Activity and movement of small mammal tick hosts at the urban fringes of Sydney, Australia
Casey L. Taylor A * , Dieter F. Hochuli
A * , Dieter F. Hochuli  A and Peter B. Banks
A and Peter B. Banks  A
A
A School of Life and Environmental Sciences, The University of Sydney, Camperdown, NSW 2006, Australia.
Wildlife Research 50(11) 927-938 https://doi.org/10.1071/WR22069
Submitted: 7 April 2022 Accepted: 22 December 2022 Published: 10 February 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Small mammals may traverse the urban fringe and use both natural and anthropogenic resources. In Australia, human commensal black rats (Rattus rattus) and native long-nosed bandicoots (Perameles nasuta) are important tick hosts, which can be found persisting at the urban fringe, leading to human–wildlife conflict.
Aims: We aimed to (1) determine the relative activity of small mammals in yards and associations with yard attributes, (2) compare activity of black rats and long-nosed bandicoots in bushland with activity in yards and (3) determine the proportion of black rats and long-nosed bandicoots that crossed the urban fringe. We predicted that native bandicoots would be more active in bushland habitats and that black rats would be more active in yards.
Methods: We used camera trapping in 56 residential yards, 18 of which were paired with adjacent bushland to measure small mammal activity in the two habitats. We recorded yard attributes and examined these associations using generalised linear models. We used isodar analysis to investigate black rat preferences of bushland habitat compared with yards, and we used Rhodamine B baiting to investigate movement at the urban fringe.
Key results: We found that black rats were the most active small mammal in residential yards and were detected in more yards than other small mammals, followed by bandicoots. Black rat activity was greater in yards adjacent to bushland, but no other yard attributes were associated with black rat and bandicoot activity. Overall, activity tended to be higher in bushland than in yards at paired locations.
Conclusions: Our findings suggest residential yards likely provide high-quality resources for long-nosed bandicoots. Low rates of movement at the urban fringe (6%), and a preference for bushland at low densities suggests that black rats may be synanthropic rather than commensal, occupying an urban niche but not depending on anthropogenic resources as expected.
Implications: Residential properties located adjacent to bushland may be exposed to increased black rat activity in yards. Future work should consider how introduced rats may be controlled in bushland to assist urban rat control efforts and avoid non-target impacts. Residential yards are likely to be important habitat for the persistence of long-nosed bandicoots in urban environments.
Keywords: anthropogenic, native wildlife, pest management, rodents, synanthropes, urban–bushland interface, urban ecology, ticks.
Introduction
Wildlife can persist at the interface between urban and natural areas (hereafter the urban fringe) by using both natural and anthropogenic resources (Blair 2001; McKinney 2002). Wildlife may be implicated in human–wildlife conflict and the spread of disease, and may suffer mortality due to pets and vehicle collisions (Banks and Smith 2015; Murray et al. 2016; McMahon et al. 2018). Many native species are attracted to anthropogenic resources; for example, coyotes visit urban yards to forage on garbage, compost, and bird seed (Murray and St. Clair 2017), occasionally leading to attacks on humans (Baker and Timm 2017). Conversely, some human commensal species (hereafter commensals) (Blair 2001; McKinney 2002) may overcome barriers to spill over, leading to colonisation of natural areas, and transitioning from human commensal to invasive (Hulme-Beaman et al. 2016). One of the world’s most widespread and destructive invasive commensal species, the black rat (Rattus rattus), has established free-living populations in some natural areas (Williams et al. 2003; King et al. 2011; Banks and Smith 2015) in the absence of competition (Stokes et al. 2009) and perceived predation (Williams et al. 2003), with negative impacts on native fauna (Banks and Hughes 2012). Mitigating these negative impacts requires an understanding of the behaviour of both native and commensal wildlife at the urban fringe (Hassell et al. 2017).
Resource availability (Hubert et al. 2011; Byers et al. 2019), and predation risk (Carthey and Banks 2012; Pettett et al. 2017) are important in determining the abundance and behaviour of native and commensal wildlife at the urban fringe. Resource availability in residential yards can provide food and shelter that attracts animals from neighbouring natural landscapes or allows some animals to become ‘resident’ of the urban area (Baker and Harris 2007). For example, native raccoons (Procyon lotor) generally prefer urban sites with anthropogenic food sources such as picnic areas with open garbage cans and dumpsters (Bozek et al. 2007). Small- to medium-sized mammals in North Carolina, USA are more active in yards compared with woodlots, likely due to chicken coops and bird feeders (Kays and Parsons 2014; Reed and Bonter 2018). Similarly, mammal species richness, diversity and relative abundance can be higher in yards and suburban forest compared with rural forest, with supplemental feeding of wildlife an important driver (Hansen et al. 2020a). Conversely, anthropogenic disturbances or predation risks posed by domestic animals may deter wildlife. For example, wood mice (Apodemus sylvaticus) are common in residential gardens in Bristol, UK but their abundance is negatively associated with distance to vegetation remnants and cat abundance (Baker et al. 2003). Similarly, raccoon, eastern grey squirrel (Sciurus carolinensis) and eastern cottontail (Sylvilagus floridanus) activity in North Carolina is negatively associated with fenced-in yards containing dogs (Kays and Parsons 2014).
A range of small- to medium-sized mammals are common at the urban fringes of Sydney – Australia’s largest city. These include long-nosed bandicoots (Perameles nasuta), ringtail possums (Pseudocheirus peregrinus), brushtail possums (Trichosurus vulpecula) (Wat et al. 2020), invasive black rats, rabbits (Oryctolagus cuniculus) and foxes (Vulpes vulpes). Bandicoots are often the source of human–wildlife conflict because they disturb lawns and gardens while foraging, and residents fear that bandicoots visiting yards will expose them to ticks (Fitzgibbon and Jones 2006; Dowle and Deane 2009; Chen 2013). Such negative perceptions of native wildlife can diminish public support for conservation efforts. On the other hand, the invasive black rat has colonised bushland remnants around Sydney (Banks et al. 2011), and has been shown to cross the urban fringe in some areas (Weerakoon 2012). This poses a potential predation risk to native wildlife and a disease risk to humans (Banks and Smith 2015). Despite their global ubiquity, black rats are historically difficult to study in urban systems (Parsons et al. 2017); therefore their ecology in urban areas beyond city centres (such as the urban fringe) remains poorly understood (Banks and Hughes 2012; Banks and Smith 2015; Parsons et al. 2017).
Globally, small mammals are important hosts of medically significant tick species, and in Australia, native bandicoots and invasive black rats are important in maintaining urban tick populations (Lydecker et al. 2019a, 2019b). Bites of Ixodes holocyclus are common on the east coast of Australia and can lead to bacterial and viral infections, debilitating allergic reactions (van Nunen 2018) and paralysis (Hall-Mendelin et al. 2011). Ticks can be frequently encountered in residential yards, including yards at the urban fringe. In Brisbane, Australia, the number of reported paralysis cases in dogs within urban areas is associated with the number and area of natural vegetation remnants within postcode boundaries, reinforcing that the urban fringe represents a high-risk area for people and pets on Australia’s East Coast (Gerasimova et al. 2018). This has prompted calls to manage ticks by removing native hosts (Chen 2013). However, we have a poor understanding of small mammal activity in residential yards and adjacent bushland, which yard features are associated with small mammal activity in yards, and whether those mammals are moving between yards and adjacent bushland in areas where ticks threaten public health. Addressing these gaps will aid decisions of where to target wildlife management efforts at the urban fringe, which is critical for managing public health risks posed by wildlife, as well as conservation threats to native species in those areas. Identifying yard attributes associated with activity is important for determining whether yards can be manipulated to encourage or discourage wildlife.
In this study, we aimed to understand ground-dwelling small mammal behaviour at the urban fringe with a focus on black rats and long-nosed bandicoots. We investigated: (1) activity in yards and associated yard attributes; (2) activity in bushland compared with adjacent yards using camera trapping; and (3) movement between yards and adjacent bushland using Rhodamine B, a non-toxic biomarker.
We predicted that rat activity would be higher in yards due to the association between black rats and anthropogenic resources in urban environments (Banks and Smith 2015), and that native mammal activity would be higher in bushland due to superior natural habitat such as nesting and refuge sites (Chambers and Dickman 2002). For yard attributes, we predicted that: (1) the presence of a vegetable garden, bird feeders, compost and chickens in yards would be associated with higher rat activity by providing food sources for rats; (2) the presence of vegetable gardens, compost, bird feeders, mulch, and chickens would be associated with higher bandicoot activity in yards by providing optimal foraging conditions or food sources (Scott et al. 1999; Hughes and Banks 2010); (3) living directly adjacent to bushland would be associated with higher bandicoot activity because bushland likely provides superior habitat (Hughes and Banks 2010; Maclagan et al. 2020); and (4) the presence of pets would be associated with lower rat and bandicoot activity due to the perceived threat of predation (Carthey and Banks 2012, 2018).
Materials and methods
Study area
This study was conducted in the Northern Beaches Local Government area of New South Wales, Australia. Sydney’s Northern Beaches is located on Australia’s East Coast where I. holocyclus is common. The Northern Beaches region comprises urban development adjoining numerous bushland remnants (Fig. 1). Some of these remnants connect to larger natural areas, including Garigal National Park and Ku-ring-gai National Park. Bushland remnants contain a diverse range of vegetation communities and native and introduced vertebrates, which can also be found in the urban matrix. Native vegetation communities include Sydney coastal dry sclerophyll forest, wet sclerophyll forest, Sydney coastal heath, and coastal floodplain wetlands (Office of Environment and Heritage Sydney 2016).
We selected study sites based on an online survey of residents’ experiences with ticks and wildlife completed by Northern Beaches residents in 2018, identifying those who agreed to participate in future field research on ticks. From these we selected a combination of yards that were adjacent to bushland (n = 35) and yards more distant from bushland (n = 21) (Fig. 1).
Camera trapping
We used ScoutGuard 560K infrared wildlife cameras to obtain an index of ground-dwelling small mammal activity in yards (n = 56). Eighteen of the 56 yards were paired sites, where cameras were placed in adjacent bushland <100 m away (n = 18). ScoutGuard cameras were originally designed for large mammals but reliably detect small mammals such as black rats (Weerakoon et al. 2014). Our past work has shown that ScoutGuard cameras detect 100% of black rats’ visits when used with a food lure (Weerakoon et al. 2014).
We placed two cameras in each yard: one facing lawn, to target mammals that forage in the open (e.g. long-nosed bandicoots), and one facing garden to target mammals that forage under cover (e.g. black rats).
For paired bushland sites (n = 18), we placed one camera in a relatively open area and one in a closed area (e.g. amongst dense vegetation) a few metres apart to replicate the camera placement in yards. Cameras were deployed at all sites for three consecutive nights. Black rat visits on the first night of camera trapping have the strongest relationship with known population size (Weerakoon et al. 2014), so we considered three nights to be sufficient for estimating activity in the two habitats. Cameras were attached to wooden stakes approximately 30–50 cm above the ground. We sprayed a cold-pressed peanut oil lure (7 mL; 10 squirts from a plastic spray bottle) 1 m from each camera in the centre of the camera field of view. Peanut oil is long-lasting and likely to keep animals investigating long enough to trigger the camera and increase the likelihood of equal detectability among species (Paull et al. 2011; Weerakoon et al. 2014).
We programmed cameras to capture 30-s high-resolution (1280 × 720) videos with a 5-min interval between recordings to reduce the chance of capturing multiple videos of the same visit (Heavener et al. 2014; Jarman and Driessen 2019; Bytheway et al. 2021). Therefore, we considered each video or detection a new visit. Cameras were set, on high sensitivity, to record videos from dusk until dawn (e.g. 1800–0600 h), when the target small mammals were most active. When visiting yards, we recorded the presence of compost bins, vegetable and/or herb gardens, chickens, cats, dogs, mulch in gardens, bird feeders, whether the yard was adjacent to bushland or fully fenced (wooden or metal privacy fencing), and whether rodent control was implemented. Compost bins varied in design, but most were enclosed or had been altered to prevent wildlife accessing the compost, with varying degrees of success based on the resident’s observations.
Rhodamine B baiting
We investigated small mammal movement between yards and adjacent bushland by placing food baits treated with Rhodamine B (a non-toxic biomarker) in yards. We then trapped small mammals in adjacent bushland three weeks later to collect guard hairs to search for the presence of Rhodamine B.
We studied movement at 14 urban fringe sites in Sydney’s Northern Beaches from August to November in 2020 (Fig. 1). We classified sites as (1) a direct interface if yards were adjacent to bushland (n = 8), (2) an indirect interface if yards were separated from bushland by road (n = 3), or (3) a mixed interface if some yards were adjacent to bushland and some were separated from the bushland by road (n = 3). Though we attempted to select sites with similar characteristics, there was some variation within categories. For example, some ‘direct interface’ sites had open space (Asset Protect Zones) between bushland and property boundaries. Asset Protection Zones are fuel-reduced areas (10–20 m) designed to protect properties from bushfires through clearing vegetation, removing leaf litter, and mowing grass.
We mixed Rhodamine B dye (hereafter RB) (ChemSupply Australia Pty Ltd, Gillman, SA, Australia) into a food bait mixture of peanut butter, rolled oats, and honey at a non-toxic concentration of 0.1% (15 mg RB/15 g bait ball) following Weerakoon (2012). A concentration of 0.1% balances RB detection in hairs with palatability in black rats (Weerakoon 2012). We placed approximately 10 bait balls in each Elliot trap (type B; 46 cm × 15.5 cm × 15 cm) and 20 bait balls in each cage trap (72 cm × 32 cm × 31 cm) and set traps across approximately 150 m of housing at each site. We used Elliot and medium-sized cage traps over bait stations used by Weerakoon (2012) to target both native and invasive rodents and long-nosed bandicoots.
The number of yards included at each site varied from 5 to 10 depending on the level of participation from residents. We placed one-to-two trap stations (one cage and one Elliot trap) in each yard totalling 212 traps across 102 yards. Because the number of yards at a site varied, the total amount of bait deployed ranged from 2324 g to 3750 g per site (average 3389 g/site). All traps were secured open using wire so that the target animals (black rats and bandicoots) could readily enter and consume bait. We placed traps in, or adjacent to, areas of low-lying vegetation, e.g. in gardens or along fences in yards with sparse vegetation. Traps were left out for four days and remaining bait was weighed to obtain an estimate of bait consumption.
Three weeks after baits were deployed in yards, we trapped small mammals in adjacent bushland to collect guard hairs for RB detection (Weerakoon et al. 2013). At each site, one transect containing 10 paired trap stations (one Elliot trap and one cage trap) was set up within 100 m of residential housing. Traps were baited with a mixture of peanut butter, rolled oats and honey, and checked at sunrise each morning.
We recorded the species, weight, and sex of each captured animal. As part of a larger project on the associations between ticks and wildlife hosts, we anaesthetised animals to estimate tick load using isoflurane vaporised in medical grade oxygen with a field anaesthetic machine. We collected guard hairs while animals were recovering from anaesthesia and thus were able to collect 25 guard hairs from all over the body using fine-tipped forceps. We stored guard hairs in plastic zip-lock bags for later examination using fluorescence microscopy. Guard hairs have been shown to be a reliable hair type for RB detection, and fluorescence microscopy is a more reliable method of RB detection compared with ambient light and UV light (Weerakoon et al. 2013).
We washed guard hairs with isopropyl alcohol for 2 min then placed hairs in distilled water for 3 min, changing baths and cleaning bowls between samples following Fisher et al. (1999) and Weerakoon et al. (2013). We allowed hairs to dry at room temperature in petri dishes for 24 h, then mounted 15–25 hairs on a large glass slide with Fluoromount-G mounting medium (Thermo Fisher Scientific Australia, Scoresby, Victoria, Australia) before placing a coverslip over them and allowing the slides to dry at room temperature for three days.
We examined hairs using a Zeiss Axiolab 5 fluorescence microscope (Zeiss, Jena, Germany) under 10× magnification with filter set 109 and a green light filter to detect RB fluorescence. Animals were classified as RB positive and thus having crossed the urban fringe if any of their sample hairs had at least one bright orange, fluorescent band (Fig. 2). We cross-checked samples with two positive controls from previous studies where individuals ingested known concentrations of RB and had definite RB bands or markings and two negative controls where individuals had not been exposed to RB (Weerakoon et al. 2013). We took images of RB positive hairs with the Axiocam 208 and Zeiss ZenPro microscope software.
Data analysis
We conducted all analyses in R (R Core Team 2021). Firstly, we used a Kruskal–Wallis test followed by Dunn’s pairwise multiple comparison test with Benjamini–Hochberg adjustments implemented by the R package FSA (Ogle et al. 2021) to compare the index of activity of the different mammals in backyards. We used a Kruskal–Wallis test because our data did not meet the normality assumptions of an ANOVA. Only visits where individual animals could be identified to species were included in analyses. We calculated activity by summing the number of visits or detection events that were separated by a 5-min interval on cameras, over the three nights for each species. Activity was our focus rather than abundance because hosts that are more active in yards (i.e. visit more) are more likely to have ticks detach that can then moult and bite residents or pets.
We then compared activity in yards with activity in bushland for the most frequently detected mammals and important tick hosts (black rats and long-nosed bandicoots), using generalised linear mixed models (GLMMs) implemented with the R package glmmADMB (Bolker et al. 2012). We fitted GLMMs with a negative binomial distribution and log link function and specified site ID as a random effect to account for the correlation between paired sites. We did not investigate possum activity further because our methods were targeted at ground-dwelling species and our other work has shown that possums are not likely to be as important tick hosts as black rats and bandicoots are Taylor (2022).
For black rats and long-nosed bandicoots, we also explored the relationship between activity indices and: (1) yard attributes, including the presence of vegetable and/or herb gardens, chickens, cats, dogs, mulch in gardens; (2) yard location (adjacent to bushland or not); and (3) whether rodent control was implemented by residents. We used GLMMs with a negative binomial distribution and log link to test the effect of yard attributes on rat and bandicoot activity, with yard as a random effect in the models. We included rat control as a predictor in the model on rat activity. Because ‘veg or herb garden’ was associated with ‘compost’ (P < 0.001), we dropped compost from all models. We excluded ‘bird feeders’ because only three yards had feeders. We did not test the effects of yard attributes on the activity of other mammals due to the very low numbers of camera visits or detection events.
Lastly, we used isodar analysis to determine whether habitat selection in black rats, the most active small mammal in yards, was density dependent. Isodar analysis involves regressing the simultaneous density in two adjoining habitats against one another (Morris 1987, 1988). The slope and intercept values of the regression can be used to understand differences between habitat types: a significant intercept indicates quantitative differences between the two habitats, for example, resource levels, and a significant slope indicates qualitative differences, for example, resource quality (Morris 1988). We calculated isodars using black rat visitations on cameras in backyards and adjacent bushland because rat visitation using our methods is a reliable estimate of broad categories of density (Weerakoon et al. 2014).
Animal ethics
All procedures were carried out with The University of Sydney Animal Ethics Committee approval (2018/1429).
Results
Overall, 780 mammal visits were recorded on cameras across 88% of residential yards sampled in this study. Invasive black rats were significantly more active (514 visits, mean ± s.d. 9.18 ± 12.40) than long-nosed bandicoots (177 visits, mean ± s.d. 3.16 ± 4.86), brushtail possums (53 visits, mean ± s.d. 0.95 ± 1.59), swamp wallabies (14 visits, mean ± s.d. 0.25 ± 1.31), brown rats (13 visits, mean ± s.d. 0.23 ± 1.24), and ringtail possums (four visits, mean ± s.d. 0.07 ± 0.26) (H = 102, d.f. = 5, P < 0.001) (Fig. 3). Low numbers of visits were recorded for introduced foxes (V. vulpes) (one visit) and rabbits (O. cuniculus) (four visits).
Black rat visits were recorded at more yards than other mammals (66%), followed by bandicoots (46%) then brushtail possums (39%), ringtail possums (7%), brown rats (7%), and wallabies (5%) (Fig. 3). All species were detected on cameras facing lawn and cameras facing garden, except the one fox visit that was detected on a camera facing a garden. There was only a significant difference in detections between lawn facing and garden facing cameras for black rats, where significantly more black rats were detected on cameras facing garden (P ≤ 0.001). There was no significant difference in black rat activity between bushland and backyard sites (estimate = 0.67, P = 0.19) nor bandicoot activity (estimate = 0.702, P = 0.13) (Fig. 4).
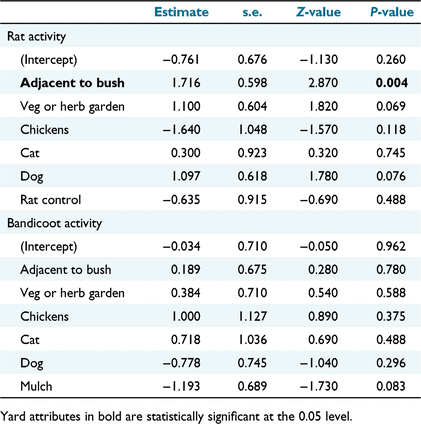
Only ‘adjacent to bush’ had a significant relationship with rat activity in residential yards (Table 1; Fig. 5), and none of the yard attributes recorded had a significant relationship with bandicoot activity in yards (Table 1). Whether a yard was fully fenced or not had no association with whether bandicoots were detected on camera (χ2 = 1.22, P = 0.27). The isodar regression (y = −0.031x + 9.393) was not statistically significant (F = 0.01, d.f. = 16, P = 0.918) (Fig. 6) but the y-intercept was significantly different from 0 (P = 0.007, 95% CI: 2.917–15.869), suggesting that black rats preferentially select bushland when at low population densities.
|
We collected guard hairs from 67 mammals trapped in bushland adjacent to yards where RB baits were deployed. Definite RB marking was characterised by bright orange/red fluorescent bands and was detected in only four individuals (6%): three black rats and one long-nosed bandicoot. We captured 40 black rats, 21 long-nosed bandicoots, three bush rats (Rattus fuscipes), two brushtail possums, and one swamp rat (Rattus lutreolus). Indeterminate fluorescence, i.e. fluorescence that could not be classified as natural or due to RB, was present in 22 other individuals. All three black rats with RB bands were female, and the long-nosed bandicoot was male. All individuals that crossed the urban–bushland interface were from ‘direct sites’ where bushland vegetation directly abutted yard boundaries. Bait consumption was highly variable and ranged from 324 g to 2245 g at a site (average 889 g/site); however, we used medium-sized cage traps that were possibly accessible to larger animals. The average amount of bait remaining at a site was 2399 g, so it is likely that, even with some degree of bait consumption by non-target species such as possums and brush-turkeys, sufficient bait remained for black rats and bandicoots to consume.
Discussion
Our findings show that some native and commensal introduced small mammals are readily using residential yards and adjacent bushland at the urban fringe near Sydney – Australia’s largest city. Black rats were the most commonly detected small mammal in yards and were more active in yards adjacent to bushland. Black rat and long-nosed bandicoot activity tended to be higher in bushland than in adjacent yards, though the difference was not significant. This suggests that the two habitats may be comparable in resource availability or that access to anthropogenic resources may outweigh the potential costs of navigating the urban matrix for these species, at least at the urban fringe. Black rat and long-nosed bandicoot activity at paired sites was highly variable overall, likely reflecting the heterogeneity in habitat quality and food sources in residential yards at the urban fringe.
The loss of many native mammals in urban areas of Australia in the last century due to habitat fragmentation and invasive predators (Burbidge and McKenzie 1989; Dickman 1996) likely explains why black rats were the most active small mammal in yards. However, greater rat activity in yards adjacent to bushland, and greater black rat activity in bushland overall compared with yards (Fig. 4), is contrary to our expectations given the historical association between black rats and anthropogenic resources. Black rats are common in bushland remnants around Sydney (Banks et al. 2011; Weerakoon 2012), but a preference for structurally complex microhabitats such as dense understorey and leaf litter compared with open grassed areas (Cox et al. 2000; Williams et al. 2003) likely explains our findings. This finding aligns with some other work globally: the risk of urban rat infestations is greater near vegetated areas in Spain (Tamayo-Uria et al. 2014), and high levels of bait consumption by urban rodents in Italy is associated with vegetation (Patergnani et al. 2010). This is likely because structurally complex vegetation provides protection from predators and supports fine-scale rat movement (Cox et al. 2000). Black rats construct dome-shaped nests using leaves, and are known to nest in trees when living in natural areas (Hooker and Innes 1995; Matsui et al. 2010). Therefore, bushland may provide superior nesting opportunities for black rats compared with residential yards. Another possible explanation for our unexpected result is that rat activity is greater in yards adjacent to bushland because of the lack of rodent control in bushland compared with yards adjacent to other yards where rodent control may take place. However, we did not collect data on rodent control in surrounding properties in this study.
Isodar analysis suggests that habitat selection by black rats was not density-dependent at the scale and time of year examined. However, the non-zero intercept implies that bushland habitat differed quantitatively from backyard habitat, for example, in the amount/availability of food or shelter, and that at low population densities rats preferred bushland habitat. A preference for bushland habitat by rats when at low population densities (Fig. 6) is possibly due to the presence of more structurally complex habitat, and/or more reliable food or nesting resources (Cox et al. 2000). Future studies could investigate density-dependent habitat selection at larger scales and across seasons.
Other yards attributes recorded in this study, such as the presence of potential predators (cats and dogs), mulch, chickens, vegetable gardens and rodent control were not associated with black rat activity or bandicoot activity. This suggests that other attributes, such as vegetation cover in yards or availability of other anthropogenic food sources, for example rubbish or pet food (Sharp 2007; Lambert et al. 2017; Maclagan et al. 2020), may be more important drivers of activity. We excluded compost from our analyses because it was associated with vegetable and herb gardens. Rat activity has been associated with the presence of compost (Himsworth et al. 2013), so although the presence of vegetable and herb gardens was not significant it warrants further investigation.
Though not significant, black rat activity was higher in yards with dogs, despite the potential threat of predation, possibly because foraging rewards outweighed predation risk (Carthey and Banks 2018). Contrary to our results, Carthey and Banks (2012) found that reported bandicoot diggings were in lower quantities and less frequent in yards with dogs compared with yards without pets. However, vegetation cover in yards may also create a sufficiently safe environment for bandicoots to forage in yards with pets (Hughes and Banks 2010; Frank et al. 2016), and should be investigated further in future studies. Though cats are known to prey on commensal rodent populations, black rats do not appear to vary their behaviour spatially or temporally in the presence of cat odour (Carthey and Banks 2018). Rats are also likely to vary their behaviour in response to cat presence rather than leave the area (Parsons et al. 2018). Taken together, this likely explains the lack of an effect of cat presence on rat activity in this study.
There was no difference in bandicoot activity in yards adjacent to bushland compared with yards away from bushland (Table 1). In contrast, a study in Tasmania found that bandicoots were more likely to be reported by residents located adjacent to bushland (Frank et al. 2016). Our finding suggests that in our study area, residential yards are likely providing sufficient food and nesting resources for bandicoots. Despite being a critical weight-range mammal (mammals weighing between 35 and 5500 g are particularly vulnerable to extinction) (Burbidge and McKenzie 1989), long-nosed bandicoots were the second most commonly recorded mammal in yards, and were detected in 46% of yards.
Bandicoots are general in their dietary and habitat requirements, which likely explains their relatively high levels of activity in urban yards, including those away from bushland. They preferentially forage in moist, open, grassed areas close to cover (Chambers and Dickman 2002; Hughes and Banks 2010), making residential yards attractive to bandicoots. Bandicoots will also use artificial materials for nesting (Dufty 1994), nest under houses (Dowle and Deane 2009) and consume anthropogenic food sources like vegetable scraps and bird seed (Scott et al. 1999). Yet, like black rats, long-nosed bandicoot activity tended to be higher in bushland than yards, though the difference was not significant. Bandicoot nests consist of dry grass and leaf litter and are typically found in dense vegetation (Scott et al. 1999; Chambers and Dickman 2002), which could explain higher bandicoot activity in bushland at the urban fringe.
Our finding that black rats and bandicoots were more active in yards at some paired locations and more active in bushland at others, suggests that perhaps resource availability in the respective habitats is variable, but that either bushland or yards may provide sufficient resources for generalist and opportunistic wildlife. Our findings may also reflect very local effects, for example, effects of resource quality or availability in individual or neighbouring yards that we were unable to sample. It is important to note that because we obtained permission to access yards by contacting residents who agreed to participate in future research on wildlife and ticks, there is a potential for sampling to be biased in some way towards residents with concerns about ticks or wildlife.
Surprisingly, only 6% of trapped rats and bandicoots were confirmed to have crossed the urban fringe in our study, suggesting very little movement between bushland and yards during the time of year sampled (late August–early November). This is substantially lower than the 67% of black rats that crossed the urban fringe in summer at other sites around Sydney (Weerakoon 2012). However, rat movement patterns are location-specific and depend on local resource availability and competition (Byers et al. 2019), so are likely to vary seasonally.
We studied rat movement in spring when the tick nymphs are attached to rats in high numbers and can be moved around the urban environment, but rat population density tends to peak in late summer following breeding (Rose 2004). This can result in transient individuals (those without permanent home ranges) and dispersing juveniles (López-Sepulcre and Kokko 2005). Radio-tracking of rats by Weerakoon (2012) revealed that most rats made only occasional forays into residential yards. Low rat movement rates have also been observed among rural habitats (e.g. 12.6% and 30.6%) in Madagascar (Rahelinirina et al. 2010). Radio-tracking of black rats at North Head, Sydney revealed variable fine-scale movement patterns, but rats did not rapidly move into nearby areas where rats had been removed, suggesting overall limited movement even in a natural setting (Hansen et al. 2020b).
Whether our findings reflect actual low rates of long-nosed bandicoot movement at the urban fringe is uncertain. Radio-tracking of urban southern brown bandicoots (Maclagan et al. 2020) and northern brown bandicoots (Fitzgibbon et al. 2011) revealed that similarly to rats, bandicoots predominantly used vegetated remnants, where they nested under dense vegetation, and only occasionally used the urban matrix to forage, such as in parks and residential yards. These results align with our finding of limited bandicoot movement between bushland and backyards. However, long-nosed bandicoots that were radio-tracked at North Head predominantly used open grassed areas and nested in all macrohabitats (grassed areas, heathland, forest swamp and scrub) where refuge was sufficient (e.g. long grass) (Scott et al. 1999). Importantly, the North Head bandicoot population is relatively isolated, and their movement patterns may not be representative of bandicoot populations in more urbanised areas within our study area. It is possible that bandicoots in our study did not consume enough bait to result in marked hairs, but other studies targeting small and medium-sized mammals have used similar concentrations (20 mg RB/15 g bait) (Hohnen et al. 2019).
The limited movement of wildlife across the urban fringe reported here suggests that there may be (1) a lower risk of parasite or zoonotic pathogen transfer than feared, at least those posed by black rats (e.g. ticks, Leptospirosis) and long-nosed bandicoots (ticks), and (2) a reduced risk of urban rodenticide for non-target wildlife in neighbouring bushland. Importantly though, if populations of wildlife in bushland and adjacent yards are largely independent from one another, or if populations predominantly reside in bushland with only a few individuals visiting nearby yards, then parasites and pathogens may be maintained within/by those populations or individuals. Therefore, these populations may pose a risk to humans and domestic animals irrespective of movement across the urban–bushland interface. Long-nosed bandicoots and black rats trapped in yards in our study area hosted ticks of all life stages (Taylor 2022). Some individuals supported high numbers (>10 ticks), which may increase the risk of tick encounters for residents and pets if ticks detach and moult to the next life stage in yards (Taylor 2022).
If populations are largely independent, then host-targeted tick management strategies, for example, could be simply targeted to urban host populations. But in terms of rat control, it is important to consider that even limited black rat movement at the urban fringe may impede urban rat control efforts; rats from surrounding bushland may gradually ‘trickle in’ to the urban matrix following localised removal (Hansen et al. 2020b). Rat control programs therefore need to consider populations in the urban matrix and urban bushland remnants. Genetic techniques and GPS tracking could shed more light on black rat movement at the urban fringe and the degree of connectivity between urban and bushland populations of black rats to inform management (Gardner-Santana et al. 2009; Byers et al. 2019).
Our research adds to a growing body of evidence that shows some commensals may not be as heavily dependent on anthropogenic resources as historically thought (Weerakoon 2012; Hulme-Beaman et al. 2016). We found black rats persisting in higher numbers in urban bushland with a preference for bushland habitat (at low population densities according to the isodar analysis). We found no evidence of regular movement (from the Rhodamine B baiting) into the urban matrix, which would have suggested dependence on anthropogenic resources or the evolution to a commensal niche as expected at the urban fringe – and exhibited in other species such as brown rats (Singleton et al. 2003; Hulme-Beaman et al. 2016). Black rats may be best described as synanthropic rather than true or even occasional commensals at the urban fringe because they can occupy urban areas but do not appear to be dependent on them (Hulme-Beaman et al. 2016).
Understanding wildlife activity at the urban fringe is critical for managing risks to humans and wildlife associated with spillover of wildlife into natural or anthropogenic habitats. We found that some small mammals were active in bushland remnants and urban yards, with black rats readily using bushland remnants and native long-nosed bandicoots readily using residential yards. However, limited movement between the two habitats suggests that animals residing in bushland may have little dependence on anthropogenic resources or that independent populations are occupying each habitat. Residential properties located adjacent to bushland may be exposed to increased black rat activity in yards, and this has important implications for management. Future work should consider how introduced rats may be safely controlled in bushland at the urban fringe to assist urban rat control efforts and avoid non-target impacts.
Data availability
The dataset for this manuscript is available at the Sydney eScholarship Repository: https://hdl.handle.net/2123/28043.
Conflicts of interest
There are no conflicts of interest.
Declaration of funding
This study was partially funded by a Paddy Pallin Science Grant (awarded to CLT). CLT was supported by a Higher Degree Research Scholarship from Northern Beaches Council.
Acknowledgements
We thank Northern Beaches Council for providing access to bushland sites and we thank Northern Beaches residents for providing access to residential yards. Thank you to Jenna Bytheway and Liam Orrock for assistance in the field. Thank you William Swan, Amelia Saul, Neville Firth, and Jenny Phuyal for providing access to fluorescent microscopes and for initial guidance in fluorescence microscopy. Thank you to the two anonymous reviewers for their helpful comments on the manuscript.
References
Baker, PJ, and Harris, S (2007). Urban mammals: what does the future hold? An analysis of the factors affecting patterns of use of residential gardens in Great Britain. Mammal Review 37, 297–315.| Urban mammals: what does the future hold? An analysis of the factors affecting patterns of use of residential gardens in Great Britain.Crossref | GoogleScholarGoogle Scholar |
Baker, RO, and Timm, RM (2017). Coyote attacks on humans, 1970–2015: implications for reducing the risks. Human–Wildlife Interactions 11, 3.
| Coyote attacks on humans, 1970–2015: implications for reducing the risks.Crossref | GoogleScholarGoogle Scholar |
Baker, PJ, Ansell, RJ, Dodds, PAA, Webber, CE, and Harris, S (2003). Factors affecting the distribution of small mammals in an urban area. Mammal Review 33, 95–100.
| Factors affecting the distribution of small mammals in an urban area.Crossref | GoogleScholarGoogle Scholar |
Banks, PB, and Hughes, NK (2012). A review of the evidence for potential impacts of black rats (Rattus rattus) on wildlife and humans in Australia. Wildlife Research 39, 78–88.
| A review of the evidence for potential impacts of black rats (Rattus rattus) on wildlife and humans in Australia.Crossref | GoogleScholarGoogle Scholar |
Banks, PB, and Smith, HM (2015). The ecological impacts of commensal species: black rats, Rattus rattus, at the urban–bushland interface. Wildlife Research 42, 86–97.
| The ecological impacts of commensal species: black rats, Rattus rattus, at the urban–bushland interface.Crossref | GoogleScholarGoogle Scholar |
Banks, P, Cleary, G, and Dickman, C (2011). Sydney’s bubonic plague outbreak 1900–1910: a disaster for foreshore wildlife? Australian Zoologist 35, 1033–1039.
| Sydney’s bubonic plague outbreak 1900–1910: a disaster for foreshore wildlife?Crossref | GoogleScholarGoogle Scholar |
Blair RB (2001) Birds and butterflies along urban gradients in two ecoregions of the United States: is urbanization creating a homogeneous fauna? In ‘Biotic homogenization’. (Eds JL Lockwood, ML McKinney) pp. 33–56. (Springer US: Boston, MA) https://doi.org/10.1007/978-1-4615-1261-5_3
Bolker B, Skaug H, Magnusson A, Nielsen A (2012) Getting started with the glmmADMB package. Available at http://glmmadmb.r-forge.r-project.org/glmmADMB.pdf
Bozek, CK, Prange, S, and Gehrt, SD (2007). The influence of anthropogenic resources on multi-scale habitat selection by raccoons. Urban Ecosystems 10, 413–425.
| The influence of anthropogenic resources on multi-scale habitat selection by raccoons.Crossref | GoogleScholarGoogle Scholar |
Burbidge, AA, and McKenzie, NL (1989). Patterns in the modern decline of western Australia’s vertebrate fauna: causes and conservation implications. Biological Conservation 50, 143–198.
| Patterns in the modern decline of western Australia’s vertebrate fauna: causes and conservation implications.Crossref | GoogleScholarGoogle Scholar |
Byers, KA, Lee, MJ, Patrick, DM, and Himsworth, CG (2019). Rats about town: a systematic review of rat movement in urban ecosystems. Frontiers in Ecology and Evolution 7, 13.
| Rats about town: a systematic review of rat movement in urban ecosystems.Crossref | GoogleScholarGoogle Scholar |
Bytheway, JP, Johnstone, KC, Price, CJ, and Banks, PB (2021). A mechanistic understanding of prebaiting to improve interaction with wildlife management devices. Pest Management Science 77, 3107–3115.
| A mechanistic understanding of prebaiting to improve interaction with wildlife management devices.Crossref | GoogleScholarGoogle Scholar |
Carthey, AJR, and Banks, PB (2012). When does an alien become a native species? A vulnerable native mammal recognizes and responds to its long-term alien predator. PLoS ONE 7, e31804.
| When does an alien become a native species? A vulnerable native mammal recognizes and responds to its long-term alien predator.Crossref | GoogleScholarGoogle Scholar |
Carthey, AJR, and Banks, PB (2018). Naïve, bold, or just hungry? An invasive exotic prey species recognises but does not respond to its predators. Biological Invasions 20, 3417–3429.
| Naïve, bold, or just hungry? An invasive exotic prey species recognises but does not respond to its predators.Crossref | GoogleScholarGoogle Scholar |
Chambers, LK, and Dickman, CR (2002). Habitat selection of the long-nosed bandicoot, Perameles nasuta (Mammalia, Peramelidae), in a patchy urban environment. Austral Ecology 27, 334–342.
| Habitat selection of the long-nosed bandicoot, Perameles nasuta (Mammalia, Peramelidae), in a patchy urban environment.Crossref | GoogleScholarGoogle Scholar |
Chen T (2013) Fox baiting program in Ku-ring-gai causes rise of bandicoots and ticks. North Shore Times, 25 July 2013. Available at https://www.dailytelegraph.com.au/newslocal/north-shore/fox-baiting-program-causes-rise-of-bandicoots-and-ticks/news-story/2c7572fb9f3408524abc3995856a87d6, or at Proquest ID: 1416380854
Cox, MPG, Dickman, CR, and Cox, WG (2000). Use of habitat by the black rat (Rattus rattus) at North Head, New South Wales: an observational and experimental study. Austral Ecology 25, 375–385.
| Use of habitat by the black rat (Rattus rattus) at North Head, New South Wales: an observational and experimental study.Crossref | GoogleScholarGoogle Scholar |
Dickman, CR (1996). Impact of exotic generalist predators on the native fauna of Australia. Wildlife Biology 2, 185–195.
| Impact of exotic generalist predators on the native fauna of Australia.Crossref | GoogleScholarGoogle Scholar |
Dowle, M, and Deane, EM (2009). Attitudes to native bandicoots in an urban environment. European Journal of Wildlife Research 55, 45–52.
| Attitudes to native bandicoots in an urban environment.Crossref | GoogleScholarGoogle Scholar |
Dufty, AC (1994). Habitat and spatial requirements of the eastern barred bandicoot (Perameles gunnii) at Hamilton, Victoria. Wildlife Research 21, 459–471.
| Habitat and spatial requirements of the eastern barred bandicoot (Perameles gunnii) at Hamilton, Victoria.Crossref | GoogleScholarGoogle Scholar |
Fisher, P, Algar, D, and Sinagra, J (1999). Use of Rhodamine B as a systemic bait marker for feral cats (Felis catus). Wildlife Research 26, 281–285.
| Use of Rhodamine B as a systemic bait marker for feral cats (Felis catus).Crossref | GoogleScholarGoogle Scholar |
Fitzgibbon, SI, and Jones, DN (2006). A community-based wildlife survey: the knowledge and attitudes of residents of suburban Brisbane, with a focus on bandicoots. Wildlife Research 33, 233–241.
| A community-based wildlife survey: the knowledge and attitudes of residents of suburban Brisbane, with a focus on bandicoots.Crossref | GoogleScholarGoogle Scholar |
Fitzgibbon, SI, Wilson, RS, and Goldizen, AW (2011). The behavioural ecology and population dynamics of a cryptic ground-dwelling mammal in an urban Australian landscape. Austral Ecology 36, 722–732.
| The behavioural ecology and population dynamics of a cryptic ground-dwelling mammal in an urban Australian landscape.Crossref | GoogleScholarGoogle Scholar |
Frank, ASK, Carthey, AJR, and Banks, PB (2016). Does historical coexistence with dingoes explain current avoidance of domestic dogs? Island bandicoots are naïve to dogs, unlike their mainland counterparts. PLoS ONE 11, e0161447.
| Does historical coexistence with dingoes explain current avoidance of domestic dogs? Island bandicoots are naïve to dogs, unlike their mainland counterparts.Crossref | GoogleScholarGoogle Scholar |
Gardner-Santana, LC, Norris, DE, Fornadel, CM, Hinson, ER, Klein, SL, and Glass, GE (2009). Commensal ecology, urban landscapes, and their influence on the genetic characteristics of city-dwelling Norway rats (Rattus norvegicus). Molecular Ecology 18, 2766–2778.
| Commensal ecology, urban landscapes, and their influence on the genetic characteristics of city-dwelling Norway rats (Rattus norvegicus).Crossref | GoogleScholarGoogle Scholar |
Gerasimova, M, Kelman, M, and Ward, MP (2018). Are recreational areas a risk factor for tick paralysis in urban environments? Veterinary Parasitology 254, 72–77.
| Are recreational areas a risk factor for tick paralysis in urban environments?Crossref | GoogleScholarGoogle Scholar |
Hall-Mendelin, S, Craig, SB, Hall, RA, O’Donoghue, P, Atwell, RB, Tulsiani, SM, and Graham, GC (2011). Tick paralysis in Australia caused by Ixodes holocyclus Neumann. Annals of Tropical Medicine & Parasitology 105, 95–106.
| Tick paralysis in Australia caused by Ixodes holocyclus Neumann.Crossref | GoogleScholarGoogle Scholar |
Hansen, CP, Parsons, AW, Kays, R, and Millspaugh, JJ (2020a). Does use of backyard resources explain the abundance of urban wildlife? Frontiers in Ecology and Evolution 8, 570771.
| Does use of backyard resources explain the abundance of urban wildlife?Crossref | GoogleScholarGoogle Scholar |
Hansen, N, Hughes, NK, Byrom, AE, and Banks, PB (2020b). Population recovery of alien black rats Rattus rattus: a test of reinvasion theory. Austral Ecology 45, 291–304.
| Population recovery of alien black rats Rattus rattus: a test of reinvasion theory.Crossref | GoogleScholarGoogle Scholar |
Hassell, JM, Begon, M, Ward, MJ, and Fèvre, EM (2017). Urbanization and disease emergence: dynamics at the wildlife–livestock–human interface. Trends in Ecology & Evolution 32, 55–67.
| Urbanization and disease emergence: dynamics at the wildlife–livestock–human interface.Crossref | GoogleScholarGoogle Scholar |
Heavener, SJ, Carthey, AJR, and Banks, PB (2014). Competitive naïveté between a highly successful invader and a functionally similar native species. Oecologia 175, 73–84.
| Competitive naïveté between a highly successful invader and a functionally similar native species.Crossref | GoogleScholarGoogle Scholar |
Himsworth, CG, Feng, AYT, Parsons, K, Kerr, T, and Patrick, DM (2013). Using experiential knowledge to understand urban rat ecology: a survey of Canadian pest control professionals. Urban Ecosystems 16, 341–350.
| Using experiential knowledge to understand urban rat ecology: a survey of Canadian pest control professionals.Crossref | GoogleScholarGoogle Scholar |
Hohnen, R, Murphy, BP, Legge, SM, Dickman, CR, and Woinarski, JCZ (2019). Uptake of ‘Eradicat’ feral cat baits by non-target species on Kangaroo Island. Wildlife Research 47, 547–556.
| Uptake of ‘Eradicat’ feral cat baits by non-target species on Kangaroo Island.Crossref | GoogleScholarGoogle Scholar |
Hooker, S, and Innes, J (1995). Ranging behaviour of forest-dwelling ship rats, Rattus rattus, and effects of poisoning with brodifacoum. New Zealand Journal of Zoology 22, 291–304.
| Ranging behaviour of forest-dwelling ship rats, Rattus rattus, and effects of poisoning with brodifacoum.Crossref | GoogleScholarGoogle Scholar |
Hubert, P, Julliard, R, Biagianti, S, and Poulle, M-L (2011). Ecological factors driving the higher hedgehog (Erinaceus europeaus) density in an urban area compared to the adjacent rural area. Landscape and Urban Planning 103, 34–43.
| Ecological factors driving the higher hedgehog (Erinaceus europeaus) density in an urban area compared to the adjacent rural area.Crossref | GoogleScholarGoogle Scholar |
Hughes, NK, and Banks, PB (2010). Heading for greener pastures? Defining the foraging preferences of urban long-nosed bandicoots. Australian Journal of Zoology 58, 341–349.
| Heading for greener pastures? Defining the foraging preferences of urban long-nosed bandicoots.Crossref | GoogleScholarGoogle Scholar |
Hulme-Beaman, A, Dobney, K, Cucchi, T, and Searle, JB (2016). An ecological and evolutionary framework for commensalism in anthropogenic environments. Trends in Ecology & Evolution 31, 633–645.
| An ecological and evolutionary framework for commensalism in anthropogenic environments.Crossref | GoogleScholarGoogle Scholar |
Jarman, P, and Driessen, M (2019). Quantitative interpretation of images of long-nosed potoroos at baited camera-traps: defining a ‘visit’. Australian Mammalogy 41, 147–149.
| Quantitative interpretation of images of long-nosed potoroos at baited camera-traps: defining a ‘visit’.Crossref | GoogleScholarGoogle Scholar |
Kays, R, and Parsons, AW (2014). Mammals in and around suburban yards, and the attraction of chicken coops. Urban Ecosystems 17, 691–705.
| Mammals in and around suburban yards, and the attraction of chicken coops.Crossref | GoogleScholarGoogle Scholar |
King, CM, Foster, S, and Miller, S (2011). Invasive European rats in Britain and New Zealand: same species, different outcomes. Journal of Zoology 285, 172–179.
| Invasive European rats in Britain and New Zealand: same species, different outcomes.Crossref | GoogleScholarGoogle Scholar |
Lambert, M, Vial, F, Pietravalle, S, and Cowan, D (2017). Results of a 15-year systematic survey of commensal rodents in English dwellings. Scientific Reports 7, 15882.
| Results of a 15-year systematic survey of commensal rodents in English dwellings.Crossref | GoogleScholarGoogle Scholar |
Lydecker, HW, Etheridge, B, Price, C, Banks, PB, and Hochuli, DF (2019a). Landscapes within landscapes: a parasite utilizes different ecological niches on the host landscapes of two host species. Acta Tropica 193, 60–65.
| Landscapes within landscapes: a parasite utilizes different ecological niches on the host landscapes of two host species.Crossref | GoogleScholarGoogle Scholar |
Lydecker, HW, Hochuli, DF, and Banks, PB (2019b). Peri-urban black rats host a rich assembly of ticks and healthier rats have more ticks. Ticks and Tick-borne Diseases 10, 749–753.
| Peri-urban black rats host a rich assembly of ticks and healthier rats have more ticks.Crossref | GoogleScholarGoogle Scholar |
López-Sepulcre, A, and Kokko, H (2005). Territorial defense, territory size, and population regulation. The American Naturalist 166, 317–325.
| Territorial defense, territory size, and population regulation.Crossref | GoogleScholarGoogle Scholar |
Maclagan, SJ, Coates, T, Hradsky, BA, Butryn, R, and Ritchie, EG (2020). Life in linear habitats: the movement ecology of an endangered mammal in a peri-urban landscape. Animal Conservation 23, 260–272.
| Life in linear habitats: the movement ecology of an endangered mammal in a peri-urban landscape.Crossref | GoogleScholarGoogle Scholar |
Matsui, S, Hisaka, M, and Takagi, M (2010). Arboreal nesting and utilization of open-cup bird nests by introduced Ship Rats Rattus rattus on an oceanic island. Bird Conservation International 20, 34–42.
| Arboreal nesting and utilization of open-cup bird nests by introduced Ship Rats Rattus rattus on an oceanic island.Crossref | GoogleScholarGoogle Scholar |
McKinney, ML (2002). Urbanization, biodiversity, and conservation. BioScience 52, 883–890.
| Urbanization, biodiversity, and conservation.Crossref | GoogleScholarGoogle Scholar |
McMahon, BJ, Morand, S, and Gray, JS (2018). Ecosystem change and zoonoses in the Anthropocene. Zoonoses and Public Health 65, 755–765.
| Ecosystem change and zoonoses in the Anthropocene.Crossref | GoogleScholarGoogle Scholar |
Morris, DW (1987). Tests of density-dependent habitat selection in a patchy environment. Ecological Monographs 57, 269–281.
| Tests of density-dependent habitat selection in a patchy environment.Crossref | GoogleScholarGoogle Scholar |
Morris, DW (1988). Habitat-dependent population regulation and community structure. Evolutionary Ecology 2, 253–269.
| Habitat-dependent population regulation and community structure.Crossref | GoogleScholarGoogle Scholar |
Murray, MH, and St. Clair, CC (2017). Predictable features attract urban coyotes to residential yards. The Journal of Wildlife Management 81, 593–600.
| Predictable features attract urban coyotes to residential yards.Crossref | GoogleScholarGoogle Scholar |
Murray, MH, Hill, J, Whyte, P, and St. Clair, CC (2016). Urban compost attracts coyotes, contains toxins, and may promote disease in urban-adapted wildlife. EcoHealth 13, 285–292.
| Urban compost attracts coyotes, contains toxins, and may promote disease in urban-adapted wildlife.Crossref | GoogleScholarGoogle Scholar |
Office of Environment and Heritage Sydney (2016) The native vegetation of the Sydney metropolitan area – Version 3.1. Available at https://datasets.seed.nsw.gov.au/dataset/the-native-vegetation-of-the-sydney-metropolitan-area-oeh-2016-vis-id-4489. [Accessed 9 February 2021]
Ogle D, Wheeler P, Dinno A (2021) FSA: fisheries stock analysis. R package version 0.8. Available at https://cran.r-project.org/web/packages/FSA/FSA.pdf. [Accessed 11 May 2021]
Parsons, MH, Banks, PB, Deutsch, MA, Corrigan, RF, and Munshi-South, J (2017). Trends in urban rat ecology: a framework to define the prevailing knowledge gaps and incentives for academia, pest management professionals (PMPs) and public health agencies to participate. Journal of Urban Ecology 3, jux005.
| Trends in urban rat ecology: a framework to define the prevailing knowledge gaps and incentives for academia, pest management professionals (PMPs) and public health agencies to participate.Crossref | GoogleScholarGoogle Scholar |
Parsons, MH, Banks, PB, Deutsch, MA, and Munshi-South, J (2018). Temporal and space-use changes by rats in response to predation by feral cats in an urban ecosystem. Frontiers in Ecology and Evolution 6, 146.
| Temporal and space-use changes by rats in response to predation by feral cats in an urban ecosystem.Crossref | GoogleScholarGoogle Scholar |
Patergnani, M, Mughini Gras, L, Poglayen, G, Gelli, A, Pasqualucci, F, Farina, M, and Stancampiano, L (2010). Environmental influence on urban rodent bait consumption. Journal of Pest Science 83, 347–359.
| Environmental influence on urban rodent bait consumption.Crossref | GoogleScholarGoogle Scholar |
Paull, DJ, Claridge, AW, and Barry, SC (2011). There’s no accounting for taste: bait attractants and infrared digital cameras for detecting small to medium ground-dwelling mammals. Wildlife Research 38, 188–195.
| There’s no accounting for taste: bait attractants and infrared digital cameras for detecting small to medium ground-dwelling mammals.Crossref | GoogleScholarGoogle Scholar |
Pettett, CE, Moorhouse, TP, Johnson, PJ, and Macdonald, DW (2017). Factors affecting hedgehog (Erinaceus europaeus) attraction to rural villages in arable landscapes. European Journal of Wildlife Research 63, 54.
| Factors affecting hedgehog (Erinaceus europaeus) attraction to rural villages in arable landscapes.Crossref | GoogleScholarGoogle Scholar |
R Core Team (2021) ‘R: a language and environment for statistical computing’. (R Foundation for Statistical Computing: Vienna, Austria) Available at https://www.R-project.org/
Rahelinirina, S, Duplantier, JM, Ratovonjato, J, Ramilijaona, O, Ratsimba, M, and Rahalison, L (2010). Study on the movement of Rattus rattus and evaluation of the plague dispersion in Madagascar. Vector-Borne and Zoonotic Diseases 10, 77–84.
| Study on the movement of Rattus rattus and evaluation of the plague dispersion in Madagascar.Crossref | GoogleScholarGoogle Scholar |
Reed, JH, and Bonter, DN (2018). Supplementing non-target taxa: bird feeding alters the local distribution of mammals. Ecological Applications 28, 761–770.
| Supplementing non-target taxa: bird feeding alters the local distribution of mammals.Crossref | GoogleScholarGoogle Scholar |
Rose T (2004) An analysis of the black rat (Rattus rattus) at Sydney’s North Head: population structure, impacts and implications for control. Honours thesis, University of New South Wales, Sydney, NSW, Australia.
Scott, LK, Hume, ID, and Dickman, CR (1999). Ecology and population biology of long-nosed bandicoots (Perameles nasuta) at North Head, Sydney Harbour National Park. Wildlife Research 26, 805–821.
| Ecology and population biology of long-nosed bandicoots (Perameles nasuta) at North Head, Sydney Harbour National Park.Crossref | GoogleScholarGoogle Scholar |
Sharp, D (2007). On rats, refuse, and recycling. Journal of Urban Health 84, 637–638.
| On rats, refuse, and recycling.Crossref | GoogleScholarGoogle Scholar |
Singleton GR, Kenney AJ, Tann CR, Sudarmaji, Nguyen QH (2003) Myth, dogma and rodent management: good stories ruined by data? In ‘Rats, Mice and People: Rodent Biology and Management’. (Australian Centre for International Agricultural Research: Canberra, ACT, Australia) Available at https://publications.csiro.au/rpr/pub?list=BRO&pid=procite:b7007510-3562-4950-a356-f8cbaf0c1775 [Accessed 5 October 2021]
Stokes, VL, Banks, PB, Pech, RP, and Williams, RL (2009). Invasion by Rattus rattus into native coastal forests of south-eastern Australia: are native small mammals at risk? Austral Ecology 34, 395–408.
| Invasion by Rattus rattus into native coastal forests of south-eastern Australia: are native small mammals at risk?Crossref | GoogleScholarGoogle Scholar |
Tamayo-Uria, I, Mateu, J, Escobar, F, and Mughini-Gras, L (2014). Risk factors and spatial distribution of urban rat infestations. Journal of Pest Science 87, 107–115.
| Risk factors and spatial distribution of urban rat infestations.Crossref | GoogleScholarGoogle Scholar |
Taylor CL (2022) Ecological drivers of human-tick encounters in urban environments. PhD thesis, The University of Sydney, NSW, Australia. Available at https://hdl.handle.net/2123/28193
van Nunen, SA (2018). Tick-induced allergies: mammalian meat allergy and tick anaphylaxis. Medical Journal of Australia 208, 316–321.
| Tick-induced allergies: mammalian meat allergy and tick anaphylaxis.Crossref | GoogleScholarGoogle Scholar |
Wat, KKY, Herath, APHM, Rus, AI, Banks, PB, and Mcarthur, C (2020). Space use by animals on the urban fringe: interactive effects of sex and personality. Behavioral Ecology 31, 330–339.
| Space use by animals on the urban fringe: interactive effects of sex and personality.Crossref | GoogleScholarGoogle Scholar |
Weerakoon MK (2012) The movements of black rats across the urban-bushland interface; a study using Rhodamine B. Masters thesis, University of New South Wales, Sydney, NSW, Australia. Available at http://unsworks.unsw.edu.au/fapi/datastream/unsworks:10324/SOURCE02?view=true [Accessed 25 June 2021]
Weerakoon, MK, Price, CJ, and Banks, PB (2013). Hair type, intake, and detection method influence Rhodamine B detectability. The Journal of Wildlife Management 77, 306–312.
| Hair type, intake, and detection method influence Rhodamine B detectability.Crossref | GoogleScholarGoogle Scholar |
Weerakoon MK, Ruffino L, Cleary GP, Heavener S, Bytheway JP, Smith HM, Banks PB (2014) Can camera traps be used to estimate small mammal population size. In ‘Camera Trapping: Wldlife Management and Research’. (Eds PD Meek, AG Ballard, PB Banks, AW Claridge, PJS Fleming, JG Sanderson, DE Swann) pp. 307–316. (CSIRO Publishing: Melbourne, Vic., Australia)
Williams RL, Singleton GR, Dickman CR (2003) The role of interspecific competition in determining macrohabitat use by the black rat and brown rat at Bradley’s Head, NSW. In ‘Rats, Mice and People: Rodent Biology and Management’. pp. 366–369. (Australian Centre for International Agricultural Research: Canberra, ACT, Australia)


