Effects of helicopter net gunning on the survival and movement behaviour of nilgai antelope
Jeremy A. Baumgardt A * , Aaron M. Foley A , Kathryn M. Sliwa A , Randy W. DeYoung A , J. Alfonso Ortega-S. A , David G. Hewitt A , Tyler A. Campbell B , John A. Goolsby C and Kim H. Lohmeyer D
A * , Aaron M. Foley A , Kathryn M. Sliwa A , Randy W. DeYoung A , J. Alfonso Ortega-S. A , David G. Hewitt A , Tyler A. Campbell B , John A. Goolsby C and Kim H. Lohmeyer D
A Caesar Kleberg Wildlife Research Institute, Texas A&M University-Kingsville, Kingsville, TX, USA.
B East Foundation, 200 Concord Plaza Drive, Suite 410, San Antonio, TX 78216, USA.
C USDA Agricultural Research Service, Cattle Fever Tick Research Laboratory, Edinburg, TX, USA.
D USDA Agricultural Research Service, Knipling-Bushland US Livestock Insect Research Laboratory, Kerrville, TX, USA.
Wildlife Research 50(11) 890-898 https://doi.org/10.1071/WR22049
Submitted: 12 March 2022 Accepted: 2 December 2022 Published: 12 January 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Research on large, terrestrial mammals often requires physical captures to attach tags or collars, collect morphological data, and collect biological samples. Choice of capture method should minimise pain and distress to the animal, minimise risk to personnel, and consider whether the method can achieve study objectives without biasing results.
Aims: We studied how capture via helicopter net-gunning affected survival, post-capture movement patterns, and space use of exotic nilgai (Boselaphus tragocamelus) in southern Texas, USA.
Methods: We estimated daily survival rates for 101 collared nilgai over 28 days, following 125 captures. We calculated mean daily movement rates and net-squared displacement for 21 recaptured nilgai for 60 days, starting 30 days before capture.
Key results: The survival probability of 125 nilgai individuals was 0.97 (95% CI = 0.92–0.99) over the 28 days following capture, with the lowest daily survival for the day after capture (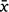 = 0.99; 95% CI = 0.96–1.00). We observed an increase of ~65% in the mean daily movement rate of 134 m/h on the first 2 days since capture, followed by a period of reduced movement out to the 5th day before returning to pre-capture levels. Analysis of net-squared displacement for 21 nilgai showed that 17 resumed pre-capture space-use patterns within a week, whereas four individuals did not return to the pre-capture range for ≥1 month.
= 0.99; 95% CI = 0.96–1.00). We observed an increase of ~65% in the mean daily movement rate of 134 m/h on the first 2 days since capture, followed by a period of reduced movement out to the 5th day before returning to pre-capture levels. Analysis of net-squared displacement for 21 nilgai showed that 17 resumed pre-capture space-use patterns within a week, whereas four individuals did not return to the pre-capture range for ≥1 month.
Conclusions: Capture-related mortality rates for nilgai using helicopter net-gunning in our study (3%) were similar or lower than those reported for similar species captured using the same method. While we were able to detect a period of elevated movement rates, followed by a recovery period of diminished movement as a result of capture, nilgai appeared to return to typical behaviour ~6 days post-capture. Most nilgai in our study also resumed typical space-use patterns within a week of capture; however, our results suggest high individual variability in their response.
Implications: We recommend using net-gunning from a helicopter as a method for capturing nilgai when conditions and where vegetation and topography allow. We suggest censoring data for a minimum of 7 days following capture for analyses related to survival and movement rates. For analyses relating to space use, we suggest inspecting net-squared displacement or some similar displacement analysis for each animal separately to account for individual variation in response and exclude data accordingly.
Keywords: Boselaphus tragocamelus, capture myopathy, mortality, movement behaviour, net-gun, net-squared displacement, survival, ungulate.
Introduction
Research on free-ranging, terrestrial large mammals often requires physical capture to attach identification markers and tracking devices (ear-tags or radio-collars), or collect biological samples. Other reasons for capture include mitigation of animal damage or disease surveillance.
Capture methods suitable for large mammals include nets (drop-net, drive net, helicopter net-gun, and rocket net), walk-in traps (corral trap, box traps), or chemical immobilisation (Schemnitz 2005). An ideal capture method is both efficient and safe for animals and humans.
Additionally, capturing should have minimal impacts on individuals such that it does not significantly bias planned analyses. For example, specific capture techniques may cause myopathy, leading to death weeks after capture for some species (Beringer et al. 1996; Breed et al. 2019), which could bias survival estimates generated from resulting data. Similarly, chemical immobilisation has been reported to affect movement rates or space use up to 10 days post-capture (Becciolini et al. 2019; Jung et al. 2019). Use of these data for estimating spatial behaviour without proper censoring could, likewise, result in biased results (Dechen Quinn et al. 2012). Thus, understanding the impacts of specific capture techniques on individuals may be critical, depending on study objectives.
Nilgai antelope (Boselaphus tragocamelus) are native to India, Nepal, and Pakistan, and were introduced into southern Texas in 1924 (Leslie 2008). Nilgai have since expanded, occupying the southern counties of Texas (Fig. 1) and northern states of Mexico (i.e. Tamaulipas, Nuevo Leon, Coahuila, and Sonora; Webber et al. 2006), with population estimates ranging from 36 000 to over 70 000 individuals (Traweek and Welch 1992; Blihovde 2020).
|
|
Since their introduction, nilgai have become a popular game animal because they are challenging to hunt, desirable for consumption, and as an exotic, are not restricted to harvest-season regulations. However, nilgai cause damage to livestock fences (Zoromski 2019) and compete for forage with livestock and native deer (Sheffield 1983; Hines 2016; Fulbright et al. 2021). Additionally, nilgai act as a host to cattle fever ticks (Rhipicephalus microplus and Rhipicephalus annulatus), which can carry protozoan parasites that cause bovine babesiosis (Cárdenas-Canales et al. 2011). Bovine babesiosis presents significant threats to the cattle industry and is common worldwide (Bock et al. 2004). The United States Department of Agriculture successfully eradicated cattle fever ticks from the USA in the mid-20th century, except for a permanent quarantine area along the Texas–Mexico border. However, outbreaks of cattle fever ticks north of the quarantine area have become common in recent years and nilgai are thought to be primarily responsible (Osbrink et al. 2022).
Little is known about nilgai ecology in their native or introduced ranges compared with many other species of large ungulate (Leslie 2008). With the increasing importance of nilgai to recreational hunting, animal damage, and disease transmission, the need for information on movements and basic ecology of nilgai has increased. As a result, we expect studies of nilgai that require capturing and fitting with tracking collars to become more frequent.
To gain approval for research that involves capture and handling of wildlife, investigators must demonstrate that the methods minimise pain and distress to the animals. In the United States, animal welfare policy for research is outlined by the Animal Welfare Act (United States Department of Agriculture (USDA) 2020), with additional guidance by the National Academy of Sciences (National Research Council 2011), and the American Veterinary Medical Association (American Veterinary Medical Association (AVMA) 2020), and recommendations from societies such as the American Society of Mammalogists (Sikes and The Animal Care and Use Committee of the American Society of Mammalogists 2016). Justification of capture techniques can be difficult for species that have received little attention. To our knowledge, there are no published guidelines for capturing and handling nilgai. Nilgai are large animals (adult males,  = 241 kg) and do not respond to feed or bait (Leslie 2008); the most common capture method on North American rangelands is the helicopter and net gun (Moczygemba et al. 2012; Foley et al. 2017). Our objective for this study was to assess the impact of capture with helicopter net-gunning on survival, movement patterns, and space use of nilgai, so as to (1) determine whether the method results in comparable mortality rates that are found to be acceptable in other ungulates, and (2) provide recommendations for censoring data for survival and movement behaviour analyses for future studies.
= 241 kg) and do not respond to feed or bait (Leslie 2008); the most common capture method on North American rangelands is the helicopter and net gun (Moczygemba et al. 2012; Foley et al. 2017). Our objective for this study was to assess the impact of capture with helicopter net-gunning on survival, movement patterns, and space use of nilgai, so as to (1) determine whether the method results in comparable mortality rates that are found to be acceptable in other ungulates, and (2) provide recommendations for censoring data for survival and movement behaviour analyses for future studies.
Materials and methods
Study area
We captured nilgai on private ranches in Kenedy, Willacy, and Cameron Counties in southern Texas (Fig. 1). The vegetation community is a mosaic of Tamaulipan thornscrub, live oak forests (Quercus virginiana), and open grasslands (Moczygemba et al. 2012; Foley et al. 2017). This area lies within the West Gulf Coast Plain physiographic province and contains little physical relief (Fenneman and Johnson 1946). Ranches were managed for different combinations of cattle production, wildlife, and dryland crops (sorghum and cotton). Because nilgai are exotic, there are no hunting regulations or harvest limits; they are legally considered property of the landowner, similar to cattle. Thus, nilgai were hunted year-round to varying degrees on all sites during the respective study periods.
Capture and handling
To assess impacts of capturing nilgai via net-gunning from a helicopter on survival, we combined data from three separate studies that employed the same capture methods. These included studies conducted in 2006–2008 (Moczygemba et al. 2012), 2015–2016 (Foley et al. 2017), and 2019–2020 (Sliwa 2021). Nilgai were captured using a two-seat helicopter (R22, Robinson Helicopters, Torrance, CA, USA) with a pilot and net-gunner. The pilot searched for individual nilgai and, when detected, flew at a low altitude and pushed the nilgai to an area with low vegetation, if necessary. When the nilgai was in an area of suitable terrain and vegetation, the gunner fired a net from the helicopter (Barrett et al. 1982).
Once netted, a ground crew using all-terrain vehicles located the animal, applied a blindfold and hobble, and removed the net. For each study, data were collected and collars were attached at the capture location; typically, nilgai were released at the capture location <15 min from the time of capture. Each captured nilgai was sexed and assigned into one of two age classes (young and adult) via tooth-wear (e.g. Severinghaus 1949). There are no formal criteria to estimate age of nilgai via tooth wear and replacement; however, sharpness of lingual crest of molars were used to distinguish between young and adult individuals (Zoromski 2019). Any nilgai that sustained an injury during capture or handling that appeared to be life-threatening (e.g. broken leg or jaw), was euthanised.
Nilgai captured during 2006 (Moczygemba et al. 2012) were captured in January or April and either fitted with a very high-frequency (VHF, Telemetry Solutions, Concord, California, USA) or a global positioning system (GPS; Televilt Tullus GPS, Lindesberg, Sweden) collar. Nilgai fitted with VHF collars were monitored twice a month via aircraft; GPS radio-collars recorded locations every 4 h (Moczygemba et al. 2012). Nilgai captured during 2015–2016 (Foley et al. 2017) were captured in April and fitted with GPS radio-collars (Lotek Wireless Inc., Newmarket, Ontario, Canada) that recorded locations at either 1-h or 13-h intervals. Nilgai captured in 2019–2020 were captured in March, June, or September and fitted with GPS collars programmed to record a location every hour (Vertex GPS, Vectronic Aerospace, Berlin, Germany). All capture and handling procedures were consistent with the recommendations of the American Society of Mammalogists (Sikes and The Animal Care and Use Committee of the American Society of Mammalogists 2016) and were approved by the Institutional Animal Care and Use Committee at the National Wildlife Research Center (Protocol QA-1363) or Texas A&M University–Kingsville (Protocols 2015-03-30, 2018-09-19) for Moczygemba et al. (2012), Foley et al. (2017), and Sliwa (2021) respectively.
Survival analysis
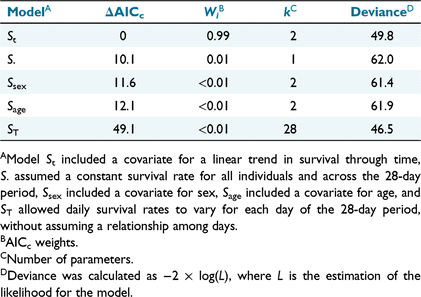
The nest-survival modelling framework in Program MARK (White and Burnham 1999) is useful for estimating survival of radio-marked animals when the exact date of mortality is unknown (Rotella et al. 2004). We used this framework to fit models to our data and produce daily survival estimates. The nest survival model requires the date when each individual was captured, the last date each was known to be alive, the last date each was monitored, and the fate of each individual at the end of the monitoring period. Capture-related mortality can be sudden and obvious, such as when an individual dies from trauma before it is released. Capture myopathy, caused by stress and physical exertion associated with capture, may lead to death days or weeks later (Beringer et al. 1996; Breed et al. 2019). Similarly, certain injuries sustained during capture, such as broken limb or spinal damage, are easily identified during handling, whereas injuries to internal organs or the head may go unnoticed and may eventually lead to death. We made no attempt to determine the cause of death and assumed that any non-hunting mortality within 28 days of capture was the result of capture and handling (Beringer et al. 1996; Jacques et al. 2009; Bengsen et al. 2021). We included individuals euthanised as a result of capture trauma in our capture-related survival analysis. We included covariates in our candidate models to test hypotheses that capture-related mortality rates varied between sexes, by age, linearly through time over the 28-day period, varied independent of days since capture, or were constant over the 28-day period for all animals. We used Akaike information criterion adjusted for small sample sizes (AICc) to identify the most parsimonious model, which we used to produce survival estimates (Burnham and Anderson 2002).
Movement analyses
Similar studies on impacts of capture on movement for red deer (Cervus elaphus; Becciolini et al. 2019) and roe deer (Capreolus capreolus; Morellet et al. 2009) have relied on location data following a single capture of individuals, requiring the authors to make assumptions regarding pre-capture behaviour (but see Neumann et al. 2011; Northrup et al. 2014; Jung et al. 2019). Foley et al. (2017), suggest that patterns of space use by nilgai are dependent on a complex social system and appear to fluctuate seasonally. Owing to our limited knowledge of nilgai ecology, we felt making assumptions of pre-capture behaviour would be imprudent. Thus, to assess the impacts of capture on the activity level and space use, we limited our analysis to the nilgai that were captured multiple times in 2019–2020, providing location data immediately before and after a capture event.
We initially captured and collared 30 individuals in March 2019; we recaptured 15 of these (four male, 11 female) in September 2019, and again recaptured six of these (two male, four female) in June 2020, for a sample size of 21 nilgai captures that we were able to record location data for immediately before and after capture. We retained only location data obtained using ≥4 satellites and excluded positions with a dilution of precision (DOP) >8 to avoid including locations with poor accuracy (D’eon and Delparte 2005). Finally, we visually inspected plots of remaining locations and removed points that appeared erroneous on the basis of extreme 1-h step lengths, coupled with large turn angles and a subsequent, similarly extreme step length back in the vicinity of the original location.
To test the hypothesis that capturing nilgai affected its movement rates, we calculated mean daily movement rates (DMR) by calculating movement rates from each hourly location (m/h) and averaging them over each 24-h period (Jung et al. 2019). We limited our analyses of movement to a 60-day period starting 30 days before capture, because we assumed that most animals would return to pre-capture behaviour and space use within 30 days following capture. This approach minimises potentially confounding impacts of seasonal changes in behaviour. We calculated the DMR from 30 days before a capture to 29 days after, averaged these calculations over all 21 nilgai, and plotted the results for visual inspection.
Net-squared displacement (NSD) is a method for describing an animal’s spatio-temporal movement patterns that have been used to identify movement patterns, such as migration and dispersal (Bunnefeld et al. 2011; Singh et al. 2016). We used the NSD to further test the hypothesis that capture affected nilgai space use and movement patterns. The calculation for NSD is simply the squared straight-line distance from a specified starting point to each subsequent location. We used the centroid of all hourly locations recorded over the 30 days prior to capture for each nilgai as the starting point to which distances to each recorded location for the 60-day period straddling a capture event were calculated. We used the 30-day centroid as our starting point because we expected this to provide a suitable baseline for the range of typical movements necessary to visually detect any substantial deviation caused by a capture event. We plotted NSD against time for each nilgai individual and visually inspected them for evidence of a deviation from typical movement behaviour that correlated with the capture, as well as to determine the time to return to typical behaviour following the capture.
Results
We used data from 101 individual nilgai representing 125 captures to assess capture-related survival, including 21 individuals captured in 2006, 35 from the 2015–2016 study, and 69 captures from 45 individuals from the 2019–2020 study (29 individuals captured a single time, eight individuals captured twice, and eight individuals captured three times). The total sample included captures of 25 young and 100 adults and included 52 males and 73 females. There was a single capture-related injury in 2015 that resulted in a broken hind leg of an adult female nilgai, which was subsequently euthanised with a penetrating captive bolt gun. Three additional mortalities occurred within 28 days of a capture from the 2019–2020 study, including an adult female that died 2 days after capture, an adult female that died 3 days after capture, and a young male that died 5 days after capture. At the time the data were recorded, it was noted that the young male was bleeding from the base of its horns, suggesting the individual sustained head trauma during capture. No evidence of trauma during capture was noted, nor was the cause of death determined for the other nilgai that died in the 28 days following capture. Except for harvested animals, we did not observe any mortality that occurred between 1 and 6 months post-capture.
Survival analysis
The most parsimonious model from our daily survival analysis suggested that survival probability varied linearly over the 28-day period, receiving 0.99 of AICc weight among the five models we considered (Table 1). According to this model, estimated daily survival rate was lowest for the first day following capture at 0.99 (95% CI = 0.96–1.00), and increased with time (Fig. 2). The derived estimate for an individual surviving the entire 28-day period following capture from this model was 0.97 (95% CI = 0.92–0.99). Our data did not support the hypothesis that capture-related mortality varied by age or sex.
|
Movement analyses
The GPS location fix rates for our 21 collared nilgai over the 60-day period was >99.9% (s.e. < 0.001). We removed five points that had a reported DOP of >8 and an additional eight points that visually appeared erroneous. This resulted in 30 224 locations for the 21 nilgai. The mean DMR over the 30 days prior to capture was 134 m/h. We observed an increase of ~65% in the mean DMR to 224 m/h on the day of capture (Day 31) and 221 m/h the following day (Day 32; Fig. 3). The mean DMR appeared to return to pre-capture levels on Days 33 and 34; however, rates then dropped to 81 and 94 m/h on days 35 and 36, respectively, before returning to pre-capture levels again on day 37.
The results from our NSD analysis showed variable responses to capture by both female and male nilgai, ranging from almost no noticeable impact up to complete change in the area used that lasted weeks (Supplementary material Figs S1, S2). Although there was a noticeable increase in NSD for most nilgai associated with the time of capture, there was high variation in the magnitude of the apparent response for both males and females. Finally, even though most disruptions in NSD associated with a capture event appeared to last <1 week, there were at least two females and two males that did not return to pre-capture space-use patterns in the month following capture (Figs S1, S2).
Discussion
Capturing with helicopter and net gun is the preferred method for many ungulates on rangelands (Webb et al. 2008; Bengsen et al. 2021; Beaver et al. 2022). However, species differ in behavioural and physiological response to capture, such that the same method may not be appropriate for all species. We observed a low mortality rate (3%) associated with capture, considering both proximate and distal causes of mortality for nilgai in our study. Mortality rates associated with net-gunning from a helicopter have been reported to be <2% for white-tailed deer (Odocoileus virginianus; Webb et al. 2008; Jacques et al. 2009), <4% for mule deer (Odocoileus hemionus; Van de Kerk et al. 2020), 9% for pronghorn (Antilocarpra americana; Jacques et al. 2009), and 10% for red deer in New Zealand (Latham et al. 2020). However, all red deer deaths associated with capture in the latter study (n = 3) were the result of the animals falling in steep terrain (Latham et al. 2020); mortality may be lower in more favourable field conditions. Our results suggested that net-gunning from a helicopter is a safe method for capturing both young and adult nilgai of both sexes. Our study has been the first evaluation of nilgai mortality associated with capture and our analysis indicated that this is an appropriate method of capture for nilgai during the conditions of our study.
Capture appeared to affect nilgai movements for several days; nilgai movement rates were elevated on the day of and the day following capture. Movement rates were within the typical range of pre-capture movement rates on 2 and 3 days following capture, then decreased to lower than typically observed on 4 and 5 days following capture. We are unaware of any other study that has identified a period of elevated movement rates, followed by a period of reduced rates. Most of the studies of impacts of capture on large mammal movement rates that we are aware of have reported elevated movement rates lasting hours for moose (Alces alces; Neumann et al. 2011), to days for bighorn sheep (Ovis canadensis; Clapp et al. 2014), mule deer (Northrup et al. 2014), bison (Bison bison; Jung et al. 2019), and female red deer (Becciolini et al. 2019). However, reduced movement rates lasting 14 days post-capture were reported for white-tailed deer (Dechen Quinn et al. 2012), and Bengsen et al. (2021) reported fallow deer had reduced activity levels in their study lasting ~10 days after capture.
We did not chemically immobilise nilgai in our study; all animals in our movement analysis were released at the location of capture and had been collared and tagged for >6 months. Thus, we suspect the deviations we observed from pre-capture activity are directly attributable to capture and handling. Further, we suspect the elevated movement rates we observed in our released nilgai were in direct response to the capturing and handling, while the decreased rates we observed up to 5 days post-capture may represent a recovery period due to the high energy exerted over the 1–3 days post-capture.
Analysis of net-squared displacement revealed that nilgai response to capture and handling in our study ranged from undetectable to a complete shift in space used for at least a month following capture. Previous studies suggest most large mammals displaced by capture had returned to pre-capture behaviour within a week (Morellet et al. 2009; Neumann et al. 2011; Northrup et al. 2014; Becciolini et al. 2019; Jung et al. 2019). We are unaware of any other example from the literature of animals that were displaced from capture for more than a few weeks; however, most studies addressing this issue have lacked the pre-capture data to document such behaviour with certainty. The enduring behaviours we detected may be unique to nilgai; nilgai have relatively large home ranges (236–7069 ha) and have been documented making long-distance movements (>40 km) that were not associated with capture (Foley et al. 2017; Sliwa 2021).
Most studies that addressed impacts of capture and handling on movement behaviour of ungulates have involved either the use of chemical immobilisation (e.g. Neumann et al. 2011; Brivio et al. 2015; Becciolini et al. 2019; Jung et al. 2019) or releasing the study animals some distance from the location of capture (e.g. Morellet et al. 2009; Northrup et al. 2014). These additional variables make it difficult, if not impossible, to tease apart responses to capture and handling from those caused by drugs and relocating. Furthermore, studies that use single capture events and lack pre-capture data cannot separate impacts of capture from any acclimation behaviour that would have resulted from being newly fitted with GPS collars and other tags. Our study is unique in that we were able to eliminate these common variables, resulting in greater confidence that our observed impacts were solely due to capture and handling.
White and Garrott (1990) recommended excluding location data for a period of up to 1 week after capture to allow animals to acclimatise to collars and tags. Our results add to the existing body of work that has suggested that the necessary acclimation period varies by species. Furthermore, our results suggest the reported observed acclimation periods with atypical movement patterns are not entirely attributable to new collars or tags and perhaps the method of capture and handling is responsible for much of this behaviour. Indeed, Brivio et al. (2015) speculated that the relatively low impacts of capture on movement behaviour of ibex (Capra ibex) in their study were likey to be due to the low stress associated with capturing using chemical immobilisation from the ground. In a study with white-tailed deer and pronghorn captured with net-guns fired from a helicopter, Jacques et al. (2009) did not consider movement behaviour, but found survival improved significantly with shorter pursuit times with the helicopter and with shorter distances the animals were released from their capture locations. We recommend capturing nilgai with a net-gun fired from a helicopter based on our survival analysis. We further recommend minimising chase time and distance as much as possible because this may reduce the overall recovery behaviour and period, thus maximising location data that is not biased by atypical behaviour caused by capturing and handling. Finally, for those using data from captured and marked nilgai, we suggest censoring a minimum of 7 days following capture for analyses related to survival and movement rates. For analyses relating to space use, we agree with Neumann et al. (2011) and Northrup et al. (2014) and suggest inspecting NSD or some similar displacement analysis from each animal separately to account for individual variation in response to capture and exclude data accordingly.
Supplementary material
Supplementary material is available online.
Data availability
The data used in this research are available at the Dryad repository under nilgai 2019 South Texas, https://doi.org/10.5061/dryad.c866t1g92.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
Research on nilgai was supported by the United States Department of Agriculture Agricultural Research Service, the Texas Animal Health Commission, the East Foundation, the San Chicago and Tio Moya leases of King Ranch, Inc., and the United States Department of Agriculture, Animal and Plant Health Inspection Service, Wildlife Services, National Wildlife Research Center.
Acknowledgements
The authors thank A. Tanner and J. Lombardi for their thoughtful review and feedback on an early draft of this paper. We also thank the Associate Editor and two reviewers for comments that further improved our paper. We are grateful to the private land stewards for access to their properties and to numerous unpaid volunteers for assistance with captures. This is manuscript 22-110 of the Caesar Kleberg Wildlife Research Institute and manuscript 077 of the East Foundation.
References
American Veterinary Medical Association (AVMA) (2020) ‘AVMA guidelines for the euthanasia of animals.’ 2020th edn. (American Veterinary Medical Association: Schaumburg, IL, USA)Barrett, MW, Nolan, JW, and Roy, LD (1982). Evaluation of a hand-held net-gun to capture large mammals. Wildlife Society Bulletin 10, 108–144.
Beaver, JT, Grantham, C, Cooksey, ML, Skow, K, Pierce, BL, and Lopez, RR (2022). Effectiveness, economics, and safety of drop nets and helicopters with net-gunning for capturing white-tailed deer. Wildlife Society Bulletin 46, e1365.
| Effectiveness, economics, and safety of drop nets and helicopters with net-gunning for capturing white-tailed deer.Crossref | GoogleScholarGoogle Scholar |
Becciolini, V, Lanini, F, and Ponzetta, MP (2019). Impact of capture and chemical immobilization on the spatial behaviour of red deer Cervus elaphus hinds. Wildlife Biology 2019, 1–8.
| Impact of capture and chemical immobilization on the spatial behaviour of red deer Cervus elaphus hinds.Crossref | GoogleScholarGoogle Scholar |
Bengsen, AJ, Hampton, JO, Comte, S, Freney, S, and Forsyth, DM (2021). Evaluation of helicopter net-gunning to capture wild fallow deer (Dama dama). Wildlife Research 48, 722–729.
| Evaluation of helicopter net-gunning to capture wild fallow deer (Dama dama).Crossref | GoogleScholarGoogle Scholar |
Beringer, J, Hansen, LP, Wilding, W, Fischer, J, and Sheriff, SL (1996). Factors affecting capture myopathy in white-tailed deer. The Journal of Wildlife Management 60, 373–380.
| Factors affecting capture myopathy in white-tailed deer.Crossref | GoogleScholarGoogle Scholar |
Blihovde WB (2020) ‘Expansion of hunting program on Laguna Atascosa National Wildlife Refuge, draft Environmental Assessment.’ (US Fish and Wildlife Service: Los Fresnos, TX, USA)
Bock, R, Jackson, L, de Vos, A, and Jorgensen, W (2004). Babesiosis of cattle. Parasitology 129, S247–S269.
| Babesiosis of cattle.Crossref | GoogleScholarGoogle Scholar |
Breed, D, Meyer, LCR, Stely, JCA, Goddard, A, Burroughs, R, and Kohn, TA (2019). Conserving wildlife in a changing world: understanding capture myopathy: a malignant outcome of stress during capture and translocation. Conservation Physiology 7, coz027.
| Conserving wildlife in a changing world: understanding capture myopathy: a malignant outcome of stress during capture and translocation.Crossref | GoogleScholarGoogle Scholar |
Brivio, F, Grignolio, S, Sica, N, Cerise, S, and Bassano, B (2015). Assessing the impact of capture on wild animals: the case study of chemical immobilisation on Alpine ibex. PLoS ONE 10, e0130957.
| Assessing the impact of capture on wild animals: the case study of chemical immobilisation on Alpine ibex.Crossref | GoogleScholarGoogle Scholar |
Bunnefeld, N, Börger, L, van Moorter, B, Rolandsen, CM, Dettki, H, Solberg, EJ, and Ericsson, G (2011). A model-driven approach to quantify migration patterns: individual, regional and yearly differences. Journal of Animal Ecology 80, 466–476.
| A model-driven approach to quantify migration patterns: individual, regional and yearly differences.Crossref | GoogleScholarGoogle Scholar |
Burnham KP, Anderson DR (2002) ‘Model selection and multimodel inference: a practical information-theoretic approach.’ 2nd edn. (Springer: New York, NY, USA)
Cárdenas-Canales, EM, Ortega-Santos, JA, Campbell, TA, García-Vázquez, Z, Cantú-Covarrubias, A, Figueroa-Millán, JV, DeYoung, RW, Hewitt, DG, and Bryant, FC (2011). Nilgai antelope in northern Mexico as a possible carrier for cattle fever ticks and Babesia bovis and Babesia bigemina. Journal of Wildlife Diseases 47, 777–779.
| Nilgai antelope in northern Mexico as a possible carrier for cattle fever ticks and Babesia bovis and Babesia bigemina.Crossref | GoogleScholarGoogle Scholar |
Clapp, JG, Beck, JL, and Gerow, KG (2014). Post-release acclimation of translocated low-elevation, non-migratory bighorn sheep. Wildlife Society Bulletin 38, 657–663.
| Post-release acclimation of translocated low-elevation, non-migratory bighorn sheep.Crossref | GoogleScholarGoogle Scholar |
Dechen Quinn, AC, Williams, DM, and Porter, WF (2012). Postcapture movement rates can inform data-censoring protocols for GPS-collared animals. Journal of Mammalogy 93, 456–463.
| Postcapture movement rates can inform data-censoring protocols for GPS-collared animals.Crossref | GoogleScholarGoogle Scholar |
D’eon, RG, and Delparte, D (2005). Effects of radio-collar position and orientation on GPS radio-collar performance, and the implications of PDOP in data screening. Journal of Applied Ecology 42, 383–388.
| Effects of radio-collar position and orientation on GPS radio-collar performance, and the implications of PDOP in data screening.Crossref | GoogleScholarGoogle Scholar |
Fenneman NM, Johnson DW (1946) ‘Physiographic divisions of the conterminous US.’ (U.S. Geological Survey: Reston, VA, USA)
Foley, AM, Goolsby, JA, Ortega-S., A, Ortega-S., JA, Pérez de León, A, Singh, NK, Schwartz, A, Ellis, D, Hewitt, DG, and Campbell, TA (2017). Movement patterns of nilgai antelope in South Texas: implications for cattle fever tick management. Preventive Veterinary Medicine 146, 166–172.
| Movement patterns of nilgai antelope in South Texas: implications for cattle fever tick management.Crossref | GoogleScholarGoogle Scholar |
Fulbright, TE, Drabek, DJ, Ortega-S, JA, Hines, SL, Sanez, R, Campbell, TA, Hewitt, DG, and Wester, DB (2021). Forb standing crop response to grazing and precipitation. Rangeland Ecology & Management 79, 175–185.
| Forb standing crop response to grazing and precipitation.Crossref | GoogleScholarGoogle Scholar |
Hines SL (2016) Cattle, deer, and nilgai interactions. PhD dissertation, Texas A&M University-Kingsville, Kingsville, TX, USA.
Jacques, CN, Jenks, JA, Deperno, CS, Sievers, JD, Grovenburg, TW, Brinkman, TJ, Swanson, CC, and Stillings, BA (2009). Evaluating ungulate mortality associated with helicopter net-gun captures in the northern Great Plains. Journal of Wildlife Management 73, 1282–1291.
| Evaluating ungulate mortality associated with helicopter net-gun captures in the northern Great Plains.Crossref | GoogleScholarGoogle Scholar |
Jung, TS, Konkolics, SM, Kukka, PM, Majchrzak, YN, Menzies, AK, Oakley, MP, Peers, MJL, and Studd, EK (2019). Short-term effect of helicopter-based capture on movements of a social ungulate. The Journal of Wildlife Management 83, 830–837.
| Short-term effect of helicopter-based capture on movements of a social ungulate.Crossref | GoogleScholarGoogle Scholar |
Latham, ADM, Davidson, B, Warburton, B, Yockney, I, and Hampton, JO (2020). Efficacy and animal welfare impacts of novel capture methods for two species of invasive wild mammals in New Zealand. Animals 10, 44.
| Efficacy and animal welfare impacts of novel capture methods for two species of invasive wild mammals in New Zealand.Crossref | GoogleScholarGoogle Scholar |
Leslie, DM (2008). Boselaphus tragocamelus (Artiodactyla: Bovidae). Mammalian Species 813, 1–16.
| Boselaphus tragocamelus (Artiodactyla: Bovidae).Crossref | GoogleScholarGoogle Scholar |
Moczygemba, JD, Hewitt, DG, Campbell, TA, Alfonso Ortega-S, J, Field, J, and Hellickson, MW (2012). Home ranges of the nilgai antelope (Boselaphus tragocamelus) in Texas. The Southwestern Naturalist 57, 26–30.
| Home ranges of the nilgai antelope (Boselaphus tragocamelus) in Texas.Crossref | GoogleScholarGoogle Scholar |
Morellet, N, Verheyden, H, Angibault, J-M, Cargnelutti, B, Lourtet, B, and Hewison, MAJ (2009). The effect of capture on ranging behaviour and activity of the European roe deer Capreolus capreolus. Wildlife Biology 15, 278–287.
| The effect of capture on ranging behaviour and activity of the European roe deer Capreolus capreolus.Crossref | GoogleScholarGoogle Scholar |
National Research Council (2011) ‘Guide for the care and use of laboratory animals.’ 8th edn. (National Academy of Sciences: Washington, DC, USA)
Neumann, W, Ericsson, G, Dettki, H, and Arnemo, JM (2011). Effect of immobilizations on the activity and space use of female moose (Alces alces). Canadian Journal of Zoology 89, 1013–1018.
| Effect of immobilizations on the activity and space use of female moose (Alces alces).Crossref | GoogleScholarGoogle Scholar |
Northrup, JM, Anderson, CR, and Wittemyer, G (2014). Effects of helicopter capture and handling on movement behavior of mule deer. The Journal of Wildlife Management 78, 731–738.
| Effects of helicopter capture and handling on movement behavior of mule deer.Crossref | GoogleScholarGoogle Scholar |
Osbrink, WLA, Thomas, DB, Lohmeyer, KH, and Temeyer, KB (2022). Climate change and alternative hosts complicate the eradication of cattle fever ticks (Acari: Ixodidae) in the southern United States, a review. Annals of the Entomological Society of America 115, 39–55.
| Climate change and alternative hosts complicate the eradication of cattle fever ticks (Acari: Ixodidae) in the southern United States, a review.Crossref | GoogleScholarGoogle Scholar |
Rotella, JJ, Dinsmore, SJ, and Shaffer, TL (2004). Modeling nest-survival data: a comparison of recently developed methods that can be implemented in MARK and SAS. Animal Biodiversity and Conservation 27, 187–205.
Schemnitz SD (2005) Capturing and handling wild animals. In ‘Techniques for wildlife investigations and management’. 6th edn. (Ed. CE Braun) pp. 239–285. (The Wildlife Society: Bethesda, MD, USA)
Severinghaus, CW (1949). Tooth development and wear as criteria of age in white-tailed deer. The Journal of Wildlife Management 13, 195–216.
| Tooth development and wear as criteria of age in white-tailed deer.Crossref | GoogleScholarGoogle Scholar |
Sheffield, WJ (1983). Food Habits of nilgai antelope in Texas. Journal of Range Management 36, 316–322.
Sikes, RS, The Animal Care and Use Committee of the American Society of Mammalogists (2016). 2016 Guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. Journal of Mammalogy 97, 663–688.
| 2016 Guidelines of the American Society of Mammalogists for the use of wild mammals in research and education.Crossref | GoogleScholarGoogle Scholar |
Singh, NJ, Allen, AM, and Ericsson, G (2016). Quantifying migration behaviour using net squared displacement approach: clarifications and caveats. PLoS ONE 11, e0149594.
| Quantifying migration behaviour using net squared displacement approach: clarifications and caveats.Crossref | GoogleScholarGoogle Scholar |
Sliwa K (2021) Nilgai movement ecology: implications for management of cattle fever ticks in South Texas. MS thesis, Range and Wildlife Management, Texas A&M University-Kingsville, Kingsville, TX, USA.
Traweek M, Welch R (1992) Exotics in Texas. PWD-BK-W7000-206 5/92. (Texas Parks and Wildlife Department)
United States Department of Agriculture (USDA) (2020) ‘Animal welfare act and animal welfare regulations.’ (United States Department of Agriculture, Animal and Plant Health Inspection Service: Washington, DC, USA)
Van de Kerk, M, McMillan, BR, Hersey, KR, Roug, A, and Larsen, RT (2020). Effect of net-gun capture on survival of mule deer. The Journal of Wildlife Management 84, 813–820.
| Effect of net-gun capture on survival of mule deer.Crossref | GoogleScholarGoogle Scholar |
Webb, SL, Lewis, JS, Hewitt, DG, Hellickson, MW, and Bryant, FC (2008). Assessing the helicopter and net gun as a capture technique for white-tailed deer. Journal of Wildlife Management 72, 310–314.
| Assessing the helicopter and net gun as a capture technique for white-tailed deer.Crossref | GoogleScholarGoogle Scholar |
Webber, M, García-Marmolejo, G, and Reyna-Hurtado, R (2006). The tragedy of the commons: wildlife management units in southeastern Mexico. Wildlife Society Bulletin 34, 1480–1488.
| The tragedy of the commons: wildlife management units in southeastern Mexico.Crossref | GoogleScholarGoogle Scholar |
White, GC, and Burnham, KP (1999). Program MARK: survival estimation from populations of marked animals. Bird Study 46, S120–S139.
| Program MARK: survival estimation from populations of marked animals.Crossref | GoogleScholarGoogle Scholar |
White GC, Garrott RA (1990) ‘Analysis of wildlife radio-tracking data.’ (Academic Press: San Diego, CA, USA)
Zoromski LD (2019) Social behavior and movement ecology of nilgai antelope. Master’s thesis, Texas A&M University-Kingsville, Kingsville, TX, USA.


