The dingo (Canis familiaris) as a secondary disperser of mycorrhizal fungal spores
Todd F. Elliott A * , C. E. Timothy Paine A , Guy-Anthony Ballard A B , Heath Milne A , Josh Van der Eyk A , Kelsey Elliott C , Paul Meek
A * , C. E. Timothy Paine A , Guy-Anthony Ballard A B , Heath Milne A , Josh Van der Eyk A , Kelsey Elliott C , Paul Meek  A D , Jeremy J. Bruhl
A D , Jeremy J. Bruhl  E and Karl Vernes
E and Karl Vernes  A
A
A Ecosystem Management, School of Environmental and Rural Science, University of New England, Armidale, NSW 2351, Australia.
B Vertebrate Pest Research Unit, Biosecurity NSW, NSW Department of Primary Industries, University of New England, PO Box U86, Armidale, NSW 2351, Australia.
C Integrative Studies Department, Warren Wilson College, Swannanoa, NC 28778, USA.
D Vertebrate Pest Research Unit, New South Wales Department of Primary Industries, Corner Gordon and Hood Street, Coffs Harbour, NSW 2450, Australia.
E Botany, School of Environmental and Rural Science, University of New England, Armidale, NSW 2351, Australia.
Abstract
Many mycorrhizal fungi are vital to nutrient acquisition in plant communities, and some taxa are reliant on animal-mediated dispersal. The majority of animals that disperse spores are relatively small and have short-distance movement patterns, but carnivores – and especially apex predators – eat many of these small mycophagists and then move greater distances. No studies to date have assessed the ecosystem services carnivores provide through long-distance spore dispersal.
In this study, we aimed to investigate whether Australia’s free-ranging dogs (Canis familiaris), including dingoes, act as long-distance spore dispersers by predating smaller mycophagous animals and then secondarily dispersing the fungi consumed by these prey species.
To answer this question, we collected dingo scats along 40 km of transects in eastern Australia and analysed the scats to determine the presence of fungal spores and prey animals. Using telemetry and passage rate data, we then developed a movement model to predict the spore dispersal potential of dingoes.
We found 16 species of mammalian prey to be eaten by dingoes, and those dingo scats contained spores of 14 genera of mycorrhizal fungi. These fungi were more likely to appear in the scats of dingoes if primary mycophagist prey mammals had been consumed. Our model predicted dingo median spore dispersal distance to be 2050 m and maximum dispersal potential to be 10 700 m.
Our study indicates that dingoes are providing a previously overlooked ecosystem service through the long-distance dispersal of mycorrhizal fungi. Many of the fungi found in this study form hypogeous (underground) fruiting bodies that are unable to independently spread spores via wind. Because dingoes move over larger areas than their prey, they are especially important to these ecosystem functions.
Our novel approach to studying an overlooked aspect of predator ecology is applicable in most terrestrial ecosystems. Similar modelling approaches could also be employed to understand the dispersal potential of both primary and secondary spore dispersers globally. Because this study highlights an unrecognised ecosystem service provided by dingoes, we hope that it will stimulate research to develop a more comprehensive understanding of other apex predators’ ecosystem functions.
Keywords: canids, carnivore ecology, diplochory, free-ranging dog, fungal ecology, mycophagy, mycorrhizae, predator ecology, spore dispersal.
Introduction
More than 600 vertebrates feed on fungi to varying degrees (Elliott et al. 2019a, 2019b, 2022). These animals directly influence ecosystems through the dispersal of fungi that form mycorrhizal associations vital to plant nutrient uptake (Dundas et al. 2018; Nuske et al. 2019; Vašutová et al. 2019). The spores of many species of mycorrhizal fungi can be dispersed long distances by air (Warner et al. 1987; Allen et al. 1989; Geml et al. 2008). However, wind dispersal is only effective for a small percentage of spores and only among species that produce certain fruiting morphologies. Estimates show that among mushrooms that forcibly discharge their spores, only about 2% of spores disperse beyond 5.2 m from the parent fruiting body (Li 2005). For fungi such as truffles that have hypogeous (belowground) or sequestrate (enclosed) fruiting morphologies, air currents are rarely a factor in their dispersal. Sequestrate fungi and their associated plant communities are thus particularly reliant on animals as dispersers, and changes in animal vectors can impact the species composition of these communities (Dundas et al. 2018; Nuske et al. 2019).
In intact or contiguous plant communities, mycorrhizal fungi commonly colonise seedlings through mycelial spread (Jonsson et al. 1999). Mycelial spread tends to be less effective in disturbed, fragmented, or degraded landscapes, and spores thus become the primary means of establishment (Trappe and Strand 1969; Peay et al. 2012). Though movement patterns vary among species and regions, fragmentation limits the movement of small mammals (Pires et al. 2002; Holland and Bennett 2009) and can thus lead to bottlenecks in the mycorrhizal diversity of fragmented systems, particularly among fungi that produce sequestrate fruiting bodies.
Because larger animals tend to have larger home ranges (Swihart et al. 1988), and those that are carnivorous often prey on smaller mycophagous species, the greater distance travelled by predators translates into a potential increase in dispersal distance. For example, in the only known study that directly examined secondary dispersal of fungi by a vertebrate, Vogilino (1895) showed toads that eat mycophagous slugs can act as secondary dispersers by moving fungal spores more widely (and into different habitats) than their prey. More recent studies have reported mycophagous prey species in the diets of vertebrate predators, or suggested that such associations were occurring (Trappe 1988; O’Malley 2013; Watson and Shaw 2018; Elliott et al. 2019a, 2019b, 2022), but none have directly studied fungal secondary dispersal through predator scat analysis.
We focus our study of secondary dispersal on dingoes (Canis familiaris), which are eastern Australia’s apex predator. In this study, we use the term dingo; however, the animals we studied are likely mixed and may include dingoes and/or free-ranging dogs with mixed dingo and domestic dog ancestry. While we do not know the ancestry of each individual in this study, the diets of these animals indicate they were behaving as wild canids. Given that this region has one of the most thoroughly studied communities of small mycophagous mammals (Claridge and May 1994; Nuske et al. 2017; Elliott et al. 2022), it is possible to test whether the presence of prey mammals known to eat fungi is correlated with the presence of spores in predator faeces. Despite the ecological importance of mycorrhizal fungi, only one previous study has applied modelling to estimate the dispersal potential of a mycophagist (Danks et al. 2020). No previous studies have modelled or investigated secondary dispersal by predators. This study aims to evaluate the role of dingoes as long-distance vectors of mycorrhizal fungal spores.
Methods
Study sites were in Cathedral Rock National Park and New England National Park in the New England Tablelands of northeastern New South Wales. The parks are relatively close to one another (~20 km), but the study sites are distinct. Vegetation description follows Specht (1970); Cathedral Rock National Park sites were composed mainly of Eucalyptus woodland grading to open forest, and New England National Park sites were dominated by wetter open and closed forests dominated by Eucalyptus. We selected two 20-km transects along fire trails in each park.
We sampled in June and July based on herbarium records and previous mycophagy studies that determined this to be the peak of the hypogeous fungi fruiting season in New England National Park (Danks et al. 2013; Elliott and Vernes 2019). We removed all carnivore scats from our transects between 12 and 13 June 2018, and returned to collect newly deposited scats between 15 and 16 July 2018 (Fig. 1). With our clearing of old scats and follow-up sampling, we could be certain that scats deposited between these dates were composed of prey animals that fed during the peak of the hypogeous fungal fruiting season. Scats were collected by two teams of two people walking side by side, each visually scanning half the width of the fire trail. Carnivore scats were stored individually in labelled paper bags.
(a) Camera trap image of a dingo carrying a swamp wallaby. © NSW DPI. (b) Camera trap image of a dingo carrying a small macropod. © NSW DPI. (c) Dingo scats deposited on a macropod carcass. (d) Micrograph illustrating fungal spores found in dingo scats.

Scats were re-examined in the lab to eliminate any misidentified scats that may have belonged to herbivores or other predators (Triggs 2004); 45 scats were selected from Cathedral Rock National Park and 37 from New England National Park. A small subsample of each of the 82 scats was softened in 70% ethanol, placed on a microscope slide and topped with a coverslip. A minimum of 12 fields of view at 400× magnification were used to determine presence or absence of fungal spores (Fig. 1). We focused solely on presence and diversity of mycorrhizal taxa; coprophilous and micro fungi were outside the scope of this study. Spores were identified to family or genus using standard micro-morphological techniques and compared with fungal collections from the same area. The remainder of each scat was sent to ScatsAbout (http://www.scatsabout.com.au) for analysis, where a reference hair collection and macroscopic/microscopic characteristics of hair and bone were used to identify prey animals (Brunner and Coman 1974). ScatsAbout also used these characters to confirm our identifications of predator species. To ensure that we had adequately sampled dingo diet at both sites, we constructed rarefaction curves of species richness against number of scat samples using the ‘specaccum’ function in the package ‘vegan’, with 10 000 permutations. The resultant plots indicated our sampling sufficiently represented dingo diet at each site. The addition of molecular tools could complement our morphological approach, but this was beyond the scope of our study. If the appropriate controls for DNA contamination were used, it would be possible to conduct DNA meta-barcoding of scats. This approach could further elucidate the taxonomy and diversity of some of the fungi being dispersed and could also help to further refine the identification of prey animals. This approach has only recently begun to be applied to studies of vertebrate mycophagy (Cloutier et al. 2019; Nuske et al. 2019; Caiafa et al. 2021; Hopkins et al. 2021; Bradshaw et al. 2022).
Telemetry data were collected from four individuals: one female and three male dingoes in nearby Guy Fawkes River National Park (G. Ballard, unpubl. data). Guy Fawkes River National Park was a more suitable location for trapping and collaring dingoes, and is in close proximity to our study sites at Cathedral Rock and New England National Park. It is also within the range of a single dingo’s home range and shares similar habitats with the scat collection sites. Animals were trapped using Victor 3 soft-catch traps (Sterling Fur Co., Ohio) with a single pair of springs and after-market in-line spring and swivel assemblies. Animals were tracked between March and August 2016 (specific start/end dates varied among individuals). Lotek Iridium Pinnacle Lite collars (Lotek, Havelock North, New Zealand) were fitted to dingoes and recorded location fixes at intervals of approximately every 30 min. The animal movement dataset contained 20 378 positions, and tracking periods ranged from 90 to 140 days, with a combined total of 497 days. Given the length of tracking periods and the frequency of location fixes, these four individuals provided us with sufficient movement data to address the dispersal questions of this study. Trapping and telemetry of dingoes was permitted under animal ethics approval ORA 1 508 002.
Fungal spore retention times are unstudied among dingoes, so we relied on passage rate data for domestic dogs. Domestic dogs are taxonomically the same species as dingoes (Jackson et al. 2017, 2021), so we based our assessment of passage rates on dog breeds within the 9.6–19.4 kg weight range of dingoes (Strahan 1983). For dog breeds within this weight range, maximum retention times are between 33.3 and 47.5 h (Banta et al. 1979; Burrows et al. 1982; Hernot et al. 2005; Childs-Sanford and Angel 2006; De Cuyper et al. 2018).
For our model, we based passage rates on data presented by Banta et al. (1979). Their thorough study used an array of markers to measure passage rates of diets through the digestive system of beagles (a breed of C. familiaris). Their results were within ranges reported by other studies (Burrows et al. 1982; Hernot et al. 2005; Childs-Sanford and Angel 2006; De Cuyper et al. 2018). From these data, we estimated that spores would not appear in dingo scats within the first 2 h after ingestion, and 100% of spores would be defecated by 42 h. The small size of fungal spores allows them to linger in digestive systems longer than other dietary items, and these rates can vary among individuals (Danks 2012). Because Banta et al. (1979) were not evaluating maximum retention time of spores but were studying a diversity of different dietary items (most of which were larger than spores), their data provide us with a conservative estimate and prevent overestimation of dispersal.
We predicted spore dispersal using a dispersal distance kernel that describes the probability of dispersal to different distances; we modified methods described by Danks et al. (2020) to fit passage rates of dogs and movement patterns of dingoes. To estimate spore passage time through the dingo digestive tract, we used data presented by Banta et al. (1979) and fitted a generalised additive model (GAM) to the log-transformed instantaneous proportion of a liquid tracer defecated by beagles. Banta et al. (1979) examined passage times of particles of varied sizes and a liquid tracer in dogs that had been fed a cereal or meat diet. We used the liquid tracer data because the particles were all far larger than fungal spores. There was no difference between passage rates of the liquid tracer in dogs fed cereal or meat, so we pooled these data. Banta et al. (1979) presented the mean and standard deviation of the proportion of tracer defecated at 2-h intervals. We accounted for uncertainty by weighting data provided to the GAM as the reciprocal of the standard deviation +1 (occasionally Banta et al.’s standard deviations were 0). The degrees of freedom in the GAM’s smoother function were adjusted to obtain an adequate fit to the data.
In estimating distance travelled during the time of passage of fungal spores, we discarded the first 60 min of track data after the animal was collared and released to account for abnormalities. We broke remaining movement data into 50-h blocks (50-h being the maximum time between ingestion of prey species and 100% defecation). If no movement was recorded between the start of the block and the location fix immediately preceding it, the animal was judged to have been resting at the start of the block. These data were discarded from analysis because dingoes would be unlikely to eat or defecate while at rest. Otherwise, we calculated each dingo’s geographic displacement from its location at the start of the block at each timepoint (i.e. the time of each position fix in the block). We used the GAM to predict probability of spore defecation at each timepoint and calculated the probability of dispersal to any distance as the product of the likelihood of defecation at each timepoint and the displacement observed at that timepoint. We present the results aggregated into 50-m bins. Analyses were performed in R 4.0.3 (R Core Team 2020).
Results
Across the two sites, more than one in four dingo scats contained mycorrhizal spores. Nine prey mammal species were in the 45 scats from Cathedral Rock National Park, and five scats (11.11%) contained evidence of eight fungal taxa (Table 1). Of the eight fungal taxa, seven were identified as ectomycorrhizal, and four were of genera that only produce sequestrate fruiting bodies. Twelve mammal prey species were present in the 37 dingo scats from New England National Park, and 18 (48.65%) contained evidence of fungal spores belonging to 11 taxa (Table 1). At New England National Park, 10 of the identified groups of fungi were ectomycorrhizal, and one was vesicular arbuscular. Seven of the identified groups primarily form sequestrate fruiting bodies. Hair analysis revealed 16 mammal prey species across both sites, plus unidentified birds and insects (Table 1). Of the prey species, 11 are known to be strongly mycophagous (Table 1).
| Prey taxa | Cathedral Rock National Park | New England National Park | Total | |
|---|---|---|---|---|
| No. of scats; N = 45 | No. of scats; N = 37 | No. of scats; N = 82 | ||
| Prey species | ||||
| Antechinus mimetes | 0 | 1 | 1 | |
| Bos taurus | 3 | 0 | 3 | |
| Isoodon macrourus | 2 | 9 | 11 | |
| Macropus giganteus | 14 | 0 | 14 | |
| Notomacropus parma | 0 | 2 | 2 | |
| Notomacropus rufogriseus | 1 | 1 | 2 | |
| Oryctolagus cuniculus | 10 | 0 | 10 | |
| Perameles nasuta | 0 | 9 | 9 | |
| Potorous tridactylus | 0 | 1 | 1 | |
| Pseudocheirus peregrinus | 16 | 12 | 28 | |
| Rattus fuscipes | 0 | 5 | 5 | |
| Rattus lutreolus | 0 | 2 | 2 | |
| Rattus rattus | 1 | 2 | 3 | |
| Trichosurus caninus | 0 | 4 | 4 | |
| Trichosurus vulpecula | 1 | 4 | 5 | |
| Wallabia bicolor | 4 | 3 | 7 | |
| Bird | 4 | 6 | 10 | |
| Beetle and cockroach | 2 | 1 | 3 | |
| Fungal taxa | N = 5 | N = 18 | N = 23 | |
|---|---|---|---|---|
| Agaricoid | 1 | 0 | 1 | |
| Amylascus | 0 | 1 | 1 | |
| Bolitoid | 0 | 1 | 1 | |
| Cortinariaceae | 3 | 4 | 7 | |
| Descolea | 1 | 8 | 9 | |
| Dingleya | 0 | 1 | 1 | |
| Elaphomyces | 0 | 4 | 4 | |
| Glomeromycota | 0 | 3 | 3 | |
| Hydnangium | 1 | 0 | 1 | |
| Hysterangium | 2 | 9 | 11 | |
| Mesophelliaceae | 0 | 2 | 2 | |
| Octaviania | 1 | 0 | 1 | |
| Rossbeevera | 1 | 5 | 6 | |
| Russulaceae | 1 | 10 | 11 |
Numbers represent the number of scats that contained the given taxon. Bolded prey species are recognised in the literature as being heavily mycophagous (see body text).
The number of mycophagous mammal species in scats was a significant predictor of the occurrence of fungal spores (GLM: z = 2.91, P < 0.01), but there was no relationship between number of non-mycophagous mammal species and fungal spores (GLM: z = 0.98, P = 0.33; Fig. 2).
The proportion of scats containing fungi when heavily mycophagous mammals were present in the diet vs when non-mycophagous mammals were present (see Table 1 for designations of heavily mycophagous vs non-mycophagous species).

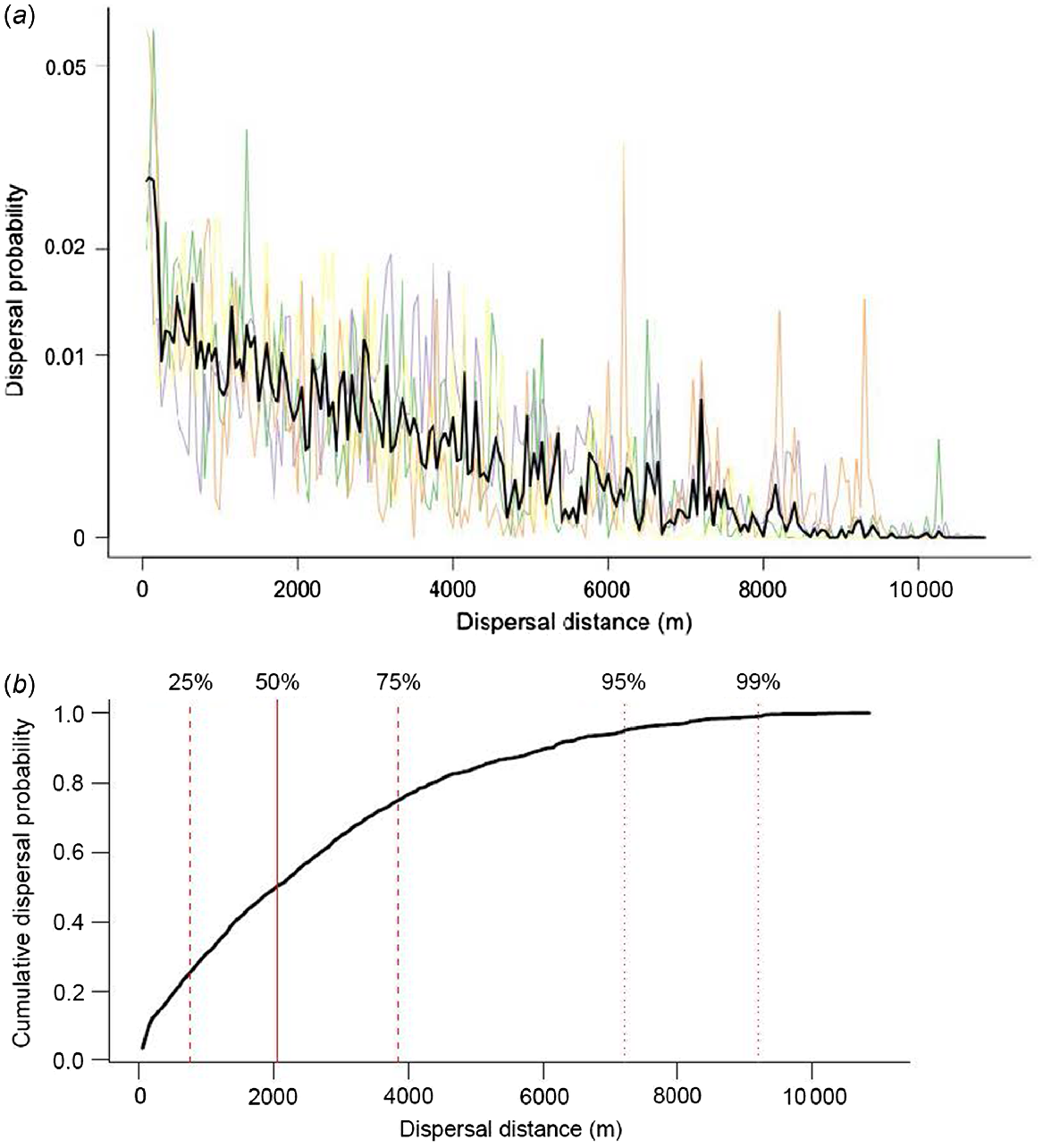
In the 50-h window between ingestion and defecation, the probability of dispersal decreased with increasing distance from point of ingestion (Fig. 3a). Median predicted distance of spore dispersal was 2050 m. From point of ingestion, 50% of spores were predicted to be dispersed >2 km, 95% <7200 m, 25% <750 m, and 75% <3850 m (Fig. 3b). The predicted overall maximum dispersal distance was 10 700 m.
(a) Probability of spore dispersal by each of the four tracked dingoes. Thin coloured lines predict dispersal distance for each individual. Thick black line represents mean prediction of probability of dispersal based on all four individuals. (b) Cumulative probability of dispersal predicted for fungal spores secondarily ingested by dingoes. Solid vertical line shows median dispersal distance, dashed vertical lines show 25% and 75% confidence intervals, and dotted vertical lines show 95% and 99% confidence intervals.
Discussion
This study is the first to empirically investigate secondary fungal dispersal by a vertebrate and the second to model estimated mammalian spore dispersal distances. We suggest that when dingoes ingest primary mycophagists, they regularly disperse spores of mycorrhizal fungi thousands of metres from where fruiting bodies appeared (Fig. 3). These dispersal events are important for both epigeous and hypogeous/sequestrate taxa (Table 1) and their associated plant communities. Many primary mycophagists also benefit from the dispersal of fungi they eat. Unlike smaller mammals, dingoes move over large areas and between fragments; in doing so, they provide a unique ecosystem service through these dispersal events. Danks et al. (2020) quantified the importance of swamp wallabies (Wallabia bicolor) as long-distance dispersers and estimated their maximum dispersal potential to be 1265 m; we predict dingoes are routinely dispersing spores more than twice this distance and potentially as far as 10 700 m. Swamp wallabies are one of the larger mycophagists, so their dispersal potential is likely far greater than that of rodents and other small mammals. As our study shows, spores can be moved much farther when a predator is involved.
We make several assumptions with our model, including: (1) prey animals that have ingested fungi are evenly distributed throughout the dingo’s range; (2) time since ingestion is the only factor that determines the predicted distance of dispersal; and (3) dingoes defecate evenly across their habitat. Data on the fine-scale distributions of each prey species would allow us to factor for even distribution, but such data were not available. Data on defecation rates of each dingo in relation to behaviour and movements would also increase accuracy, but it was not possible for us to determine these differences or collect such data within the context of this study. Our observations during scat collection indicate that dingoes do not defecate evenly across their habitat, but we could not factor for this within our study design.
Dingoes have a very diverse diet (Doherty et al. 2019), so we cannot rule out the possibility that they may occasionally ingest fungi. But we could find no reports of direct fungal consumption by dingoes in the literature, and there are very few reports of mycophagy by other species of large canid predators. Our data show that the presence of spores in scats increases substantially with the presence of mycophagous prey mammals (Fig. 2). However, when dingoes are hunting in sites that have primary fungal consumers, they would also have access to the fungi eaten by these small mammals. We therefore cannot unequivocally rule out the possibility that primary mycophagy could also occur.
Our interpretation that dingoes contribute substantially to long-distance spore dispersal rests upon the assumption that the spores they disperse are viable. We did not test spore viability in this study due to the extensive research on spore resiliency and positive impacts of spore scarification by animal digestive systems (Vašutová et al. 2019; Elliott et al. 2022). Some studies have suggested that longer transit times and/or harsher digestive systems may actually be beneficial for the germination rates of some mycorrhizal fungal spores (Zambonelli et al. 2017; Ori et al. 2021). Our microanalysis of spores present in the scats included in this study showed no evidence of detrimental spore degradation, and many previous studies of spore viability post vertebrate ingestion also support our assumption of viability (Tay et al. 2018; Vašutová et al. 2019; Aguirre et al. 2021; Caiafa et al. 2021; Ori et al. 2021). We can find no evidence that the digestive system of any mammal negatively impacts mycorrhizal spore viability, and at least 40 mammal species have been experimentally shown to disperse viable spores (Elliott et al. 2022). The Pampas fox (Pseudalopex gymnocercus) is the closest relative to the dingo that has been studied, and Aguirre et al. (2021) showed it to disperse viable mycorrhizal fungal spores in its scats. Based on our examination of spores found in dingo scats and what has been widely shown by numerous studies of spore viability among other animals, we predict that spores would survive – and perhaps even benefit from – transit through the digestive system of dingoes.
The historic impacts of dingoes as long-distance spore dispersers in Australia are unknown. The dingo is a relative newcomer to the continent, with current archaeological evidence estimating its arrival to be between 3500 and 4000 years ago (Jackson et al. 2017). Prior to the arrival of dingoes, it is likely that other large predators (e.g. the thylacine; Thylacinus cynocephalus) performed similar long-distance spore dispersal services. But between the fragmentation of much of the Australian landscape and the extinction/loss in abundance of many of the larger fungal specialist mammals – particularly members of the Potoroidae – dingoes and possibly other secondary dispersers are likely far more important than they once were for long-distance dispersal.
Our objectives in this study were to introduce a previously unrecognised ecosystem service provided by dingoes and an unexplored area of study in predator ecology. It is likely that numerous other carnivores – including many members of the Canidae, Dasyuridae, Felidae and Mustelidae – frequently prey on mycophagous mammals and provide similar secondary spore dispersal. We also suspect that raptors and snakes eat small mycophagous mammals and that they and insectivorous mammals, reptiles, amphibians and birds may also be secondary dispersers. We hope this study will inspire further investigation into the complex associations between fungi, mycophagists and their predators.
Acknowledgements
The hair analysis by Georgeanna Story of ScatsAbout contributed greatly to this study. Tani Cooper provided valuable assistance with GPS data. Craig Johnson provided access to and assistance with microscopes at the University of New England. Peter Fleming and Steve Jackson provided taxonomic and nomenclatural insights on dingoes.
References
Aguirre F, Nouhra E, Urcelay C (2021) Native and non-native mammals disperse exotic ectomycorrhizal fungi at long distances from pine plantations. Fungal Ecology 49, 101012.
| Crossref | Google Scholar |
Allen MF, Hipps LE, Wooldridge GL (1989) Wind dispersal and subsequent establishment of VA mycorrhizal fungi across a successional arid landscape. Landscape Ecology 2, 165-171.
| Crossref | Google Scholar |
Banta CA, Clemens ET, Krinsky MM, Sheffy BE (1979) Sites of organic acid production and patterns of digesta movement in the gastrointestinal tract of dogs. The Journal of Nutrition 109, 1592-1600.
| Crossref | Google Scholar |
Bradshaw AJ, Autumn KC, Rickart EA, Dentinger BTM (2022) On the origin of feces: fungal diversity, distribution, and conservation implications from feces of small mammals. Environmental DNA 2022, 608-626.
| Crossref | Google Scholar |
Burrows CF, Kronfeld DS, Banta CA, Merritt AM (1982) Effects of fiber on digestibility and transit time in dogs. The Journal of Nutrition 112, 1726-1732.
| Crossref | Google Scholar |
Caiafa MV, Jusino MA, Wilkie AC, Díaz IA, Sieving KE, Smith ME (2021) Discovering the role of Patagonian birds in the dispersal of truffles and other mycorrhizal fungi. Current Biology 31, 5558-5570.e3.
| Crossref | Google Scholar |
Childs-Sanford SE, Angel CR (2006) Transit time and digestibility of two experimental diets in the maned wolf (Chrysocyon brachyurus) and domestic dog (Canis lupus). Zoo Biology 25, 369-381.
| Crossref | Google Scholar |
Claridge AW, May TW (1994) Mycophagy among Australian mammals. Austral Ecology 19, 251-275.
| Crossref | Google Scholar |
Cloutier VB, Piché Y, Fortin JA, Bérubé JA, Glémet H, Desrochers A (2019) A novel approach for tracing mycophagous small mammals and documenting their fungal diets. Botany 97, 475-785.
| Crossref | Google Scholar |
Danks MA (2012) Gut-retention time in mycophagous mammals: a review and a study of truffle-like fungal spore retention in the swamp wallaby. Fungal Ecology 5, 200-210.
| Crossref | Google Scholar |
Danks M, Lebel T, Vernes K, Andrew N (2013) Truffle-like fungi sporocarps in a eucalypt-dominated landscape: patterns in diversity and community structure. Fungal Diversity 58, 143-157.
| Crossref | Google Scholar |
Danks MA, Simpson N, Elliott TF, Paine CET, Vernes K (2020) Modeling mycorrhizal fungi dispersal by the mycophagous swamp wallaby (Wallabia bicolor). Ecology and Evolution 10, 12920-12928.
| Crossref | Google Scholar |
De Cuyper A, Hesta M, Tibosch S, Wanke C, Clauss M, Janssens GPJ (2018) How does dietary particle size affect carnivore gastrointestinal transit: a dog model. Journal of Animal Physiology and Animal Nutrition 102, e615-e622.
| Crossref | Google Scholar |
Doherty TS, Davis NE, Dickman CR, Forsyth DM, Letnic M, Nimmo DG, et al. (2019) Continental patterns in the diet of a top predator: Australia’s dingo. Mammal Review 49, 31-44.
| Crossref | Google Scholar |
Dundas SJ, Hopkins AJM, Ruthrof KX, Tay NE, Burgess TI, Hardy GESJ, Fleming PA (2018) Digging mammals contribute to rhizosphere fungal community composition and seedling growth. Biodiversity and Conservation 27, 3071-3086.
| Crossref | Google Scholar |
Elliott TF, Vernes K (2019) Superb lyrebird Menura novaehollandiae mycophagy, truffles and soil disturbance. Ibis 161, 198-204.
| Crossref | Google Scholar |
Elliott TF, Jusino MA, Trappe JM, Lepp H, Ballard G-A, Bruhl JJ, Vernes K (2019a) A global review of the ecological significance of symbiotic associations between birds and fungi. Fungal Diversity 98, 161-194.
| Crossref | Google Scholar |
Elliott TF, Bower DS, Vernes K (2019b) Reptilian mycophagy: a global review of mutually beneficial associations between reptiles and macrofungi. Mycosphere 10, 776-797.
| Crossref | Google Scholar |
Elliott TF, Truong C, Jackson SM, Zúñiga CL, Trappe JM, Vernes K (2022) Mammalian mycophagy: a global review of ecosystem interactions between mammals and fungi. Fungal Systematics and Evolution 9, 99-159.
| Crossref | Google Scholar |
Geml J, Tulloss RE, Laursen GA, Sazanova NA, Taylor DL (2008) Evidence for strong inter- and intracontinental phylogeographic structure in Amanita muscaria, a wind-dispersed ectomycorrhizal basidiomycete. Molecular Phylogenetics and Evolution 48, 694-701.
| Crossref | Google Scholar |
Hernot DC, Biourge VC, Martin LJ, Dumon HJ, Nguyen PG (2005) Relationship between total transit time and faecal quality in adult dogs differing in body size. Journal of Animal Physiology and Animal Nutrition 89, 189-193.
| Crossref | Google Scholar |
Holland GJ, Bennett AF (2009) Differing responses to landscape change: implications for small mammal assemblages in forest fragments. Biodiversity and Conservation 18, 2997-3016.
| Crossref | Google Scholar |
Hopkins AJM, Tay NE, Bryant GL, Ruthrof KX, Valentine LE, Kobryn H, Burgess TI, Richardson BB, Hardy GESJ, Fleming PA (2021) Urban remnant size alters fungal functional groups dispersed by a digging mammal. Biodiversity and Conservation 30, 3983-4003.
| Crossref | Google Scholar |
Jackson SM, Groves CP, Fleming PJS, Aplin KP, Eldridge MDB, Gonzalez A, Helgen KM (2017) The wayward dog: is the Australian native dog or dingo a distinct species? Zootaxa 4317, 201-224.
| Crossref | Google Scholar |
Jackson SM, Fleming PJS, Eldridge MDB, Archer M, Ingleby S, Johnson RN, Helgen KM (2021) Taxonomy of the dingo: it’s an ancient dog. Australian Zoologist 41, 347-357.
| Crossref | Google Scholar |
Jonsson L, Dahlberg A, Nilsson M-C, Kårén O, Zackrisson O (1999) Continuity of ectomycorrhizal fungi in self-regenerating boreal Pinus sylvestris forests studied by comparing mycobiont diversity on seedlings and mature trees. New Phytologist 142, 151-162.
| Crossref | Google Scholar |
Li D-W (2005) Release and dispersal of basidiospores from Amanita muscaria var. alba and their infiltration into a residence. Mycological Research 109, 1235-1242.
| Crossref | Google Scholar |
Nuske SJ, Vernes K, May TW, Claridge AW, Congdon BC, Krockenberger A, Abell SE (2017) Redundancy among mammalian fungal dispersers and the importance of declining specialists. Fungal Ecology 27, 1-13.
| Crossref | Google Scholar |
Nuske SJ, Anslan S, Tedersoo L, Congdon BC, Abell SE (2019) Ectomycorrhizal fungal communities are dominated by mammalian dispersed truffle-like taxa in north-east Australian woodlands. Mycorrhiza 29, 181-193.
| Crossref | Google Scholar |
Ori F, Menotta M, Leonardi M, Amicucci A, Zambonelli A, Covès H, Selosse M-A, Schneider-Maunoury L, Pacioni G, Iotti M (2021) Effect of slug mycophagy on Tuber aestivum spores. Fungal Biology 125, 796-805.
| Crossref | Google Scholar |
Peay KG, Schubert MG, Nguyen NH, Bruns TD (2012) Measuring ectomycorrhizal fungal dispersal: macroecological patterns driven by microscopic propagules. Molecular Ecology 21, 4122-4136.
| Crossref | Google Scholar |
Pires AS, Koeler Lira P, Fernandez FAS, Schittini GM, Oliveira LC (2002) Frequency of movements of small mammals among Atlantic Coastal Forest fragments in Brazil. Biological Conservation 108, 229-237.
| Crossref | Google Scholar |
Swihart RK, Slade NA, Bergstrom BJ (1988) Relating body size to the rate of home range use in mammals. Ecology 69, 393-399.
| Crossref | Google Scholar |
Tay NE, Hopkins AJM, Ruthrof KX, Burgess T, Hardy GES, Fleming PA (2018) The tripartite relationship between a bioturbator, mycorrhizal fungi, and a key Mediterranean forest tree. Austral Ecology 43, 742-751.
| Crossref | Google Scholar |
Trappe JM (1988) Lessons from alpine fungi. Mycologia 80, 1-10.
| Crossref | Google Scholar |
Trappe JM, Strand RF (1969) Mycorrhizal deficiency in a Douglas-fir region nursery. Forest Science 15, 381-389.
| Google Scholar |
Vašutová M, Mleczko P, López-García A, Maček I, Boros G, Ševčík J, Fujii S, Hackenberger D, Tuf IH, Hornung E, Páll-Gergely B, Kjøller R (2019) Taxi drivers: the role of animals in transporting mycorrhizal fungi. Mycorrhiza 29, 413-434.
| Crossref | Google Scholar |
Vogilino P (1895) Richerche intorno all’ azione delle lumache e dei rospi nello sviluppo di Agaricini. Nuovo Giornale Botanico 27, 181-185 [In Italian].
| Google Scholar |
Warner NJ, Allen MF, MacMahon JA (1987) Dispersal agents of vesicular–arbuscular mycorrhizal fungi in a disturbed arid ecosystem. Mycologia 79, 721-730.
| Crossref | Google Scholar |
Watson DM, Shaw D (2018) Veiled polypore (Cryptoporus volvatus) as a foraging substrate for the white-headed woodpecker (Picoides albolarvatus). Northwestern Naturalist 99, 58-62.
| Crossref | Google Scholar |


