The influence of camera-trap flash type on the behavioural response, detection rate and individual recognition of Eld’s deer
Rachel Ladd A * , Paul Meek
A * , Paul Meek  A B and Luke K.-P. Leung A
A B and Luke K.-P. Leung A
A School of Agriculture and Food Sciences, The University of Queensland, Gatton, Qld 4343, Australia.
B Vertebrate Pest Research Unit, NSW Department of Primary Industries, PO Box 530, Coffs Harbour, NSW 2450, Australia.
Wildlife Research - https://doi.org/10.1071/WR22055
Submitted: 20 March 2022 Accepted: 2 August 2022 Published online: 31 August 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Camera traps are available with infrared or white flash, with the former being more commonly used. However, white flash produces colour night-time photographs that can be critically useful for both species and individual identification. White flash was thought to cause more disturbance to wildlife than was infrared and this may lead to camera avoidance. Evaluating the extent of this response, and differences between the flash types, is useful to develop improved survey designs.
Aims: This research aimed to quantify the behavioural responses of Eld’s deer to white and infrared flash, to determine whether white-flash cameras were suitable for use in population surveys of this species.
Methods: A behavioural ethogram was used to quantify the responses of the deer to the two flash types, as well as the responses of different sex-age classes and group sizes when encountering a camera trap. Additionally, the detection rate for white flash and infrared flash cameras was compared through time, to determine any pattern of avoidance.
Key results: While deer were more likely to observe and be startled by white flash than infrared, this did not adversely affect the detection of the deer, with no significant change in the detection rate between the two different flash types over time. Group size was found not to influence behavioural response when encountering camera traps, whereas different age–sex classes of deer showed very few differences in response to camera traps.
Conclusions: White flash cameras were found to be suitable for Eld’s deer population surveys and were beneficial in providing colour night-time photos that allow for spotted female deer to be individually identified.
Implications: Practitioners should not be concerned about the influence of white flash when using camera traps to monitor populations of Eld’s deer, and using white flash is recommended when individual identification is required.
Keywords: animal behaviour, avoidance, camera trap, deer, detection, flash type, monitoring bias, wildlife monitoring.
Introduction
Camera traps are a valuable tool for monitoring long-term trends in wildlife populations. There is a need to optimise and refine camera trap methods to ensure that the most reliable and informative results are achieved (Kelly 2008), particularly for populations that are challenging to monitor, such as Eld’s deer (Rucervus eldii), an endangered species with several small populations. Eld’s deer is a dry tropical forest specialist endemic to Southeast Asia, with three officially recognised, geographically distinct subspecies. The exact status of the species in Cambodia is less well understood owing to the difficulty in surveying the small, scattered populations remaining (Ladd et al. 2022). Optimising camera trapping surveys will improve the rigour of population estimates, resulting in better conservation outcomes through more informed planning and decision-making.
Both spatial and non-spatial forms of capture–recapture, and its derivative mark–recapture, analyses are increasingly popular methods for estimating density and abundance from camera trap surveys (Borchers and Efford 2008; Royle et al. 2009; Chandler and Royle 2013). However, these methods require either all, or a subset of individuals within the population, to be uniquely identifiable (Royle et al. 2009; Chandler and Royle 2013; Efford and Hunter 2018). Although similar approaches have been developed for unmarked species, the estimates are typically much less precise (Rowcliffe et al. 2008; Chandler and Royle 2013; Ramsey et al. 2015). Many carnivores, and, in particular, felids often have distinct pelage patterns, allowing identification from naturally occurring marks, such as a tiger’s (Panthera tigris) stripes (Karanth 1995). The use of natural marks has also been used for species without such obvious markings, such as pumas (Puma concolor) and chimpanzees (Pan troglodytes), by utilising more subtle characteristics (Kelly et al. 2008; Després-Einspenner et al. 2017). Artificial markings, such as ear tags or collars, can also be used to identify a proportion of the population. However, this can add significant logistical and resourcing challenges, as well as introducing a risk to the target species through handling. The use of natural markings for identification is highly preferable for Eld’s deer because of limited management resources (Ladd et al. 2022). Only adult males are routinely identifiable by their antlers. A subset of the adult female population retains pelage spot patterns, which can be used for identification during a survey period, and in both males and females, scars and ear nicks may provide additional markings. Unfortunately, the spot patterns are not visible in the black-and-white photos produced by infrared flash cameras at night, when the deer are most commonly detected (R. Ladd, unpubl. data).
Earlier in the development of camera traps, the standard flash was a white light xenon flash. However, there were reports of avoidance of white flash camera traps by some target species such as kinkajous (Potos flavus) and tiger (Wegge et al. 2004; Schipper 2007). Technological advances led to the development of infrared flash cameras, and they have become the most widely used camera trap for ecological research and monitoring, with marketing claims that animals cannot see the infrared light (Meek and Pittet 2012). However, the part of the infrared spectrum used by camera traps generally falls within the range visible to many species, particularly the nocturnal–crepuscular species often targeted by camera trap studies (Newbold and King 2009; Meek et al. 2014). Despite concerns of greater disturbance, the colour night-time photographs produced by white flash cameras (either xenon or newer LEDs) can be essential for the identification of some species that have similar morphology (Meek and Pittet 2012). They also ensure that the more subtle pelage patterns of some species are visible and clear, which is essential for individual identification. For Eld’s deer, colour night photos are needed to see individual spot patterns in a subset of the population, and this visibility will potentially lead to a higher number of identifications and less ambiguity in their identities. However, if Eld’s deer has a strongly negative response to white flash cameras and avoid them, the reduced detections will eliminate any benefits of increased identification.
There have been a few investigations into the behavioural responses of animals to camera traps (e.g. Glen et al. 2013; Gregory et al. 2014; Meek et al. 2016). However, it was not possible to discern from these studies which stimuli the animals are responding to. Camera traps produce sounds and light, as well as being a novel object in the environment, and animals could respond to any one or a combination of these stimuli (Meek et al. 2014). Arboreal deployment of infrared camera traps in a tropical forest found no reduction in detections over time, suggesting that the cameras do not cause significant disturbance (Gregory et al. 2014). However, an investigation of responses of Australian predators found that the majority of cats (Felis catus), foxes (Vulpes vuples) and wild dogs (Canis familiaris) observed and responded to the camera traps, with similar response rates between day and night except for cats, which were more likely to detect the camera during the day (Meek et al. 2016).
Explicit examination of the effects of different flash types is similarly limited, but differences have been found both within and among species. Stoats (Mustela erminea) were found to exhibit signs of wariness regardless of flash type, whereas domestic cats exhibited variable individual responses during a controlled study (Glen et al. 2013). In a field study, there was no reduced detection rate of cats by white flash camera traps, and no indication of more negative responses to white flash than to infrared (Taggart et al. 2020). No differences in detection rate were found in a comparison of white, standard infrared and high-wavelength infrared flash camera traps for red deer (Cervus elaphus) and roe deer (Capreolus capreolus; Henrich et al. 2020). However, behavioural response differences were found among species and among sites for the two different infrared flash camera types and day-time detections, although no comparison was made with white flash (Henrich et al. 2020). A comparison of differences in detection probability at infrared or white flash camera traps for five common metropolitan species in the United States found infrared flash cameras resulted in a higher detection probability for some species than did white flash cameras; however, this was significant only for one species, the brown rat (Rattus norvegicus; Herrera et al. 2021). These studies indicated the need for information specific to the targeted species population about their behavioural response to camera trap flash type and consideration of behaviour when choosing a flash type for objectives of a particular study.
In this study, we quantify the behavioural responses of Eld’s deer to white and infrared flash cameras in a wildlife sanctuary in Cambodia during a camera trapping survey. We hypothesise that (1) behavioural reactions will occur most frequently when encountering white flash cameras, less often when encountering infrared flash cameras, and occur least often when no flash fires during the day; (2) responses to camera traps will differ depending on the age, sex and group size of the deer; and (3) over time, after negative reactions, avoidance of camera traps will lead to a decrease in the detection rate. The results will be used to develop improved survey methods for monitoring Eld’s deer.
Materials and methods
A camera trap survey was conducted in Siem Pang Wildlife Sanctuary (SPWS), Stung Treng Province, Cambodia, to estimate the population size of Eld’s deer (Ladd 2022). The sanctuary includes deciduous dipterocarp, semi-evergreen and riverine forest habitats at low elevations (<350 m asl). This survey was undertaken entirely within the deciduous dipterocarp forest (DDF) in the south of the sanctuary. The area has strongly seasonal rainfall, with the rainy season occurring May–October and the dry season occurring November–April.
Data were collected from late December 2019 to early June 2020 across two adjacent blocks, surveyed consecutively, to allow for a higher density of camera trap sites within the survey area. Logistical challenges meant that white flash camera traps were available in the second half of the survey only and these were added to the second survey block to increase the number of camera trap sites. The addition of white flash cameras to the original infrared flash cameras allowed for the investigation of differences in behavioural responses and detection rates between the two different flash types, discussed in the present paper. A comparison of the two camera trap types will inform future surveys of the utility of white flash and whether they provide an advantage for identifying individual Eld’s deer.
A grid was used to demarcate the survey area, with cells of 1 km2, and the area was divided into two adjacent blocks, surveyed consecutively; Block 1 consisted of 32 sites active from late December to mid-March, and Block 2 consisted of 51 sites active from mid-March to early June (Fig. 1). One camera trap was positioned within 200 m of the centre of each 1 km2 cell, resulting in an average trap spacing of 862 m. The exact camera location was selected on the basis of advice of a local ranger, so as to maximise Eld’s deer photo captures. However, cameras were not aimed directly towards a water source or other attractive feature, so as to avoid creating a subset of naturally lured sites, as well as to reduce the likelihood of human interference. In Block 1, Reconyx Hyperfire HC600 infrared cameras were used. In Block 2, 31 Reconyx Hyperfire HC600 infrared flash and 20 Reconyx HP2W white flash cameras were used. The two camera trap flash types were randomly allocated to sites in the block. No confounding factors across the two consecutively surveyed blocks were defined because the site is homogenous and population sampling by camera trap will always have covariates that may influence detection but are too difficult to identify and measure, as such they are not typically addressed in camera trapping studies.
Cameras were mounted on trees, approximately 0.8 m above the ground and vegetation within 2 m of the cameras was slashed to minimise the risk of fire damage and to ensure an open field of view. Five photographs were taken per trigger, with no delay between triggers, and the cameras were set for high sensitivity. The HC600 cameras were set to fast-shutter night mode, whereas the HP2W cameras were set with a high flash output, 1/480th shutter speed and Max ISO of 3200. The HP2W were also put into the Hyperfire legacy PIR type to minimise detection differences between the two models. Memory cards were collected and batteries changed twice during each deployment block.
Animal detections were assigned metadata tags identifying the species present by using the program ExifPro 2.1 (Kowalski 2013). Eld’s deer detections were delineated into temporally independent events by using a 6-min interval, with researcher discretion used in some cases of obviously new individuals not part of a group (R. Ladd, P. Meek, L. Leung, unpubl. data). All detection events of Eld’s deer were also assigned secondary metadata tags with the sex, age class and group size of the individuals captured. Events in which the deer responses could not be observed, such as when the face of the animal was not photographed or the animal was too distant, were excluded from the analysis. For events containing multiple individuals, each animal had their behaviour scored separately.
An ethogram enables the accurate and consistent recording of observed behaviours, with a typical ethogram consisting of a list of behaviours exhibited by the target species, which have descriptive definitions (Martin and Bateson 2007). To describe the behavioural responses of the deer to the camera traps, including their awareness of the traps, movements and actions exhibited, an ethogram was developed, adapted from Meek et al. (2016) (Table 1). Additionally, the direction in which the animal approached the camera was also noted when it could be determined. The use of an ethogram and a single observer (RL) to conduct the behavioural assessment ensured consistency and reduced the possibility of observer bias and misinterpretation of behaviour. Data from both survey blocks were analysed using the ethogram.
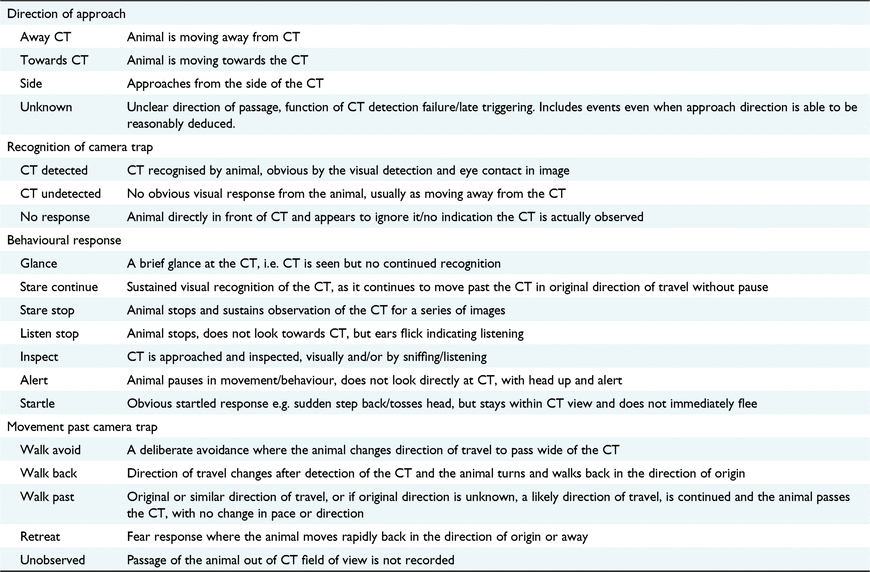
|
Recognition of the camera trap was categorised as the animal detecting the camera, not detecting it, or giving no indication that they have detected it, despite being orientated to observe the camera trap. In the case of white flash cameras, it was assumed that when the flash triggers at night, all deer in the vicinity of the camera would notice the light, but the deer may not exhibit an observable response. Therefore, for white flash cameras activating at night, deer are assumed to have always detected the camera trap and were tagged as either detecting the camera or as exhibiting no response, whereas an individual encountering an infrared flash may alternately be tagged as not detecting the camera. Behavioural responses also included the individual’s reaction to the light, even when they did not explicitly recognise the device causing the flash. For example, the animal is not observed looking towards the camera trap, but may instead pause or look around in the opposite direction of the camera. All animals that either did not detect the camera trap or gave no response to it were assumed to continue past the camera without being influenced by it.
The quantitative behaviours were grouped into response categories for analysis, similar to those used by Meek et al. (2016), including the following: Observe, where the animal displayed a visual change in behaviour that indicated observation of the camera, including awareness of the camera trap without looking directly at it; Startle, a response in which a change in facial expression or behaviour occurred, e.g. changing posture, direction of travel or stopping, indicating awareness and increased alertness; Approach, where the animal detected the camera trap and changes behaviour to move towards or investigate the camera; Repulsion, where the camera is detected and the animal does not continue in the original direction of travel, walking back, away or a complete flight response; and lastly, Continue, in which the animal resumes passage in its original direction, or a close approximation to the original direction, after detection. A single event of a lone individual could therefore potentially be included in four out of five categories when describing their response, with only Repulsion and Continue responses not able to occur within the same event.
Analysis was conducted in R ver. 4.0.4 (R Core Team 2021). Behavioural responses were analysed as proportions of the population sample, with confidence intervals (95%), by using a likelihood-ratio test in the R program ‘binom’ (Dorai-Raj 2014) according to flash type to test Hypothesis 1, and according to sex, age class, and group size to test Hypothesis 2. Results are presented as percentage values with 95% confidence intervals, with a significant difference occurring when the confidence intervals between a set of values do not overlap.
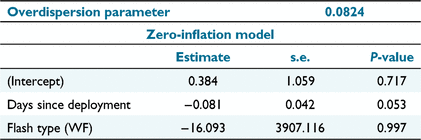
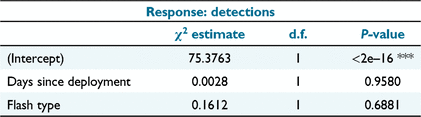
To test whether the camera trapping rate declined within flash types (Hypothesis 3), we counted the number of events per 24 h interval at each location, beginning at midnight after camera trap placement. A possible influence of the flash type and days since deployment on the number of events per day was assessed using a negative binomial generalised linear mixed model (GLMM), with camera trap location as a random effect and no interaction term, using the R package ‘glmmTMB’ (Brooks et al. 2017). Only data collected in Block 2, where white flash and infrared flash cameras were operating simultaneously, were used to fit the GLMM model to negate any possible temporal effects between the two consecutively surveyed blocks. The significance (α = 0.05) of the variables of the zero-inflated model were assessed by analysis of deviance (Type III Wald chi-squared test) by using the R package ‘car’ (Fox and Weisberg 2019).
Ethics statement
Research for this study was conducted with an animal ethics approval from the University of Queensland’s Native and Exotic Wildlife and Marine Animals Ethics Committee (SAFS/072/16 and ANRFA/086/19/Cambodia).
Results
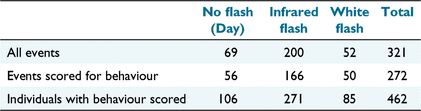
In total, 321 independent events of Eld’s deer were recorded (Table 2). Of these, 272 were of at least one individual suitable for scoring behaviour. This resulted in a total of 462 individuals scored for behavioural response. There were more than three times as many individuals scored for behaviour captured by infrared flash than white flash, and more day-time detections than white flash detections (Table 2). White flash camera traps lead to improved identification of adult females because their identification depends on the visibility of pelage spot patterns that are not perceptible by infrared flash. Including day-time detections, adult female detections by white flash cameras were individually identified 13% of the time, compared with only 3% of infrared flash camera detections of adult females. Adult females were also more than twice as likely to be detected than were adult males. Deer approached the camera from behind, so that they were recorded walking directly away from it 12% of the time. Deer approached directly towards the camera 7% of the time and came from the side 40% of the time. The direction of approach was unclear for 41% of individuals.
|
For both infrared flash detections and day-time detections with no flash, the percentage of deer that did not detect the camera were high, at 76% and 79% respectively, and when combined with the deer that made no response, the percentage of deer that did not observably react on encountering an infrared or day-time camera trap increases to 89% and 88% respectively (Fig. 2). Although it was assumed that all individuals would detect the white flash, 44% of individuals exhibited no response to the light. A higher percentage of individuals exhibited a behavioural response when encountering white flash cameras. However, there was no significant difference in the percentage of individuals that approached the camera, or in the percentage of individuals exhibiting a repulsion response for white flash and infrared flash cameras. A repulsion response, which included back-tracking, changing direction to pass the camera at a greater distance or a flight response, occurred only once during the day time when no flash was triggered. When no flash or infrared flash was used, nearly all deer, namely 99% and 97% respectively, continued past the camera with no repulsion response. The percentage of deer encountering white flash cameras that continued past was significantly lower than for infrared or no flash, but at 78% it was still a high percentage of deer.
|
|
Few significant effects were found within Hypothesis 2, namely, fawns and juveniles were the least likely age class to exhibit a behavioural response to camera traps, and showed significant differences with adult females in both the Observe and Startle behaviour categories (see Supplementary material, Fig. 1). No other significant differences were detected between the age and sex classes. Similarly, no differences in behavioural responses to camera traps were found regardless of group size (see Supplementary material, Fig. 2).
The GLMM was successfully fitted (Table 3) and the analysis of deviance indicated that there was no significant effect of flash type or days since deployment on the detection rate (Table 4). This indicates that there was no decline in trapping rate as a result of avoidance of cameras, regardless of flash type, as proposed by Hypothesis 3.
|
|
Discussion
Our experiment has provided solid evidence that there is no difference between infrared and no flash day-time behavioural response rates of Eld’s deer. However, white flash camera traps do result in an increased response rate. The overall sample size of this study was constrained by the low abundance of this rare deer. Examining the types of responses the deer had would be further supported by a larger sample. The higher Observe and Startle responses to white flash cameras was expected, given deer are assumed to always detect the camera; however, 44% of deer exhibited no discernible response to the light. Very few individuals approached a camera trap, regardless of flash type, which suggests Eld’s deer exhibit little curiosity, and are likely to exhibit a certain degree of wariness, towards camera traps. A repulsion response was recorded more often for individuals encountering white flash cameras than for those encountering infrared flash cameras, but the difference was not significant. However, it should be noted that the overall proportion of deer that experienced a repulsion response was very low (∼3% of all detections). Furthermore, only six individuals (1.3%) took flight overall, occurring at least once for each flash type.
Our data provided little support for Hypothesis 2, with differences found only between adult females and fawns and juveniles in relation to Observe and Startle responses. Males were more likely, but not significantly so, to react with a repulsion response. Eld’s deer were most often, and with similar frequencies, detected alone or in pairs, with a maximum group size of seven being recorded on two occasions. Eld’s deer are typically solitary, with females being associated mainly with their fawns. However, they do congregate during the rut in the dry season, approximately in April (Aung et al. 2001), and this time period was sampled during the survey. A larger group size may reduce Startle and Repulsion behaviours because of a sense of safety in numbers (one responds, they all respond). Although a decreasing trend in Startle behaviours with an increasing group size was shown in the data, it was not significant.
Behavioural responses are likely to vary according to individual traits, and there were some notable behaviours being recorded by individuals that were identifiable from natural markings. This included an adult male who encountered the same white flash camera at night approximately two weeks apart and, in both detections, a strong flight response was observed. With two negative responses and a lack of further detections, this may indicate that this particular individual was attempting to avoid the camera trap. In contrast, an identifiable adult female with a fawn encountered the same infrared camera twice, at night, within a couple of days. On the first detection she was observed being startled and changing direction to avoid passing in front of the camera. On the second encounter, two days later, the same adult female and fawn showed no response, despite facing the camera. This indicates that some individuals may initially exhibit a negative response to the camera, but then become habituated to the camera. Individual responses to camera traps have also been recorded by Meek et al. (2016), such as a particular wild dog always keeping distant from the camera trap, even when that meant diverging from its social group, and individually recognisable cats initially approaching the camera trap and then on later visits, ignoring it. Although generalisations about species responses to camera traps can be made, it is important to note that this is unlikely to be true for all individuals in the population.
A possible short-coming of this study was that there was no independent verification of behavioural responses, with only the responses of individuals that are both detected by and recorded by the camera trap available. It is possible that more extreme negative reactions have been missed. Such strong repulsion responses may cause the individual to move out of the field of view before they can be recorded. Deer may also react to the sight and smell of the camera, as suggested in other wildlife studies (Séquin et al. 2003; Wegge et al. 2004; Larrucea et al. 2007; Muñoz et al. 2014). This reaction may take place before the flash fires, and they avoid the camera before they can trigger it and be recorded. Therefore, it is possible that an under-representation of negative responses exists in the data. For example, some encounters consisted only of parts of an individual being recorded as it withdraws from the camera, which cannot be behaviourally quantified, or if an individual avoids the camera earlier in the encounter and fails to trigger it at all. However, if this is an issue, it is affecting both flash types similarly as no significant difference in detection rate was found. This problem was overcome by Glen et al. (2013) in their assessment of camera traps in a controlled environment where video cameras were operated continuously to record all animal encounters with a camera trap inside their pens. However, for a large, rare animal such as Eld’s deer, such a setup is unlikely to be feasible.
The advantages of white flash cameras, namely that night photographs are both coloured and sharply focussed as opposed to pixelated monochrome images from infrared, can readily be considered without undue concern for negative reactions from Eld’s deer. The white flash cameras allowed for improved image quality and the increased identification of adult females with spot patterns. This is an important consideration for capture–recapture and mark–recapture studies that rely on individual identification from natural markings. However, consideration must be given to the potentially greater risk of theft and interference when using white flash cameras (Meek and Pittet 2012), because they are more noticeable at night. We had no theft or interference incidents for either camera flash type in this survey; however, there have been a few camera trap thefts and vandalism in Seim Pang Wildlife Sanctuary in previous years. The relative risk of potential data and camera loss through such problems needs to be balanced with the advantages of colour night-time photos for identification.
Conclusions
Camera traps are an important and widely used tool in conservation management and ecological study; hence, it is essential that we understand the influence this tool has on the behaviour and detections of the animals under study. There are pros and cons of the different flash types in terms of the quality of the photographs produced and their influence on animal behaviour, and this needs to be carefully considered when making a selection. This study provides field data on the influence of flash type on the behaviour and detection rate of Eld’s deer that helps guide optimised camera trap methodology for future studies of population trends. We assert that white flash cameras are suitable for studies of demography and monitoring Eld’s deer and are recommended over infrared camera traps when individual identification is a requirement of the study.
Supplementary material
Supplementary material is available online.
Data availability
Data collected and analysed in this study are archived in the library of the University of Queensland, Gatton, Queensland, Australia.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
Rachel Ladd was supported by an Australian Government Research Training Program (RTP) Stipend and RTP Fee-Offset Scholarship through the University of Queensland. We thank Conservation Force for providing funding for this project.
Acknowledgements
We thank BirdLife International Cambodia Programme and Rising Phoenix Co. Ltd for providing field support, and in particular Jonathan C. Eames for facilitating this work. Statistical advice was given by Vincent Mellor.
References
Aung, M, McShea, WJ, Htung, S, Than, A, Soe, TM, Monfort, S, and Wemmer, C (2001). Ecology and social organization of a tropical deer (Cervus eldi thamin). Journal of Mammalogy 82, 836–847.| Ecology and social organization of a tropical deer (Cervus eldi thamin).Crossref | GoogleScholarGoogle Scholar |
Borchers, DL, and Efford, MG (2008). Spatially explicit maximum likelihood methods for capture–recapture studies. Biometrics 64, 377–385.
| Spatially explicit maximum likelihood methods for capture–recapture studies.Crossref | GoogleScholarGoogle Scholar |
Brooks, ME, Kristensen, K, van Benthem, KJ, Magnusson, A, Berg, CW, Nielsen, A, Skaug, HJ, Maechler, M, and Bolker, BM (2017). glmmTMB balances speed and flexibility among packages for zero-inglated generalized linear mixed modeling. The R Journal 9, 378–400.
| glmmTMB balances speed and flexibility among packages for zero-inglated generalized linear mixed modeling.Crossref | GoogleScholarGoogle Scholar |
Chandler, RB, and Royle, JA (2013). Spatially explicit models for inference about density in unmarked or partially marked populations. The Annals of Applied Statistics 7, 936–954.
| Spatially explicit models for inference about density in unmarked or partially marked populations.Crossref | GoogleScholarGoogle Scholar |
Després-Einspenner, M-L, Howe, EJ, Drapeau, P, and Kühl, HS (2017). An empirical evaluation of camera trapping and spatially explicit capture–recapture models for estimating chimpanzee density. American Journal of Primatology 79, e22647.
| An empirical evaluation of camera trapping and spatially explicit capture–recapture models for estimating chimpanzee density.Crossref | GoogleScholarGoogle Scholar |
Dorai-Raj S (2014) binom: binomial confidence intervals for several parameterizations. R package (version 1.1-1). Available at http://CRAN.R-project.org/package=binom
Efford, MG, and Hunter, CM (2018). Spatial capture–mark–resight estimation of animal population density. Biometrics 74, 411–420.
| Spatial capture–mark–resight estimation of animal population density.Crossref | GoogleScholarGoogle Scholar |
Fox J, Weisberg S (2019) ‘An R companion to applied regression.’ 3rd edn. (Sage: Thousand Oaks, CA, USA) Available at https://socialsciences.mcmaster.ca/jfox/Books/Companion/
Glen, AS, Cockburn, S, Nichols, M, Ekanayake, J, and Warburton, B (2013). Optimising camera traps for monitoring small mammals. PLoS ONE 8, e67940.
| Optimising camera traps for monitoring small mammals.Crossref | GoogleScholarGoogle Scholar |
Gregory, T, Carrasco Rueda, F, Deichmann, J, Kolowski, J, Alonso, A, and Fisher, D (2014). Arboreal camera trapping: taking a proven method to new heights. Methods in Ecology and Evolution 5, 443–451.
| Arboreal camera trapping: taking a proven method to new heights.Crossref | GoogleScholarGoogle Scholar |
Henrich, M, Niederlechner, S, Kröschel, M, Thoma, S, Dormann, CF, Hartig, F, Heurich, M, Rowcliffe, M, and Wearn, O (2020). The influence of camera trap flash type on the behavioural reactions and trapping rates of red deer and roe deer. Remote Sensing in Ecology and Conservation 6, 399–410.
| The influence of camera trap flash type on the behavioural reactions and trapping rates of red deer and roe deer.Crossref | GoogleScholarGoogle Scholar |
Herrera, DJ, Moore, SM, Herrmann, V, McShea, WJ, and Cove, MV (2021). A shot in the dark: white and infrared LED flash camera traps yield similar detection probabilities for common urban mammal species. Hystrix, the Italian Journal of Mammalogy 32, 72–75.
| A shot in the dark: white and infrared LED flash camera traps yield similar detection probabilities for common urban mammal species.Crossref | GoogleScholarGoogle Scholar |
Karanth, KU (1995). Estimating tiger Panthera tigris populations from camera-trap data using capture–recpature models. Biological Conservation 71, 333–338.
| Estimating tiger Panthera tigris populations from camera-trap data using capture–recpature models.Crossref | GoogleScholarGoogle Scholar |
Kelly, MJ (2008). Design, evaluate, refine: camera trap studies for elusive species. Animal Conservation 11, 182–184.
| Design, evaluate, refine: camera trap studies for elusive species.Crossref | GoogleScholarGoogle Scholar |
Kelly, MJ, Noss, AJ, Di Bitetti, MS, Maffei, L, Arispe, RL, Paviolo, A, De Angelo, CD, and Di Blanco, YE (2008). Estimating Puma densities from camera trpping across three study sites: Bolivia, Argentina and Belize. Journal of Mammalogy 89, 408–418.
| Estimating Puma densities from camera trpping across three study sites: Bolivia, Argentina and Belize.Crossref | GoogleScholarGoogle Scholar |
Kowalski M (2013) ExifPro photo browser. (version 2.1). Available at http://www.exifpro.com
Ladd R (2022) ‘Deer, Dogs, Demographics and Detection: Conservation Management of Eld’s Deer (Rucervus eldii) in Cambodia.’ (PhD Thesis, School of Agriculture and Food Science, The University of Queensland)
Ladd, R, Crouthers, R, Brook, S, and Eames, JC (2022). Reviewing the status and demise of the endangered Eld’s deer and identifying priority sites and conservation actions in Cambodia. Mammalia , .
| Reviewing the status and demise of the endangered Eld’s deer and identifying priority sites and conservation actions in Cambodia.Crossref | GoogleScholarGoogle Scholar |
Larrucea, ES, Brussard, PF, Jaeger, MM, and Barrett, RH (2007). Cameras, coyotes, and the assumption of equal detectability. Journal of Wildlife Management 71, 1682–1689.
| Cameras, coyotes, and the assumption of equal detectability.Crossref | GoogleScholarGoogle Scholar |
Martin P, Bateson P (2007) ‘Measuring behaviour: an introductory guide.’ (Cambridge University Press: Cambridge, UK)
Meek, PD, and Pittet, A (2012). User-based design specifications for the ultimate camera trap for wildlife research. Wildlife Research 39, 649–660.
| User-based design specifications for the ultimate camera trap for wildlife research.Crossref | GoogleScholarGoogle Scholar |
Meek, PD, Ballard, G-A, Fleming, PJS, Schaefer, M, Williams, W, and Falzon, G (2014). Camera traps can be heard and seen by animals. PLoS ONE 9, e110832.
| Camera traps can be heard and seen by animals.Crossref | GoogleScholarGoogle Scholar |
Meek, P, Ballard, G, Fleming, P, and Falzon, G (2016). Are we getting the full picture? Animal responses to camera traps and implications for predator studies. Ecology and Evolution 6, 3216–3225.
| Are we getting the full picture? Animal responses to camera traps and implications for predator studies.Crossref | GoogleScholarGoogle Scholar |
Muñoz, D, Kapfer, J, and Olfenbuttel, C (2014). Do available products to mask human scent influence camera trap survey results? Wildlife Biology 20, 246–252.
| Do available products to mask human scent influence camera trap survey results?Crossref | GoogleScholarGoogle Scholar |
Newbold, HG, and King, CM (2009). Can a predator see ‘invisible’ light? Infrared vision in ferrets (Mustela furo). Wildlife Research 36, 309–318.
| Can a predator see ‘invisible’ light? Infrared vision in ferrets (Mustela furo).Crossref | GoogleScholarGoogle Scholar |
Ramsey, DSL, Caley, PA, and Robley, A (2015). Estimating population density from presence-absence data using a spatially explicit model. The Journal of Wildlife Management 79, 491–499.
| Estimating population density from presence-absence data using a spatially explicit model.Crossref | GoogleScholarGoogle Scholar |
R Core Team (2021) ‘R: A language and envrionment for statistical computing.’ (R Foundation for Statistical Computing: Vienna, Austria) Available at https://www.R-project.org/
Rowcliffe, JM, Field, J, Turvey, ST, and Carbone, C (2008). Estimating animal density using camera traps without the need for individual recognition. Journal of Applied Ecology 45, 1228–1236.
| Estimating animal density using camera traps without the need for individual recognition.Crossref | GoogleScholarGoogle Scholar |
Royle, JA, Nichols, JD, Karanth, KU, and Gopalaswamy, AM (2009). A hierarchical model for estimating density in camera-trap studies. Journal of Applied Ecology 46, 118–127.
| A hierarchical model for estimating density in camera-trap studies.Crossref | GoogleScholarGoogle Scholar |
Schipper, J (2007). Camera-trap avoidance by Kinkajous Potos flavous: rethinking the ‘non-invasive’ paradigm. Small Carnivore Conservation 36, 38–41.
Séquin, ES, Jaeger, MM, Brussard, PF, and Barrett, RH (2003). Wariness of coyotes to camera traps relative to social status and territory boundaries. Canadian Journal of Zoology 81, 2015–2025.
| Wariness of coyotes to camera traps relative to social status and territory boundaries.Crossref | GoogleScholarGoogle Scholar |
Taggart, PL, Peacock, DE, and Fancourt, BA (2020). Camera trap flash-type does not influence the behaviour of feral cats (Felis catus). Australian Mammalogy 42, 220–222.
| Camera trap flash-type does not influence the behaviour of feral cats (Felis catus).Crossref | GoogleScholarGoogle Scholar |
Wegge, P, Pokheral, CP, and Jnawali, SR (2004). Effects of trapping effort and trap shyness on estimates of tiger abundance from camera trap studies. Animal Conservation 7, 251–256.
| Effects of trapping effort and trap shyness on estimates of tiger abundance from camera trap studies.Crossref | GoogleScholarGoogle Scholar |


