Do high recapture rates indicate representative sampling? The relationship between recapture probability, risk-taking, and personality
Kyla Chloe Johnstone A * , Clare McArthur
A * , Clare McArthur  A and Peter Bruce Banks A
A and Peter Bruce Banks A
A School of Life and Environmental Sciences, The University of Sydney, Sydney, NSW 2006, Australia.
Wildlife Research 50(11) 954-964 https://doi.org/10.1071/WR22046
Submitted: 9 March 2022 Accepted: 22 December 2022 Published: 2 February 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Monitoring programs provide valuable information on wildlife populations, thereby underpinning strategies for conservation and control. For threatened species, where every animal represents a substantial portion of the population, representative sampling is vital. One fundamental challenge during sampling is understanding drivers of survey bias; for instance, behavioural heterogeneity in trap response. Methods such as capture–mark–recapture have long been used to estimate capture and recapture heterogeneity; yet, this method, like many others, is able to gather data only from the trappable and re-trappable portion of the population; a problem that presents a particular challenge for small or vulnerable populations. A greater understanding of why biases arise can result in improved survey methods, more reliable survey data and increased modelling accuracy.
Aims: We focus on an endangered species with unusually high recapture probabilities (0.78–0.92), namely, the mountain pygmy-possum (Burramys parvus). Specifically, we examine whether, within a single trapping session, a recapture bias exists either as a function of past trapping experience or personality.
Methods: We tested whether recapture probability differs among cohorts with different capture histories (‘known’ animals captured during trapping sessions in previous years vs ‘new’ animals trapped for the first time in this study). We also tested for individual personality, general risk-taking behaviour during foraging, and subsequent links to recapture probability.
Key results: Recapture probability was significantly affected by cohort. New animals had lower probabilities of recapture and took fewer risks during foraging than did known animals. Although personality did not significantly influence recapture probability, it did influence risk-taking during foraging.
Conclusions: Despite high recapture probability within the populations, captures were significantly skewed towards a subset of the population, likely being due to different perceptions of risk among individuals.
Implications: Understanding potential sources of bias during live-capture surveys is the initial step towards modifying and improving surveys to reduce sampling biases and to ensure representative population sampling.
Keywords: Burramys parvus, personality, population estimates, recapture probability, risk and reward, risk-taking, survey bias, trapping.
Introduction
Robust and representative population estimates are vital for effective conservation actions, particularly for tracking population changes in endangered species, where every animal may represent a substantial portion of the extant population. Consequently, any bias has considerable implications for accuracy of population estimate and subsequent conservation decisions. For most species, it is impossible to count every individual in a population, and, so, total population size is estimated from capture or count data. For instance, the capture–mark–recapture method (and associated statistical models) often uses live-capture trapping results to estimate population size, survival rates, recruitment, and population growth (e.g. Otis et al. 1978; Besbeas et al. 2002; Wilson et al. 2007). Recapture is crucial to population estimates and underpins many models used to estimate population parameters. Although algorithms can be used to account for heterogeneity in recapture (and initial capture), a more robust approach may be to understand and accommodate potential biases into the trapping method itself.
Importantly, the act of entering a trap is a decision made by an animal, and so animal behaviour is likely to play a central role in trappability (Garvey et al. 2020). Entering traps and other enclosed devices can be perceived as a risky behaviour, with animals responding in a risk-sensitive manner (Johnstone et al. 2021a). This risk-sensitive decision can also be influenced by prior trapping experience (Linhart et al. 2012; Roche et al. 2013; Camacho et al. 2017), and whereas some animals are likely to re-enter traps, others may take longer or may not be recaptured (Balph 1968) and can vary on the individual level. Animal personality, consistent, among-individual differences in behaviour (Gosling 2001), can influence risk-sensitive decisions (Carter et al. 2010; Cole and Quinn 2014). Traits, including boldness, activity and docility, are associated with life-history traits and individual fitness (see table 1 in Biro and Stamps 2008) and can drive heterogeneity in trappability, skewing population sampling. In some species, increased boldness and activity correlate with ease of capture, whereas extreme shyness can link with active avoidance of traps (Boon et al. 2008; Carter et al. 2012).
Although capture heterogeneity during surveys is a common occurrence, high recapture rates are often associated with (1) little potential for bias, and (2) robust population estimates with low error (Krebs 1999; O’brien et al. 2005). However, these assumptions have not been properly tested and the potential for behavioural heterogeneity to drive differences in recapture probability has not been explored in the context of high recapture rates.
In this paper, we focus on the Endangered mountain pygmy-possum (Burramys parvus), a small (40 g) marsupial with reportedly high recapture probability (0.78–0.92; Broome 2001a). The mountain pygmy-possum (hereafter pygmy-possum) is endemic to the alpine and subalpine regions of south-eastern Australia (Happold 1989), and in New South Wales (NSW) it persists in isolated populations in Kosciuszko National Park. Here, we test for potential mechanisms affecting recapture within a single trapping session (i.e. four nights). We first tested whether trap response differs between population cohorts (differing in previous trapping experience). Such differences can arise due to age (DomèNech and Senar 1997) or prior experience with traps (Linhart et al. 2012). Given that personality can influence individual responses to the perceived risks and rewards associated with traps (Garvey et al. 2020; Johnstone et al. 2021a), we quantified pygmy-possum personality and tested for any personality effect on trap response.
As an indirect driver of trap response, we also tested whether risk-taking differed between cohorts or was influenced by personality. We quantified the risk-taking behaviour of trapped and marked individuals that visited feeding stations (i.e. feeders) with different risk–reward treatments. We examined (1) variation among individuals in visit frequency, and (2) behaviour at feeders. We anticipated that visiting and foraging from feeders would present a perceived risk either comparable (risky feeders) or lower (safe feeders) than the perceived risk of entering a trap. We also anticipated that visit frequency would be greater at feeders with high-preference food than at feeders with low-preference food. As personality and experience can influence risk-taking, we also predicted that either factor may link with increased visits or increased foraging at risky feeders.
Materials and methods
Study area and species
This study was conducted in Kosciuszko National Park, NSW, Australia during the 2017 pygmy-possum survey. As part of the National Recovery Plan and Saving Our Species Recovery Project, the NSW Department of Planning, Industry and Environment run annual four-night surveys in November/December across multiple sites. To maximise our sample size, we surveyed subpopulations at two independent sites (8.5 km apart), namely, the first, at Charlotte Pass (3.4 ha, 1740–1765 m), and the second, at Lower Blue Cow (1.78 ha, 1800–1850 m). Both sites are rocky boulderfields consisting of boulder piles reaching over 2 m deep, periglacial blockstreams, and other boulder formations (Rosengren and Peterson 1989; Broome 2001a). Crevices between boulders provide shelter from larger predators, including introduced cats (Felis catus) and foxes (Vulpes vulpes), and native raptors (Green and Osborne 1981; Broome et al. 2012). No movement between the two sites has been recorded (Broome 2001a) and, during the breeding season, when surveys are conducted, animals are reasonably sedentary (Broome 2001a). Young are born in November/December and are trappable by 12 months (Broome 2001b). With a life-span of roughly 3 years, individuals are trappable over multiple surveys, although males aged five and females reaching 11 years have been recorded (Broome 2001a).
Factors affecting recapture probability
To test whether trap response differed between the population cohorts or was influenced by individual personality, we surveyed sites over four consecutive nights (one annual monitoring session). Elliott traps (Charlotte Pass = 100, Lower Blue Cow = 35) baited with walnuts were set ~10 m apart in crevices between boulders. For each capture, we recorded existing microchip IDs of known animals (trapped in previous years) and microchipped all new animals (trapped for the first time in this session). Animals were sexed and weighed, but we did not estimate age as it is difficult to differentiate between subadults (1 year old) and mature (>2 years) animals (Broome 2001a). Individuals were also uniquely fur clipped (Charlotte Pass = 25 of 38, Lower Blue Cow = 15 of 15) for later identification on camera. Because the number of clear, unique marks is limited, given the size of a pygmy-possum (40 g), at Charlotte Pass, 13 randomly selected individuals were given a common mark to identify them as a trapped animal on camera (Supplementary material Fig. S1). All Lower Blue Cow captures were uniquely marked.
Quantifying personality
At capture we also tested for personality by using the immobility test (Martin and Réale 2008). Individuals were tested once per capture, with a maximum of three tests per individual. Once the pygmy-possum was in a handling bag, the bag was suspended, and we calculated the cumulative time an animal spent immobile in 60 s. Limited access to this threatened species meant that all repeat tests were conducted within a four-night span. However, in other marsupials and small mammals, personality quantified in the short term has been consistent with traits quantified over weeks (Mella et al. 2016), or even months (Wat et al. 2020; Johnstone et al. 2021b).
Factors affecting risk-taking at feeders
To test whether risk-taking differed between cohorts or was influenced by personality, we ran an experimental feeding trial (1–2 days post-trapping) by using novel feeders (constructed of chicken-wire; holes 10 mm × 10 mm, height 150 mm, maximum width 120 mm; Fig. S2). We presented four feeder treatments, differing in risk (two levels: exposed, sheltered) and reward (two levels: high-preference, low-preference). Eight replicates of each treatment (total number of feeders = 32) were set at Charlotte Pass and four replicates (total number of feeders = 16) at Lower Blue Cow. We manipulated feeder risk (Fig. 1) by setting feeders on the top of boulders (exposed feeders) where animals are at high risk of predation (Broome et al. 2012), or in the crevices between boulders (sheltered feeders), where feeders were likely to be perceived as safer. As novel objects, all feeders were likely to be perceived with some degree of risk, at least initially (Cowan 1977). The feeder design prevented the rapid removal of food, and animals had to make a risk versus reward trade-off to forage. We manipulated feeder reward by using either 40 g of walnuts (high-preference food) or raisins (low-preference food) mashed into 10 g bait balls. All feeders were set within 5 m of a previous trap position and left for three nights. A remote-sensing camera (SG560K-12mHD) was set ~1.5 m from feeders to film behaviour and help identify individuals (see Supplementary methods 1 – Camera set up). A pilot study conducted at a separate site tested feeder designs and food preferences, from which we selected the feeder and foods used in this study.
|
|
Quantifying visits to and behaviours at feeders
To analyse individual risk-taking behaviour, we quantified (1) the frequency of visits per feeder and (2) the proportion of time (in-sight) individuals allocated to different behaviours (Supplementary material Table S1), all as a function of feeder risk and reward. We measured behaviours related to risk-taking, namely, approach (in motion, approaches feeder or head is angled towards feeder), locomotion (in motion, but not focused on feeder), investigation (not in motion, focused on and within one body length of the feeder), and total time foraging (consuming or attempting to remove food from a device); and behaviours related to cautionary responses, including vigilance (not moving, but alert) and not moving (not moving but not vigilant). We included total time foraging (rather than the proportion of time) as a measure of the absolute value of time spent at a feeder. We scored video behaviours using the software JWatcher (Blumstein and Daniel 2007), and because pygmy-possums rarely remained at feeders for the full video duration (60 s), we considered each video to be a separate visit. (For full details, see Supplementary methods 2 – Quantifying behaviours at feeders).
Statistical analysis
Quantifying personality
All analysis was conducted in JMP Pro 13 (SAS Institute, Cary, NC, USA) unless otherwise specified. Behaviour from the immobility test was analysed as a potential personality trait by using the GLIMMIX procedure in SAS (following Dingemanse and Dochtermann 2013). Models were fitted with a Gaussian distribution, and we checked for normality and homogeneity of variance. We compared two reduced models with fixed-effect assemblages (sex, test order), with the second including individual identity as a random factor. We used the −2Log-likelihood (−2LL) and Akaike information criterion corrected for small sample sizes (AICc) to determine whether including individual identity improved the model fit, and, if it did, the behaviour was considered a significant trait. To test for trait plasticity, we compared the second model with a third model, which included the interaction between individual identity and test order as a random effect. Finally, we calculated trait repeatability following Dingemanse and Dochtermann (2013).
Ten individuals were tested only once (i.e. were not recaptured) and to determine whether they could be included in the analysis, we tested whether, in recaptured individuals (n = 43), immobility differed between repeat tests. A mixed model analysis with test order as a fixed effect and individual identity as a random factor showed no significant effect of test order on immobility (P = 1.00). Therefore, all individuals (n = 53) were included in the analyses.
Testing the influence of cohort and personality on the probability of recapture
To test whether personality (immobility) differed between the cohorts, we applied a square-root transformation to time immobile and ran a general linear model with cohort as a fixed effect. We also tested whether the probability of recapture during our survey was influenced by population cohort (known vs new animals) or personality. We ran a logistic regression with a binary response variable (recaptured: yes, no) and included cohort, immobility, sex and site as fixed effects.
Testing the influence of cohort and personality on risk-taking at feeders
We tested whether cohort or personality influenced (1) visit frequency to feeders, and (2) behaviour at feeders. We included exposed and sheltered feeders with high-preference food (feeders with low-preference food had too few visits to gain meaningful data). We focused on the behaviour of animals in their initial interactions with a feeder by limiting our analysis to a maximum of the first three visits per feeder risk (i.e. a maximum of three visits to exposed feeders and three visits to sheltered feeders per individual). To examine whether marked (captured) animals were taking greater risks than were unmarked (not captured) animals, we looked at visit frequency to exposed and sheltered feeders (with high-preference food). We ran a relative risk test (calculated as the number of visits to feeders by marked pygmy-possums / number of visits to feeders by unmarked pygmy-possums, per feeder risk).
We also tested whether visit frequency to (high-preference food) feeders by identified (i.e. uniquely marked) pygmy-possums (n = 40) was influenced by feeder risk (two levels: exposed, sheltered), cohort (two levels: known, new), personality, or the interaction between feeder risk and cohort or personality. We used a linear mixed model approach and included individual identity as a random factor, because individuals could visit exposed and sheltered feeders. For each model, we checked for normality and homogeneity of variance. For significant effects we ran pair-wise comparisons using a Tukey–Kramer adjustment. As a complementary test, we tested whether capture frequency affected the relative visits to exposed and sheltered feeders (using the difference in total number of visits between exposed and sheltered feeders, with high-preference food feeders only). We ran a one-way ANOVA and included all visits from identified individuals that visited at least one feeder (n = 32), with number of times trapped and relative visits as fixed effects. For individuals (n = 13) that visited exposed and sheltered feeders with high-preference food, we also tested whether risk-taking (approach, locomotion, investigation, total time foraging) and cautionary behaviours (vigilance and not moving) were influenced by feeder risk, either cohort or personality, or the interaction. We used the mean proportion of time an individual allocated to behaviours across all (maximum of three) visits, per feeder risk. For total time foraging, we used the mean time individuals spent foraging across all (maximum of three) visits, per feeder risk.
To test whether the population cohorts differed in their overall behaviour at feeders, we used a permutational multivariate ANOVA (PERMANOVA, in Primer ver. 6 and PERMANOVA+) and included feeder risk, cohort and the interaction as fixed factors, and individual identity nested in cohort as a random factor. We used the conservative Type III sums of squares, with fixed effects summed to zero and permutation of residuals under a reduced model, with 9999 permutations. To test whether personality influenced behaviour, we used a general linear model with a Poisson distribution and log-link function for non-gaussian data. We ran a separate model for each behaviour at exposed and sheltered feeders and included immobility as a fixed effect.
Ethics approval
All research was conducted in accordance with The University of Sydney Animal Ethics (Permits: 2017/1247, 991129/01) and NSW National Parks and Wildlife Service Scientific Licence (SL100835).
Results
We captured 53 pygmy-possums (29 females, 24 males) over the four trapping nights, and, as expected, a high proportion (0.81, n = 43) was recaptured during the survey (Fig. 2a, b, Table S2). Similarly, of the 40 uniquely marked individuals, most were recaptured (0.88; Table S2). In total, 25 of the 40 marked individuals were new animals (trapped for the first time in this session) and 15 were known animals (trapped in previous years). Overall, most (58%) individuals were captured in the first night (Fig. 2a, b), after which additional captures declined (over the four trapping nights, 31, 11, 7 and 4 individuals respectively, were captured for the first time in this session). Similarly, most (47%) individuals were recaptured on the second night; over the second, third and fourth nights, 25, 13 and 5 individuals were recaptured respectively. Because this was a short survey, recaptures might have increased, given additional trapping nights, although this would have limited impact on our findings.
Mean body weight (±s.e.) was similar between the sexes (mean female = 39.3 g ± 0.89 g, mean male = 39.3 g ± 0.93 g) and there was no sex bias in the group of animals that was not recaptured (female n = 5, male n = 5). On average, females were captured 3.2 times and males 2.5 times. Captures of known animals were skewed slightly (0.7) towards females (14/21), whereas captures of new animals were roughly equal (15 females, 17 males). Known animals were also slightly heavier than were new animals (mean weight (±s.e.): 42.5 g ± 0.71 g and 37.3 g ± 0.75 g respectively).
Personality, quantified through the immobility test (i.e. time spent immobile in the handling bag), was a significant (LRT = 18.51, P < 0.001) and repeatable (r = 0.40) trait. Although males spent more time immobile than did females (mean time ± s.e.: males = 3.00 ± 1.61, females = −2.61 ± 1.52, F1,51 = 7.70, P = 0.01), personality did not differ between the population cohorts (F = 3.20, P = 0.90). The recapture probability being significantly influenced by cohort was greater for known animals than for new animals (likelihood ratio χ2 = 5.24, P = 0.02; Table S2). Recapture probability was not significantly influenced by personality (LR χ2 = 0.05, P = 0.83), sex (LR χ2 = 0.01, P = 0.94) or site (LR χ2 = 0.05, P = 0.82).
We analysed 1566 videos (i.e. visits) of pygmy-possums at feeders. Overall, there was a clear rank preference (in number of visits) among the four risk–reward treatments (Fig. 3). Feeders were ranked first by reward, then by risk, with visit number being greatest at sheltered, high-preference feeders and lowest at exposed, low-preference feeders. Of these visits, 746 were by marked (unique and common marks) animals trapped in this session. However, 312 visits were by unmarked animals (i.e. not trapped in this session), and 508 visits were by animals where the presence/absence of a mark could not be confirmed. Whereas visits to exposed feeders (with high-preference food) by marked and unmarked animals was comparable (relative risk = 1.1), marked animals were twice (relative risk = 2.0) as likely to visit sheltered feeders (with high-preference food) than were unmarked animals. Of the 40 uniquely marked individuals, 32 visited a feeder at least once (range = 1–45; mean = 15.44, s.e. ± 2.31), and 13 visited both exposed and sheltered feeders.
Visit frequency to feeders was influenced by the interaction between population cohort and feeder risk (F1,36 = 6.51, P = 0.02). Known animals revisited exposed and sheltered feeders similarly, whereas new animals were more risk-adverse and tended to revisit only sheltered feeders (Fig. 4). We found no significant effect of personality on visit frequency (F1,36 = 1.09, P = 0.30) or interactive effect of personality and feeder risk (F1,36 = 1.26, P = 0.27). There was also no significant effect of capture frequency on the relative frequency of visits to feeders (F3,28 = 0.15, P = 0.93).
|
|
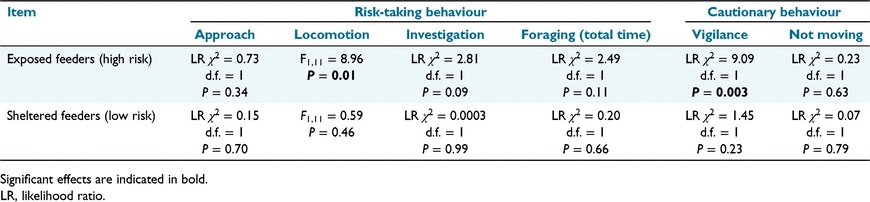
Of the behaviours recorded at feeders, locomotion and approach were positively correlated (r = 0.65), and total time spent foraging was negatively correlated with approach (r = −0.74), locomotion (r = −0.63), and investigation (r = −0.51). Our PERMANOVA showed that behaviour at feeders was not significantly affected by population cohort, feeder risk, or the interaction (pseudo F1,11 ≥ 0.13, P ≥ 0.28). However, in analysing behaviours separately by using general linear models, personality significantly affected risk-taking behaviour, but only at exposed feeders. Individuals that were more immobile also spent more time being vigilant (F1,11 = 9.09, d.f. = 1, P = 0.003) and less time in locomotion (F1,11 = 8.96, P = 0.01), than did less immobile individuals (Fig. 5a, b). There was no significant effect of personality on any other behaviour at feeders (Fig. 5c–f; Table 1).
Discussion
Determining the mechanisms underpinning differences in trap response following initial capture is one step towards addressing a substantial source of bias in live-capture surveys. Recapture rates during our trapping session were high, suggesting that most of the populations had been caught. However, recapture probability differed by cohort, and known animals trapped in previous years had a significantly greater recapture probability than did new animals trapped for the first time. We anticipated risk-assessment as a potential mechanism underpinning recapture probability (e.g. Johnstone et al. 2021a) and the cohorts, indeed, differed in risk-taking behaviours. Known animals visited exposed (risky) and sheltered (safe) feeders similarly, whereas new animals were more risk-adverse, visiting exposed feeders far less frequently than sheltered feeders. Although personality (immobility) did not influence recapture, it did influence general risk-taking behaviours at exposed feeders. Individuals that were more immobile spent more time vigilant and less time in locomotion than did individuals that were less immobile. Notably, where recapture history could be determined (i.e. an animal was marked or unmarked), 29.5% of visits to feeders were by unmarked (i.e. not captured) animals. Together, our findings suggest that despite high recapture rates, within a single trapping session (1) differences in risk-taking proclivity between cohorts may influence recapture probability to drive a sample bias, and (2) a substantial portion of the population may go unsampled.
Despite our high recapture rates, the probability of recapture within our trapping session was substantially lower for new animals (trapped for the first time) than for known animals (trapped in previous years). Known animals were adults with prior trapping experience and had persisted in the population for at least 1 year. In contrast, new animals would have consisted mostly of subadults with no prior experience with traps, along with a small proportion of adults (Broome 2001a). Age and prior trapping experience can influence trap response (Camacho et al. 2017); adults are often more difficult to trap over time, whereas captures are greater for younger, trap-naive animals (Daly 1980; Camacho et al. 2017). Interestingly, our short study found the opposite effect.
We considered several factors that may have contributed towards the differences in trappability between our cohorts. Differences may have been a function of low trap sensitivity to the lighter weight of young new animals (e.g. Anthony et al. 2005). However, because subadult pygmy-possums often weigh the same as adults (Broome 2001a), trap sensitivity was unlikely to influence our study. Second, competition for a high-value food (walnuts) during a period when animals are building fat reserves for hibernation may have led to known adults dominating traps. However, traps were not saturated and any resource guarding would have been negated once the dominant animal was captured. Individuals also had opportunity to encounter multiple traps (Broome 2001a) and, throughout our trapping session, individuals were often caught in different traps. Third, we considered the timing of the survey and the possibility that new adults may have avoided capture in previous years by dispersing before surveys began or recently entered the populations from unsurveyed sites. However, animals are generally trapped at the same site each year (Broome 2001a), and within the scope of our study. It was not possible to explore any effects of annual dispersal. Finally, we considered the potential for increased trappability, given a longer trapping session. Although the annual trapping survey (as with this study) only spans four nights, by mid-way, 72% of animals were recaptured and 81% were recaught after four nights. This outcome suggested that few (but potentially some) additional animals would have been recaught if the survey were extended. Accounting for the above factors, we considered it to be most likely that the differences in recapture between the cohorts arose from behavioural differences.
Contrary to our prediction, we found that personality, per se, did not significantly affect recapture probability. Even though immobility can negatively correlate with boldness (Réale et al. 2000), activity, and aggression (Taylor et al. 2012), traits that can directly influence trappability (Boon et al. 2008; Carter et al. 2012), we found no evidence of a personality bias. However, not all traits influence trappability (Garamszegi et al. 2009) and some studies have found no discernible effect of personality on trappability (Michelangeli et al. 2016; Jolly et al. 2019). Considering our high recapture rates, it is possible that traps are not perceived as risky and there is no link between personality and recapture probability. But as we measured personality only along a single trait axis, the influence of additional traits would need to be assessed before we could conclude that personality, in general, does not drive a sample bias in this species.
Personality did influence behaviour in a high-risk context (i.e. at exposed feeders). But rather than driving avoidance of high-risk scenarios, personality influenced how individuals managed the risk, and immobility was associated with vigilant and explorative behaviours. More immobile individuals were risk adverse and these individuals were more vigilant, a cautionary behaviour often measured in response to predation risk (Brown 1999), and less active (less time in locomotion) at feeders, a response associated with risk-taking (Wat et al. 2020). To manage foraging risk–reward trade-offs, animals can decide where to spend their foraging time (i.e. selecting safe over risky patches), or can allocate different amounts of time to vigilance depending on the patch risk (Brown 1999). We found that patch selection differed between cohorts, and mitigating risk at risky patches (i.e. exposed feeders) was influenced by personality. Fundamentally, wildlife detection is dependent on animals visiting a given device (e.g. live traps), and subsequently interacting with it (i.e. entering the trap). Given that personality influenced cautionary and investigative behaviours at high-risk exposed feeders, our results lend support to other research (Carter et al. 2012; Johnstone et al. 2021a) that has demonstrated the influence of personality on detection probability when using approaches that detect specific animal behaviours.
More generally, our results suggest that risk-taking behaviour may explain the differences in recapture probability between the cohorts. Overall, pygmy-possums visited sheltered feeders significantly more often than they did exposed feeders (Fig. 3), suggesting that they perceived sheltered feeders as safer microhabitats. These responses are consistent with other prey species. Both house mice (Mus musculus; Ylönen et al. 2002) and field voles (Microtus agrestis; Korpimaki et al. 1996) favour vegetated or sheltered microhabitats under potential or realised levels of predation risk. Importantly, whereas known individuals revisited exposed and sheltered feeders similarly, new individuals revisited sheltered feeders, but typically visited exposed feeders only once, suggesting that these animals were reducing their risk-taking, despite the great reward.
Although younger animals are typically less risk-averse than are adults (Fairbanks 1993; Bergman and Kitchen 2009), in some cases, this pattern may be reversed. For example, in the endangered alalā (Corvus hawaiiensis), younger individuals are more neophobic than are adults (Greggor et al. 2020), likely owing to heightened predation risk (e.g. at fledging). Similarly, nestling pygmy-possums are at risk from nest raiding by Antechinus sp. (NSW National Parks and Wildlife Service 2002), and inexperienced subadults may initially avoid unfamiliar or potentially risky situations (such as traps and exposed feeders). Risk aversion can dissipate with age (Greggor et al. 2020), because time and experience allow animals to make more informed decisions (Trimmer et al. 2011), and risk avoidance may decrease in pygmy-possums as they mature and trappability may subsequently increase in future trapping sessions. Together, our results suggest that exposed feeders and, to a lesser degree, live-traps were perceived as risky by pygmy-possums, at least following the first encounter. However, we surmise that known animals with greater life experience either associated traps and exposed feeders with little risk or were generally less risk-averse, resulting in a greater recapture probability.
Importantly, we noted that a larger-than-expected proportion (~0.30) of visits to feeders were by unmarked pygmy-possums (i.e. untrapped in this session). Although these animals may have been trapped in previous years and may be captured in future surveys, when a large part of a population goes undetected, the accuracy of population estimates decreases, and if many animals have capture probabilities close to zero, the actual population size may be greatly underestimated (Pollock and Otto 1983). Trapping the untrappable has long been a wicked problem in wildlife management (Bisi et al. 2011; Biro 2013; Garvey et al. 2020) and was observed here only because of our use of wildlife cameras. How much this untrappability was driven by personality or risk aversion is unknown because these individuals were not assessable within this trapping session. Although additional trapping sessions may alleviate some bias, novel or complementary (Garvey et al. 2020) methods of capture or monitoring could be useful to address this knowledge gap.
As with all short studies, the interpretation of our results would benefit from replication. Although it is likely that a substantial number of animals went undetected during this survey, these individuals may have been trappable in previous (or in subsequent) years. Long-term surveying (as is conducted on these populations) is crucial for providing robust and reliable population data for wildlife managers to act on. However, experimental studies such as ours provide useful insights that may benefit from further investigation. For instance, although a single monitoring method is beneficial in providing consistent and comparative long-term data, no method is without bias (Biro 2013) and complementary or comparative methods may more effectively provide representative population samples, a factor that should never be discounted, especially when working on endangered species.
Our results indicated that even for species with high recapture rates, heterogeneity in trappability can arise during population sampling, likely owing to risk-sensitive decision-making by individuals. This heterogeneity can bias population estimates towards the most detectable individuals and overlook those that avoid capture. To address these biases during survey sessions, wildlife managers can seek to accommodate the different motivations of individuals that vary in experience and personality (Garvey et al. 2020), for instance, by using a suite of traps or sampling methods (Wilson et al. 2011; Johnstone et al. 2021c), running extended sessions where possible to account for trap-shy animals, and conducting repeated trapping sessions over multiple seasons or years. Understanding drivers of detection biases is a crucial step towards reducing sample bias during surveys and to increase representative population sampling.
Supplementary material
Supplementary material is available online.
Data availability
The data used to generate the results in this paper are available and accessible via figshare.com (https://doi.org/10.6084/m9.figshare.21598899).
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
Research was funded by the Holsworth Wildlife Research Endowment and the Ecological Society of Australia.
Acknowledgements
Our gratitude goes to L. Broome at the Department of Planning and Environment for access and the opportunity to work with the mountain pygmy-possum, to H. Bates for feedback on the project proposal and to the possum trappers who assisted during this project. We thank two anonymous reviewers for their constructive comments on drafts of this paper. KCJ and PBB conceptualised and designed the research; KCJ conducted the field research and collected data. All authors contributed to the statistical analysis. KCJ drafted the manuscript, and critical revisions were made by PBB and CM. This paper forms part of the PhD thesis of Kyla C Johnstone (2021).
References
Anthony, NM, Ribic, CA, Bautz, R, and Garland, T (2005). Comparative effectiveness of Longworth and Sherman live traps. Wildlife Society Bulletin 33, 1018–1026.| Comparative effectiveness of Longworth and Sherman live traps.Crossref | GoogleScholarGoogle Scholar |
Balph, DF (1968). Behavioral responses of unconfined Uinta ground squirrels to trapping. The Journal of Wildlife Management 32, 778–794.
| Behavioral responses of unconfined Uinta ground squirrels to trapping.Crossref | GoogleScholarGoogle Scholar |
Bergman, TJ, and Kitchen, DM (2009). Comparing responses to novel objects in wild baboons (Papio ursinus) and geladas (Theropithecus gelada). Animal Cognition 12, 63–73.
| Comparing responses to novel objects in wild baboons (Papio ursinus) and geladas (Theropithecus gelada).Crossref | GoogleScholarGoogle Scholar |
Besbeas, P, Freeman, SN, Morgan, BJT, and Catchpole, EA (2002). Integrating mark–recapture–recovery and census data to estimate animal abundance and demographic parameters. Biometrics 58, 540–547.
| Integrating mark–recapture–recovery and census data to estimate animal abundance and demographic parameters.Crossref | GoogleScholarGoogle Scholar |
Biro, PA (2013). Are most samples of animals systematically biased? Consistent individual trait differences bias samples despite random sampling. Oecologia 171, 339–345.
| Are most samples of animals systematically biased? Consistent individual trait differences bias samples despite random sampling.Crossref | GoogleScholarGoogle Scholar |
Biro, PA, and Stamps, JA (2008). Are animal personality traits linked to life-history productivity? Trends in Ecology & Evolution 23, 361–368.
| Are animal personality traits linked to life-history productivity?Crossref | GoogleScholarGoogle Scholar |
Bisi, F, Newey, S, Nodari, M, Wauters, LA, Harrison, A, Thirgood, S, and Martinoli, A (2011). The strong and the hungry: bias in capture methods for mountain hares Lepus timidus. Wildlife Biology 17, 311–316.
| The strong and the hungry: bias in capture methods for mountain hares Lepus timidus.Crossref | GoogleScholarGoogle Scholar |
Blumstein DT, Daniel JC (2007) ‘Quantifying behavior the JWatcher way.’ (Sinauer Associates Incorporated: Sunderland, MA, USA)
Boon, AK, Réale, D, and Boutin, S (2008). Personality, habitat use, and their consequences for survival in North American red squirrels Tamiasciurus hudsonicus. Oikos 117, 1321–1328.
| Personality, habitat use, and their consequences for survival in North American red squirrels Tamiasciurus hudsonicus.Crossref | GoogleScholarGoogle Scholar |
Broome, LS (2001a). Intersite differences in population demography of Mountain Pygmy-possums Burramys parvus Broom (1986–1998): implications for metapopulation conservation and ski resorts in Koskiuszko National Park, Australia. Biological Conservation 102, 309–323.
| Intersite differences in population demography of Mountain Pygmy-possums Burramys parvus Broom (1986–1998): implications for metapopulation conservation and ski resorts in Koskiuszko National Park, Australia.Crossref | GoogleScholarGoogle Scholar |
Broome, LS (2001b). Density, home range, seasonal movements and habitat use of the mountain pygmy-possum Burramys parvus (Marsupialia: Burramyidae) at Mount Blue Cow, Kosciuszko National Park. Austral Ecology 26, 275–292.
| Density, home range, seasonal movements and habitat use of the mountain pygmy-possum Burramys parvus (Marsupialia: Burramyidae) at Mount Blue Cow, Kosciuszko National Park.Crossref | GoogleScholarGoogle Scholar |
Broome L, Archer M, Bates H, Shi H, Geiser F, McAllan B, Heinze D, Hand S, Evans T, Jackson S (2012) A brief review of the life history of, and threats to, Burramys parvus with a prehistory-based proposal for ensuring that it has a future. In ‘Wildlife and climate change: towards robust conservation strategies for Australian fauna’. (Eds D Lunney, P Hutchings) pp. 114–126. (Royal Zoological Society of NSW: Sydney, NSW, Australia)
Brown, JS (1999). Vigilance, patch use and habitat selection: foraging under predation risk. Evolutionary Ecology Research 1, 49–71.
Camacho, C, Canal, D, and Potti, J (2017). Lifelong effects of trapping experience lead to age-biased sampling: lessons from a wild bird population. Animal Behaviour 130, 133–139.
| Lifelong effects of trapping experience lead to age-biased sampling: lessons from a wild bird population.Crossref | GoogleScholarGoogle Scholar |
Carter, AJ, Goldizen, AW, and Tromp, SA (2010). Agamas exhibit behavioral syndromes: bolder males bask and feed more but may suffer higher predation. Behavioral Ecology 21, 655–661.
| Agamas exhibit behavioral syndromes: bolder males bask and feed more but may suffer higher predation.Crossref | GoogleScholarGoogle Scholar |
Carter, AJ, Heinsohn, R, Goldizen, AW, and Biro, PA (2012). Boldness, trappability and sampling bias in wild lizards. Animal Behaviour 83, 1051–1058.
| Boldness, trappability and sampling bias in wild lizards.Crossref | GoogleScholarGoogle Scholar |
Cole, EF, and Quinn, JL (2014). Shy birds play it safe: personality in captivity predicts risk responsiveness during reproduction in the wild. Biology Letters 10, 20140178.
| Shy birds play it safe: personality in captivity predicts risk responsiveness during reproduction in the wild.Crossref | GoogleScholarGoogle Scholar |
Cowan, PE (1977). Neophobia and neophilia: new-object and new-place reactions of three Rattus species. Journal of Comparative and Physiological Psychology 91, 63–71.
| Neophobia and neophilia: new-object and new-place reactions of three Rattus species.Crossref | GoogleScholarGoogle Scholar |
Daly, JC (1980). Age, sex and season: factors which determine the trap response of the European wild rabbit, Oryctolagus cuniculus. Wildlife Research 7, 421–432.
| Age, sex and season: factors which determine the trap response of the European wild rabbit, Oryctolagus cuniculus.Crossref | GoogleScholarGoogle Scholar |
Dingemanse, NJ, and Dochtermann, NA (2013). Quantifying individual variation in behaviour: mixed-effect modelling approaches. Journal of Animal Ecology 82, 39–54.
| Quantifying individual variation in behaviour: mixed-effect modelling approaches.Crossref | GoogleScholarGoogle Scholar |
DomèNech, J, and Senar, JC (1997). Trapping methods can bias age ratio in samples of passerine populations. Bird Study 44, 348–354.
| Trapping methods can bias age ratio in samples of passerine populations.Crossref | GoogleScholarGoogle Scholar |
Fairbanks, LA (1993). Risk-taking by juvenile vervet monkeys. Behaviour 124, 57–72.
| Risk-taking by juvenile vervet monkeys.Crossref | GoogleScholarGoogle Scholar |
Garamszegi, LZ, Eens, M, and Török, J (2009). Behavioural syndromes and trappability in free-living collared flycatchers, Ficedula albicollis. Animal Behaviour 77, 803–812.
| Behavioural syndromes and trappability in free-living collared flycatchers, Ficedula albicollis.Crossref | GoogleScholarGoogle Scholar |
Garvey, PM, Banks, PB, Suraci, JP, Bodey, TW, Glen, AS, Jones, CJ, McArthur, C, Norbury, GL, Price, CJ, Russell, JC, and Sih, A (2020). Leveraging motivations, personality, and sensory cues for vertebrate pest management. Trends in Ecology & Evolution 35, 990–1000.
| Leveraging motivations, personality, and sensory cues for vertebrate pest management.Crossref | GoogleScholarGoogle Scholar |
Gosling, SD (2001). From mice to men: what can we learn about personality from animal research? Psychological Bulletin 127, 45–86.
| From mice to men: what can we learn about personality from animal research?Crossref | GoogleScholarGoogle Scholar |
Green, K, and Osborne, WS (1981). The diet of foxes, Vulpes vulpes (L.), in relation to abundance of prey above the winter snowline in New South Wales. Wildlife Research 8, 349–360.
| The diet of foxes, Vulpes vulpes (L.), in relation to abundance of prey above the winter snowline in New South Wales.Crossref | GoogleScholarGoogle Scholar |
Greggor, AL, Masuda, B, Flanagan, AM, and Swaisgood, RR (2020). Age-related patterns of neophobia in an endangered island crow: implications for conservation and natural history. Animal Behaviour 160, 61–68.
| Age-related patterns of neophobia in an endangered island crow: implications for conservation and natural history.Crossref | GoogleScholarGoogle Scholar |
Happold DCD (1989) Small mammals of the Australian Alps. In ‘The Scientific Significance of the Australian Alps’. (Ed. R Good) pp. 221–239. (Australian Academy of Science and NSW National Parks and Wildlife Service)
Johnstone, KC, McArthur, C, and Banks, PB (2021a). Behavioural drivers of survey bias: interactive effects of personality, the perceived risk and device properties. Oecologia 197, 117–127.
| Behavioural drivers of survey bias: interactive effects of personality, the perceived risk and device properties.Crossref | GoogleScholarGoogle Scholar |
Johnstone, KC, McArthur, C, and Banks, PB (2021b). Testing transgenerational transfer of personality in managed wildlife populations: a house mouse control experiment. Ecological Applications 31, e02247.
| Testing transgenerational transfer of personality in managed wildlife populations: a house mouse control experiment.Crossref | GoogleScholarGoogle Scholar |
Johnstone, KC, McArthur, C, and Banks, PB (2021c). Catch me if you can: personality drives technique-specific biases during live-capture trapping. Wildlife Research 48, 713–721.
| Catch me if you can: personality drives technique-specific biases during live-capture trapping.Crossref | GoogleScholarGoogle Scholar |
Jolly, CJ, Webb, JK, Gillespie, GR, Hughes, NK, and Phillips, BL (2019). Bias averted: personality may not influence trappability. Behavioral Ecology and Sociobiology 73, 129.
| Bias averted: personality may not influence trappability.Crossref | GoogleScholarGoogle Scholar |
Korpimaki, E, Koivunen, V, and Hakkarainen, H (1996). Microhabitat use and behavior of voles under weasel and raptor predation risk: predator facilitation? Behavioral Ecology 7, 30–34.
| Microhabitat use and behavior of voles under weasel and raptor predation risk: predator facilitation?Crossref | GoogleScholarGoogle Scholar |
Krebs CJ (1999) ‘Ecological methodology.’ 2nd edn. (Addison-Welsey Educational Publishers: Menlo Park, CA, USA)
Linhart, P, Fuchs, R, Poláková, S, and Slabbekoorn, H (2012). Once bitten twice shy: long-term behavioural changes caused by trapping experience in willow warblers Phylloscopus trochilus. Journal of Avian Biology 43, 186–192.
| Once bitten twice shy: long-term behavioural changes caused by trapping experience in willow warblers Phylloscopus trochilus.Crossref | GoogleScholarGoogle Scholar |
Martin, JGA, and Réale, D (2008). Temperament, risk assessment and habituation to novelty in eastern chipmunks, Tamias striatus. Animal Behaviour 75, 309–318.
| Temperament, risk assessment and habituation to novelty in eastern chipmunks, Tamias striatus.Crossref | GoogleScholarGoogle Scholar |
Mella, VSA, Krucler, J, Sunderasan, L, Hawkins, J, Herath, APHM, Johnstone, KC, Troxell-Smith, SM, Banks, PB, and McArthur, C (2016). Effective field-based methods to quantify personality in brushtail possums (Trichosurus vulpecula). Wildlife Research 43, 332–340.
| Effective field-based methods to quantify personality in brushtail possums (Trichosurus vulpecula).Crossref | GoogleScholarGoogle Scholar |
Michelangeli, M, Wong, BBM, and Chapple, DG (2016). It’s a trap: sampling bias due to animal personality is not always inevitable. Behavioral Ecology 27, 62–67.
| It’s a trap: sampling bias due to animal personality is not always inevitable.Crossref | GoogleScholarGoogle Scholar |
NSW National Parks and Wildlife Service (2002) ‘Approved recovery plan for the Mountain Pygmy-possum, Burramys parvus.’ (NSW National Parks and Wildlife Service: Hurstville, NSW, Australia)
O’brien, S, Robert, B, and Tiandry, H (2005). Consequences of violating the recapture duration assumption of mark–recapture models: a test using simulated and empirical data from an endangered tortoise population. Journal of Applied Ecology 42, 1096–1104.
| Consequences of violating the recapture duration assumption of mark–recapture models: a test using simulated and empirical data from an endangered tortoise population.Crossref | GoogleScholarGoogle Scholar |
Otis, DL, Burnham, KP, White, GC, and Anderson, DR (1978). Statistical inference from capture data on closed animal populations. Wildlife Monographs 62, 3–135.
Pollock, KH, and Otto, MC (1983). Robust estimation of population size in closed animal populations from capture-recapture experiments. Biometrics 39, 1035–1049.
| Robust estimation of population size in closed animal populations from capture-recapture experiments.Crossref | GoogleScholarGoogle Scholar |
Réale, D, Gallant, BY, Leblanc, M, and Festa-Bianchet, M (2000). Consistency of temperament in bighorn ewes and correlates with behaviour and life history. Animal Behaviour 60, 589–597.
| Consistency of temperament in bighorn ewes and correlates with behaviour and life history.Crossref | GoogleScholarGoogle Scholar |
Roche, EA, Brown, CR, Brown, MB, and Lear, KM (2013). Recapture heterogeneity in cliff swallows: increased exposure to mist nets leads to net avoidance. PLoS ONE 8, e58092.
| Recapture heterogeneity in cliff swallows: increased exposure to mist nets leads to net avoidance.Crossref | GoogleScholarGoogle Scholar |
Rosengren NJ, Peterson JA (1989) Heritage values and the geological and geomorphological significance of the Australian alpine zone. In ‘The scientific significance of the Australian alps’. (Ed. RB Good) pp. 187–204. (Australian Alps Liaison Committee/Australian Academy of Science)
Taylor, RW, Boon, AK, Dantzer, B, Réale, D, Humphries, MM, Boutin, S, Gorrell, JC, Coltman, DW, and McAdam, AG (2012). Low heritabilities, but genetic and maternal correlations between red squirrel behaviours. Journal of Evolutionary Biology 25, 614–624.
| Low heritabilities, but genetic and maternal correlations between red squirrel behaviours.Crossref | GoogleScholarGoogle Scholar |
Trimmer, PC, Houston, AI, Marshall, JAR, Mendl, MT, Paul, ES, and McNamara, JM (2011). Decision-making under uncertainty: biases and Bayesians. Animal Cognition 14, 465–476.
| Decision-making under uncertainty: biases and Bayesians.Crossref | GoogleScholarGoogle Scholar |
Wat, KKY, Herath, APHM, Rus, AI, Banks, PB, and McArthur, C (2020). Space use by animals on the urban fringe: interactive effects of sex and personality. Behavioral Ecology 31, 330–339.
| Space use by animals on the urban fringe: interactive effects of sex and personality.Crossref | GoogleScholarGoogle Scholar |
Wilson, DJ, Efford, MG, Brown, SJ, Williamson, JF, and McElrea, GJ (2007). Estimating density of ship rats in New Zealand forests by capture-mark-recapture trapping. New Zealand Journal of Ecology 31, 47–59.
Wilson, ADM, Binder, TR, McGrath, KP, Cooke, SJ, Godin, J-GJ, and Kraft, C (2011). Capture technique and fish personality: angling targets timid bluegill sunfish, Lepomis macrochirus. Canadian Journal of Fisheries and Aquatic Sciences 68, 749–757.
| Capture technique and fish personality: angling targets timid bluegill sunfish, Lepomis macrochirus.Crossref | GoogleScholarGoogle Scholar |
Ylönen, H, Jacob, J, Davies, MJ, and Singleton, GR (2002). Predation risk and habitat selection of Australian house mice, Mus domesticus, during an incipient plague: desperate behaviour due to food depletion. Oikos 99, 284–289.
| Predation risk and habitat selection of Australian house mice, Mus domesticus, during an incipient plague: desperate behaviour due to food depletion.Crossref | GoogleScholarGoogle Scholar |