from 62 trials in which two baits without capsules were simultaneously offered to 16 individually housed devils. The occurrence of observed behaviours was not mutually exclusive. Additionally, interactions with the two different baits in each trial were grouped, rather than separating behaviours for each bait. The ‘bait crushed’ in jaws behaviour comprised the largest proportion of time among state events (37%; Table 3). Among observable state events (i.e. excluding ‘bait interaction – obstructed view’), ‘bait held in paws’ was the next most frequently occurring behaviour (19%), followed by ‘bait sniffed’ (12%). Devils displayed a total of seven different point behaviours and 424 point events in bait-only trials (Table 3). ‘Bait approached’ was the most commonly occurring behaviour (51%). ‘Bait contacted’ also represented a considerable proportion of behaviours (40%). ‘Scratch mouth’, ‘scent mark’, and ‘scratch body’ behaviours made up lesser proportions of point events in both groups.
|
In total, 164 state behaviours were recorded from 29 capsule bait trials in fish baits with 11 devils. ‘Bait crushed’ (42%) and ‘bait held in paws’ (26%) again comprised the largest proportion of state behaviours. In total, 67 point events were recorded from capsule bait trials. ‘Bait approached’ was the most commonly occurring point event (40%; Table 3). ‘Bait contacted’ comprised the next-largest proportion of point events (37%). ‘Bait capsule punctured’ comprised 16% of events.
Wild-devil bait trials
Macropods were by far the most common animals observed (60%, 822/1361) in videos across 289 nights where bait was present and not present at the six baited field sites and one unbaited field site (Table S3); pademelons were the most common macropod, accounting for 56% of the total visits (768/1361). Possums were the next-most observed animals at 11% (156/1361) of total visits. Eastern quolls and devils made up 7% (99/1361) and 5% (67/1361) of the total animals observed respectively.
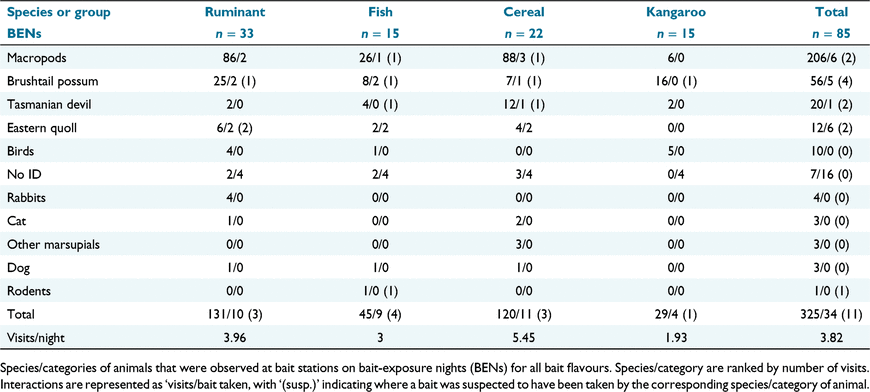
In the subset of footage from 85 bait-exposure nights (BENs) when baits were present, six sites were visited on 325 occasions by individuals from 10 species or higher taxonomic classifications (e.g. macropods). Among identifiable animals, 63% (206/325) of animals observed at bait stations when baits were present were macropods. Brushtail possums, devils, and eastern quolls accounted for 17% (56/325), 6% (20/325), and 4% (12/325) respectively, of the visitors on BENs (Table 4). Rabbits, cats, dogs, and other marsupials were observed infrequently, with rodents being observed the least but potentially underestimated because of their smaller size.
|
Nineteen per cent of baits (16/85) were classified as taken by ‘No ID’ because they were removed from the bait station but the animal responsible could not be identified (Table 4). In total, 53% (45/85) of baits were removed from bait stations over 85 BENs, with 34 baits being confirmed as ‘taken’ and 11 baits classed as ‘suspected taken’. Seventy-six per cent (34/45) of the bait removals occurred on the first night the bates were placed in the field (Fig. 4). Among identifiable animals, brushtail possums were responsible for the 20% (9/45) of the confirmed/suspected bait removals. Pademelons and eastern quolls were each responsible for 18% (8/45) of the confirmed or suspected bait removals. Devils were confirmed or suspected to remove 7% (3/45) of the removed baits.
Bait stations with cereal baits were visited most frequently (5.4 visits/night) and comprised the largest number of devil visits (12) of all four bait flavours. The cereal bait also had the most confirmed bait removals (11), including the only confirmed devil bait removal. Devils were also suspected to have removed one cereal and one fish bait, but no ruminant or kangaroo meat baits. Ruminant-bait stations were the second-most visited bait station (3.9 visits/night), with the second-most baits being confirmed taken (10) and three baits suspected taken. Ruminant- and kangaroo meat-bait stations had the fewest visits by devils. Fish bait and kangaroo meat stations had 3 and 1.9 visits per night respectively. Nine fish baits were confirmed taken from these bait stations, with four baits being suspected taken (13 suspected/confirmed).
Dispenser trials
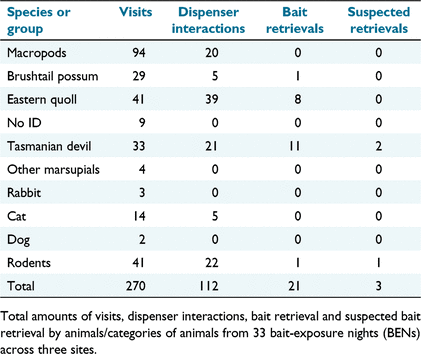
As a result of the higher-density non-target species taking most baits before lower-density devils visited the bait stations, automated baited dispensers were evaluated at three of the field sites. Automated bait dispensers were visited 270 times by animals from 10 categories over 33 BENs across three sites (Table 5). Macropods were again the most frequent visitors (94), followed by eastern quolls (41), rodents (41), Tasmanian devils (33), brushtail possums (29), and cats (14). Rabbits, dogs, other marsupials and unidentifiable animals were infrequent visitors to dispenser sites. In total, 112 dispenser interactions were observed by animals from five categories. Eastern quolls were responsible for the most dispenser interactions (39), followed by rodents (22), Tasmanian devils (21), macropods (20), brushtail possums (5) and cats (5). Devils accounted for 8% (21/270) of visits to bait dispensers, compared with 6% (20/325) of visits to ground bait stations in the prior trials. However, devils accounted for 54% (13/24) of the confirmed and suspected bait removals from the dispensers (Fig. 5), compared with only 7% (3/45) of the confirmed and suspected removals of ground baits. The percentage of baits retrieved by eastern quolls was 33% (8/24) from dispensers, compared with 18% (8/45) of ground baits. The bait dispensers decreased the percentage of baits retrieved by possums and no macropods retrieved a bait from the dispenser, despite being the most common visitors in the dispenser videos.
|
Discussion
An oral-bait vaccination strategy has been proposed for combatting the disease-induced decline in the wild devil population (Flies et al. 2020). However, very little information is available to assess the feasibility of this strategy from a vaccine-bait delivery viewpoint (Hughes et al. 2011; Mallick et al. 2016). The present study showed that manufactured baits consisting primarily of ruminant or fish meat were palatable to both captive and wild devils. However, in a field setting, bait modifications or automated dispensers are needed to reduce consumption by non-target species and to improve specificity for devils.
In contrast to previous research that reported that devils were overwhelmingly the most frequent visitors to ruminant-based bait stations and consumed the majority of baits in the field (Hughes et al. 2011), macropods and brushtail possums often arrived first and consumed the most baits placed on the ground in our study. We hypothesise that this difference is due, in part, to differences in species abundance and ecology between the studies. The Hughes et al. (2011) study occurred in a more remote part of northern Tasmania where devil density was high prior to devil facial tumour disease arriving in the area. Local devil populations have declined by approximately 80% across most of the state since that time (Lazenby et al. 2018; Cunningham et al. 2021). Most ground baits were removed on the first night, with more abundant species such as pademelons and possums removing the baits before devils had an opportunity to locate and consume the bait.
We emphasise that field sites used in this pilot study were peri-urban and additional testing in other habitat types will be needed to develop effective regional baiting strategies. Future studies should occur at sites where devil and competitor population densities have been quantified. Additionally, baiting near landscape and habitat features preferred by devils could also help improve uptake of ground baits by devils. For example, devils often travel along fence lines, whereas quolls are less likely to follow fence lines (Andersen et al. 2017).
The reduced devil density has been associated with other ecological changes, such as reduced fear behaviour in possums (Cunningham et al. 2019), which could also be associated with an increased uptake by possums and other non-target species. Lower devil density may also coincide with an overabundance of devil food in the environment (e.g. macropods and possums as roadkill). Overabundance of devil food may lead to reduced devil foraging and interest in small food items such as baits. Future studies at larger scales should be performed at long-term monitoring sites to assess the effects of devil density on the proportion of baits retrieved by devils.
Thirty-six per cent (16/45) of ground baits were removed by unknown species. It is likely that some of the unknown removals were by small animals that did not trigger the cameras. For example, rats are known to be present at one of the sites and a small burrow to the buried bait was observed, but no rat was recorded on video removing the bait. Additionally, foggy and rainy nights are likely to have contributed to some of the unknown bait removals by obscuring the animal that was otherwise in frame. Using the same camera model at each site would have been preferable and possibly could have reduced the number of baits removed by unknown species. However, it is unlikely that using the same camera model at each site would change the major conclusions of the study.
Our use of automated bait dispensers increased the percentage of baits being onsumed by devils nearly 10-fold in this pilot study. Importantly, the design of the dispenser decreased confirmed and suspected bait removals by macropods from 18% to 0%. This preliminary study also suggests that modifications to the dispenser position, such as raising the bait receptable higher off the ground, could reduce uptake by small species. For example, we observed one wild rat retrieve a bait at 200 mm above the ground; however, what appeared to be the same rat was unable to retrieve a bait once the receptacle of the dispenser had been raised to 350 mm. Eastern quolls were able to retrieve baits from a height of 350 mm but this often required a lunge to reach the bait, whereas devils could reach in and grab the bait at 350 mm. Future studies should test additional dispenser heights and refinement to the receptacle designs and non-target filters to further increase bait uptake by devils.
Dispensers suffer from the limitation that some individuals could repeatedly visit the dispensers throughout the night. We were unable to definitively identify individual devils in the dispenser footage, but it is very likely that some individual devils and quolls were able to retrieve more than one bait per night. Discriminatory sensor camera technology, as used in Felixer grooming traps (Read et al. 2019), may be used in conjunction with dispensers so that baits are released only when activation sensors recognise the shape of a devil. Alternatively, topical chemical deterrents could be used with baits to limit the ability of individual animals to monopolise baits or food resources (Johnson et al. 2022).
During wild trials, a cat was recorded passing by a dispenser site with a prey animal in its mouth. The prey species could not be definitively identified in the video, but the prey species was about the size of a rat. Rats had previously been recorded interacting with the dispenser. If future research shows that dispenser sites attract large numbers of prey animals, then predation by invasive predators could be an issue that would need to be addressed. For example, dispensers may be set up for a brief period at a location.
Increasing target uptake could potentially be accomplished through habitat-based and seasonal baiting programs based on devil behavioural and movement ecology, as has been simulated for racoons (McClure et al. 2022). Likewise, non-target uptake by herbivores and omnivores could potentially be reduced by baiting during periods when other food sources are readily available. Current knowledge about spatial and temporal distributions of non-target species such as pademelons (le Mar 2002), brushtail possums (le Mar 2002; le Mar and McArthur 2005; Hollings et al. 2015), and eastern quolls (Fancourt 2010; Hollings et al. 2013) would assist in timing bait distribution to correspond with periods when there is a greater ratio of target to non-target species.
Similar to the 2011 study that tested placebo ruminant-meat baits with captive devils (Hughes et al. 2011), we observed that male devils were more likely to consume baits than were female devils at two captive-devil facilities. A recent study suggested that males have larger home-range sizes than do females in areas affected by DFT1 (Comte et al. 2020), which could further lead to disproportionate uptake of baits by male devils. Studies of devil contact networks have shown that males are more likely to receive bite wounds than are females (Hamilton et al. 2019). The wounds create the potential for pathogen transmission and are more commonly inflicted during the mating season when males mate-guard females. High uptake of an effective vaccine by males could prevent transmitted tumour cells from establishing in new male hosts or progressing towards more severe disease post-infection. Previous research has also shown that males with severe disease become increasingly socially isolated, likely owing to the debilitating effects of the disease. Healthy, vaccinated male devils would be expected to be more competitive for mates and could ultimately reduce disease prevalence in females by reducing contact with diseased males.
Maximising delivery of the vaccine component of the bait is also critical for an effective OBV. For example, if the vaccine component can be easily separated from the bait matrix, then ingestion of the vaccine will be reduced. The results of capsule trials showed that most captive devils will eat and fully consume capsule-loaded fish bait. However, in many trials the capsule became separated from the bait matrix, which led to the bait being consumed but not the capsule. A uniform distribution of the vaccine throughout the bait matrix would force vaccine ingestion with the consumption of the bait matrix.
Feeding behaviour of the target species is also important for an effective vaccine. A study observing bait-feeding behaviours of captive raccoons and skunks with placebo baits found that raccoons were more likely to ‘chew the entire bait and hold the bait in their paws when eating’ than were skunks (Johnson et al. 2016). Comparatively, skunks were observed to pin the bait on the ground and possibly chew the bait more than do raccoons, increasing the chance of liquid vaccine dispersing onto the ground. In agreement with a prior prey-processing study of captive devils (Pollock et al. 2022), we found that captive and wild devils were likely to hold the bait in their paws and crush the bait.
In summary, the outcomes of this study lay the foundation for an OBV delivery system if an effective DFT1/2 vaccine can be developed. A bait that is less attractive to non-target species will be needed before large-scale ground-bait campaigns could be implemented because of the cost of OBVs. Flavour palatability and feeding behaviour information may be used to optimise bait-matrix formulations by designing a palatable bait that cannot be easily consumed by non-target species. However, our study also suggests that bait dispensers could be used effectively at regional levels as a means of reducing non-target uptake and increasing the specificity of delivering currently available bait formulations to devils.
Supplementary material
Supplementary material is available online.
Data availability
Data and videos related to this paper will be publicly available through the Supplementary material and the University of Tasmania Research Data Portal: https://rdp.utas.edu.au/ using data key ‘RD-EOBDMFTDH’.
Conflicts of interest
An associated bait research project by the authors is supported by funding from Animal Control Technologies Australia.
Declaration of funding
This research was supported by the Australian Research Council (ARC) DECRA grant #DE180100484 and ARC Discovery grant #DP180100520, University of Tasmania Foundation through funds raised by the Save the Tasmanian Devil Appeal, Wildcare Tasmania, a Charitable organisation from the Principality of Liechtenstein, and a Select Foundation Senior Research Fellowship. Baits were supplied by Animal Control Technologies Australia.
Author contributions
ASF, RJP, SBA, SD, and TJS designed the study. ASF, JMM, RP, and SD performed the experiments. ASF, NMFJ, and SD performed the data analysis. ASF, ATG, NMFJ, RJP, SD, and TJS interpreted the results. ASF and SD wrote the manuscript, and all authors edited the manuscript.
Acknowledgements
We thank Ginny Ralph for ongoing care of captive Tasmanian devils. We thank Androo Kelly and the knowledgeable keepers at the Trowunna Wildlife Sanctuary. We thank Dave Schaap and Drew Lee and other members of the Tasmanian Government’s Save the Tasmanian Devil Program for coordinating access to captive devils. We thank Dr Linton Staples for providing baits and a wealth of knowledge of baiting practices. We thank Associate Professor Chris Burridge, Professor Menna Jones, Professor Greg Woods, and Associate Professor Bruce Lyons for the comments and support on the Honours thesis associated with this study. We thank Dr Marissa Parrott for providing the base ethogram and comments on methods. We thank Dr Barrett Wolfe for statistical advice. We thank Robb Meijers and Tim Long for assistance with field trials.
References
Andersen, GE, Johnson, CN, and Jones, ME (2016). Sympatric predator odour reveals a competitive relationship in size-structured mammalian carnivores. Behavioral Ecology and Sociobiology 70, 1831–1841.| Sympatric predator odour reveals a competitive relationship in size-structured mammalian carnivores.Crossref | GoogleScholarGoogle Scholar |
Andersen, GE, Johnson, CN, Barmuta, LA, and Jones, ME (2017). Use of anthropogenic linear features by two medium-sized carnivores in reserved and agricultural landscapes. Scientific Reports 7, 11624.
| Use of anthropogenic linear features by two medium-sized carnivores in reserved and agricultural landscapes.Crossref | GoogleScholarGoogle Scholar |
Ballesteros, C, Vicente, J, Morriss, G, Jockney, I, Rodríguez, O, Gortázar, C, and de la Fuente, J (2011). Acceptance and palatability for domestic and wildlife hosts of baits designed to deliver a tuberculosis vaccine to wild boar piglets. Preventive Veterinary Medicine 98, 198–203.
| Acceptance and palatability for domestic and wildlife hosts of baits designed to deliver a tuberculosis vaccine to wild boar piglets.Crossref | GoogleScholarGoogle Scholar |
Bürkner, P-C (2017). brms: an R package for Bayesian multilevel models using Stan. Journal of Statistical Software 80, 1–28.
| brms: an R package for Bayesian multilevel models using Stan.Crossref | GoogleScholarGoogle Scholar |
Comte, S, Carver, S, Hamede, R, and Jones, M (2020). Changes in spatial organization following an acute epizootic: Tasmanian devils and their transmissible cancer. Global Ecology and Conservation 22, e00993.
| Changes in spatial organization following an acute epizootic: Tasmanian devils and their transmissible cancer.Crossref | GoogleScholarGoogle Scholar |
Cunningham, CX, Johnson, CN, Hollings, T, Kreger, K, and Jones, ME (2019). Trophic rewilding establishes a landscape of fear: Tasmanian devil introduction increases risk-sensitive foraging in a key prey species. Ecography 42, 2053–2059.
| Trophic rewilding establishes a landscape of fear: Tasmanian devil introduction increases risk-sensitive foraging in a key prey species.Crossref | GoogleScholarGoogle Scholar |
Cunningham, CX, Johnson, CN, and Jones, ME (2020). A native apex predator limits an invasive mesopredator and protects native prey: Tasmanian devils protecting bandicoots from cats. Ecology Letters 23, 711–721.
| A native apex predator limits an invasive mesopredator and protects native prey: Tasmanian devils protecting bandicoots from cats.Crossref | GoogleScholarGoogle Scholar |
Cunningham, CX, Comte, S, McCallum, H, Hamilton, DG, Hamede, R, Storfer, A, Hollings, T, Ruiz-Aravena, M, Kerlin, DH, Brook, BW, Hocking, G, and Jones, ME (2021). Quantifying 25 years of disease-caused declines in Tasmanian devil populations: host density drives spatial pathogen spread. Ecology Letters 24, 958–969.
| Quantifying 25 years of disease-caused declines in Tasmanian devil populations: host density drives spatial pathogen spread.Crossref | GoogleScholarGoogle Scholar |
Delong, ER, Delong, DM, and Clarke-Pearson, DL (1988). Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44, 837–845.
| Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach.Crossref | GoogleScholarGoogle Scholar |
Drawert, B, Flies, AS, Matthew, A, Powell, M, and Rumsey, B (2022). “Saving the Devils Is in the Details”. Letters in Biomathematics 9, 121–140.
Fancourt B (2010) Spatial and temporal variation in declining eastern quoll (Dasyurus viverrinus) populations in Tasmania, BSc (Hons) thesis, University of Tasmania.
Flies, AS, Flies, EJ, Fox, S, Gilbert, AT, Johnson, SR, Liu, G-S, Lyons, AB, Patchett, AL, Pemberton, D, and Pye, RJ (2020). An oral bait vaccination approach for the Tasmanian devil facial tumor diseases. Expert Review of Vaccines 19, 1–10.
| An oral bait vaccination approach for the Tasmanian devil facial tumor diseases.Crossref | GoogleScholarGoogle Scholar |
Friard, O, and Gamba, M (2016). BORIS : a free, versatile open-source event-logging software for video/audio coding and live observations. Methods in Ecology and Evolution 7, 1325–1330.
| BORIS : a free, versatile open-source event-logging software for video/audio coding and live observations.Crossref | GoogleScholarGoogle Scholar |
Gabry J, Mahr T (2022) ‘Bayesplot: plotting for Bayesian models. R package, R package version 1.9.0.’ Available at https://mc-stan.org/bayesplot/
Hamede, R, Madsen, T, McCallum, H, Storfer, A, Hohenlohe, PA, Siddle, H, Kaufman, J, Giraudeau, M, Jones, M, Thomas, F, and Ujvari, B (2021). Darwin, the devil, and the management of transmissible cancers. Conservation Biology 35, 748–751.
| Darwin, the devil, and the management of transmissible cancers.Crossref | GoogleScholarGoogle Scholar |
Hamilton, DG, Jones, ME, Cameron, EZ, McCallum, H, Storfer, A, Hohenlohe, PA, and Hamede, RK (2019). Rate of intersexual interactions affects injury likelihood in Tasmanian devil contact networks. Behavioral Ecology 30, 1087–1095.
| Rate of intersexual interactions affects injury likelihood in Tasmanian devil contact networks.Crossref | GoogleScholarGoogle Scholar |
Hobday, AJ, and Minstrell, ML (2008). Distribution and abundance of roadkill on Tasmanian highways: human management options. Wildlife Research 35, 712–726.
| Distribution and abundance of roadkill on Tasmanian highways: human management options.Crossref | GoogleScholarGoogle Scholar |
Holderness-Roddam, B, and McQuillan, PB (2014). Domestic dogs (Canis familiaris) as a predator and disturbance agent of wildlife in Tasmania. Australasian Journal of Environmental Management 21, 441–452.
| Domestic dogs (Canis familiaris) as a predator and disturbance agent of wildlife in Tasmania.Crossref | GoogleScholarGoogle Scholar |
Hollings, T, Jones, M, Mooney, N, and McCallum, H (2013). Trophic cascades following the disease-induced decline of an apex predator, the Tasmanian devil. Conservation Biology 28, 63–75.
| Trophic cascades following the disease-induced decline of an apex predator, the Tasmanian devil.Crossref | GoogleScholarGoogle Scholar |
Hollings, T, McCallum, H, Kreger, K, Mooney, N, and Jones, M (2015). Relaxation of risk-sensitive behaviour of prey following disease-induced decline of an apex predator, the Tasmanian devil. Proceedings of the Royal Society B: Biological Sciences 282, 20150124.
| Relaxation of risk-sensitive behaviour of prey following disease-induced decline of an apex predator, the Tasmanian devil.Crossref | GoogleScholarGoogle Scholar |
Hughes, C, Gaffney, R, and Dickman, CR (2011). A preliminary study assessing risk to Tasmanian devils from poisoning for red foxes. The Journal of Wildlife Management 75, 385–392.
| A preliminary study assessing risk to Tasmanian devils from poisoning for red foxes.Crossref | GoogleScholarGoogle Scholar |
Johnson, SR, Crider, NJ, Weyer, GA, and Tosh, RD Johnson, SR, Crider, NJ, Weyer, GA, and Tosh, RD (2016). Bait development for oral delivery of pharmaceuticals to raccoons (Procyon lotor) and striped skunks (Mephitis mephitis). Journal of Wildlife Diseases 52, 893–901.
| Bait development for oral delivery of pharmaceuticals to raccoons (Procyon lotor) and striped skunks (Mephitis mephitis).Crossref | GoogleScholarGoogle Scholar |
Johnson, SR, Deliberto, ST, Urchek, K, Gilbert, AT, and Werner, SJ (2022). Concentration-response of an anthraquinone-based repellent for raccoons (Procyon lotor). Applied Animal Behaviour Science 251, 105628.
| Concentration-response of an anthraquinone-based repellent for raccoons (Procyon lotor).Crossref | GoogleScholarGoogle Scholar |
Jones, ME (2000). Road upgrade, road mortality and remedial measures: impacts on a population of eastern quolls and Tasmanian devils. Wildlife Research 27, 289–296.
| Road upgrade, road mortality and remedial measures: impacts on a population of eastern quolls and Tasmanian devils.Crossref | GoogleScholarGoogle Scholar |
Jones, ME, Paetkau, D, Geffen, E, and Moritz, C (2004). Genetic diversity and population structure of Tasmanian devils, the largest marsupial carnivore. Molecular Ecology 13, 2197–2209.
| Genetic diversity and population structure of Tasmanian devils, the largest marsupial carnivore.Crossref | GoogleScholarGoogle Scholar |
Kayigwe, AN, Darby, JM, Lyons, AB, Patchett, AL, Lisowski, L, Liu, G-S, and Flies, AS (2022). A human adenovirus encoding IFN-γ can transduce Tasmanian devil facial tumour cells and upregulate MHC-I. Journal of General Virology , .
| A human adenovirus encoding IFN-γ can transduce Tasmanian devil facial tumour cells and upregulate MHC-I.Crossref | GoogleScholarGoogle Scholar |
Lamp L (2021) Implementation of an agent-based model for devil facial tumor disease in Tasmanian devils, and evaluation of interventions. Master of Science, University of Saskatchewan. Available at https://harvest.usask.ca/handle/10388/13666
Lawrence C, Wiersma HF (2019) DFTD is a killer but what about other threats. In ‘Saving the Tasmanian devil: recovery through science-based management’. (Eds CJ Hogg, S Fox, D Pemberton, K Belov) pp. 131–138. (CSIRO Publishing: Melbourne, Vic., Australia)
Lazenby, BT, Tobler, MW, Brown, WE, Hawkins, CE, Hocking, GJ, Hume, F, Huxtable, S, Iles, P, Jones, ME, Lawrence, C, Thalmann, S, Wise, P, Williams, H, Fox, S, and Pemberton, D (2018). Density trends and demographic signals uncover the long-term impact of transmissible cancer in Tasmanian devils. Journal of Applied Ecology 55, 1368–1379.
| Density trends and demographic signals uncover the long-term impact of transmissible cancer in Tasmanian devils.Crossref | GoogleScholarGoogle Scholar |
le Mar K (2002) Spatial organisation and habitat selection patterns of three marsupial herbivores within a patchy forestry environment, PhD thesis, University of Tasmania.
le Mar, K, and McArthur, C (2005). Comparison of habitat selection by two sympatric macropods, Thylogale billardierii and Macropus rufogriseus rufogriseus, in a patchy eucalypt-forestry environment. Austral Ecology 30, 674–683.
| Comparison of habitat selection by two sympatric macropods, Thylogale billardierii and Macropus rufogriseus rufogriseus, in a patchy eucalypt-forestry environment.Crossref | GoogleScholarGoogle Scholar |
Mallick, S, Pauza, M, Eason, C, Mooney, N, Gaffney, R, and Harris, S (2016). Assessment of non-target risks from sodium fluoroacetate (1080), para-aminopropiophenone (PAPP) and sodium cyanide (NaCN) for fox-incursion response in Tasmania. Wildlife Research 43, 140–152.
| Assessment of non-target risks from sodium fluoroacetate (1080), para-aminopropiophenone (PAPP) and sodium cyanide (NaCN) for fox-incursion response in Tasmania.Crossref | GoogleScholarGoogle Scholar |
McClure, KM, Bastille-Rousseau, G, Davis, AJ, Stengel, CA, Nelson, KM, Chipman, RB, Wittemyer, G, Abdo, Z, Gilbert, AT, and Pepin, KM (2022). Accounting for animal movement improves vaccination strategies against wildlife disease in heterogeneous landscapes. Ecological Applications 32, e2568.
| Accounting for animal movement improves vaccination strategies against wildlife disease in heterogeneous landscapes.Crossref | GoogleScholarGoogle Scholar |
Müller, TF, Schröder, R, Wysocki, P, Mettenleiter, TC, and Freuling, CM (2015). Spatio-temporal use of oral rabies vaccines in fox rabies elimination programmes in Europe. PLoS Neglected Tropical Diseases 9, e0003953.
| Spatio-temporal use of oral rabies vaccines in fox rabies elimination programmes in Europe.Crossref | GoogleScholarGoogle Scholar |
Palphramand, K, Delahay, R, Robertson, A, Gowtage, S, Williams, GA, McDonald, RA, Chambers, M, and Carter, SP (2017). Field evaluation of candidate baits for oral delivery of BCG vaccine to European badgers, Meles meles. Vaccine 35, 4402–4407.
| Field evaluation of candidate baits for oral delivery of BCG vaccine to European badgers, Meles meles.Crossref | GoogleScholarGoogle Scholar |
Pearse, AM, and Swift, K (2006). Transmission of devil facial-tumour disease. Nature 439, 549.
| Transmission of devil facial-tumour disease.Crossref | GoogleScholarGoogle Scholar |
Pollock, TI, Hocking, DP, Hunter, DO, Parrott, ML, Zabinskas, M, and Evans, AR (2022). Torn limb from limb: the ethology of prey-processing in Tasmanian devils (Sarcophilus harrisii). Australian Mammalogy 44, 126–138.
| Torn limb from limb: the ethology of prey-processing in Tasmanian devils (Sarcophilus harrisii).Crossref | GoogleScholarGoogle Scholar |
Pye, RJ, Pemberton, D, Tovar, C, Tubio, JMC, Dun, KA, Fox, S, Darby, J, Hayes, D, Knowles, GW, Kreiss, A, Siddle, HVT, Swift, K, Lyons, AB, Murchison, EP, and Woods, GM (2016a). A second transmissible cancer in Tasmanian devils. Proceedings of the National Academy of Sciences 113, 374–379.
| A second transmissible cancer in Tasmanian devils.Crossref | GoogleScholarGoogle Scholar |
Pye, R, Hamede, R, Siddle, Hv, Caldwell, A, Knowles, GW, Swift, K, Kreiss, A, Jones, ME, Lyons, AB, and Woods, GM (2016b). Demonstration of immune responses against devil facial tumour disease in wild Tasmanian devils. Biology Letters 12, 20160553.
| Demonstration of immune responses against devil facial tumour disease in wild Tasmanian devils.Crossref | GoogleScholarGoogle Scholar |
Pye, R, Patchett, A, McLennan, E, Thomson, R, Carver, S, Fox, S, Pemberton, D, Kreiss, A, Baz Morelli, A, Silva, A, Pearse, MJ, Corcoran, LM, Belov, K, Hogg, CJ, Woods, GM, and Lyons, AB (2018). Immunization strategies producing a humoral IgG immune response against devil facial tumor disease in the majority of Tasmanian devils destined for wild release. Frontiers in Immunology 9, 259.
| Immunization strategies producing a humoral IgG immune response against devil facial tumor disease in the majority of Tasmanian devils destined for wild release.Crossref | GoogleScholarGoogle Scholar |
Pye, R, Darby, J, Flies, AS, Fox, S, Carver, S, Elmer, J, Swift, K, Hogg, C, Pemberton, D, Woods, G, and Lyons, AB (2021). Post-release immune responses of Tasmanian devils vaccinated with an experimental devil facial tumour disease vaccine. Wildlife Research 48, 701–712.
| Post-release immune responses of Tasmanian devils vaccinated with an experimental devil facial tumour disease vaccine.Crossref | GoogleScholarGoogle Scholar |
R Core Team (2021) ‘R: a language and for statistical computing.’ (R Foundation for Statistical Computing)
Read, JL, Bowden, T, Hodgens, P, Hess, M, Mcgregor, H, and Moseby, K (2019). Target specificity of the felixer grooming ‘trap’. Wildlife Society Bulletin 43, 112–120.
| Target specificity of the felixer grooming ‘trap’.Crossref | GoogleScholarGoogle Scholar |
Rocke, TE, Tripp, DW, Russell, RE, Abbott, RC, Richgels, KL, Matchett, MR, Biggins, DE, Griebel, R, Schroeder, G, Grassel, SM, Pipkin, DR, Cordova, J, Kavalunas, A, Maxfield, B, Boulerice, J, and Miller, MW (2017). Sylvatic Plague Vaccine Partially Protects Prairie Dogs (Cynomys spp.) in Field Trials. EcoHealth 14, 438–450.
| Sylvatic Plague Vaccine Partially Protects Prairie Dogs (Cynomys spp.) in Field Trials.Crossref | GoogleScholarGoogle Scholar |
Scoleri, VP, Johnson, CN, Vertigan, P, and Jones, ME (2020). Conservation trade-offs: island introduction of a threatened predator suppresses invasive mesopredators but eliminates a seabird colony. Biological Conservation 248, 108635.
| Conservation trade-offs: island introduction of a threatened predator suppresses invasive mesopredators but eliminates a seabird colony.Crossref | GoogleScholarGoogle Scholar |
Smyser, TJ, Redding, JV, Bevis, CM, Page, LK, and Swihart, RK (2015). Development of an automated dispenser for the delivery of medicinal or vaccine-laden baits to raccoons (Procyon lotor). Journal of Wildlife Diseases 51, 513–518.
| Development of an automated dispenser for the delivery of medicinal or vaccine-laden baits to raccoons (Procyon lotor).Crossref | GoogleScholarGoogle Scholar |
Thalmann, S, Peck, S, Wise, P, Potts, JM, Clarke, J, and Richley, J (2016). Translocation of a top-order carnivore: tracking the initial survival, spatial movement, home-range establishment and habitat use of Tasmanian devils on Maria Island. Australian Mammalogy 38, 68–79.
| Translocation of a top-order carnivore: tracking the initial survival, spatial movement, home-range establishment and habitat use of Tasmanian devils on Maria Island.Crossref | GoogleScholarGoogle Scholar |
Tovar, C, Pye, RJ, Kreiss, A, Cheng, Y, Brown, GK, Darby, J, Malley, RC, Siddle, HVT, Skjødt, K, Kaufman, J, Silva, A, Baz Morelli, A, Papenfuss, AT, Corcoran, LM, Murphy, JM, Pearse, MJ, Belov, K, Lyons, AB, and Woods, GM (2017). Regression of devil facial tumour disease following immunotherapy in immunised Tasmanian devils. Scientific Reports 7, 43827.
| Regression of devil facial tumour disease following immunotherapy in immunised Tasmanian devils.Crossref | GoogleScholarGoogle Scholar |
Vehtari, A, Gabry, J, Yao, Y, and Gelman, A (2018). loo: efficient leave-one-out cross-validation and WAIC for Bayesian models. R package version 2, 1003.
Wickham H (2016) ‘ggplot2: elegant graphics for data analysis.’ (Springer)
Woinarski, JCZ, Braby, MF, Burbidge, AA, Coates, D, Garnett, ST, Fensham, RJ, Legge, SM, McKenzie, NL, Silcock, JL, and Murphy, BP (2019). Reading the black book: the number, timing, distribution and causes of listed extinctions in Australia. Biological Conservation 239, 108261.
| Reading the black book: the number, timing, distribution and causes of listed extinctions in Australia.Crossref | GoogleScholarGoogle Scholar |
† A preprint version of this article is available at https://lettersinbiomath.journals.publicknowledgeproject.org/index.php/lib/article/view/555.


