A lesson for planning rodent eradications: interference of invasive slugs during the Gough Island mouse eradication attempt in 2021
Araceli Samaniego A * , Wes Jolley B and Pete McClelland C
A * , Wes Jolley B and Pete McClelland C
A Manaaki Whenua – Landcare Research, 231 Morrin Road, St Johns, Auckland 1072, New Zealand.
B Island Conservation, 2100 Delaware Avenue, Suite 1, Santa Cruz, CA 95060, USA.
C Royal Society for the Protection of Birds, The Lodge, Sandy, Bedfordshire, SG19 2DL, UK.
Wildlife Research 50(5) 344-355 https://doi.org/10.1071/WR22024
Submitted: 16 February 2022 Accepted: 13 June 2022 Published: 8 August 2022
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: House mice (Mus musculus) are the main drivers of biodiversity declines on Gough Island (6500 ha; 40°21′S, 009°53′W), central South Atlantic. A mouse eradication operation was planned, the largest global attempt targeting only this species. Understanding and managing challenges of operating at such scales are crucial for maximising the chance of eradication success. The Gough Island mouse eradication attempt was implemented between June and August 2021, after years of planning and trials. We expected poor weather and negligible non-target bait consumption.
Aims: We aimed to assess the impact of expected and unexpected challenges faced during the eradication operation on Gough Island, namely poor weather and rapid bait disappearance.
Methods: We set up bait degradation plots across the primary habitats to monitor the impact of expected heavy rain on bait pellets. In contrast, bait availability monitoring and slug laboratory trials were set up ad hoc in response to unexpected observations of high bait consumption by invasive slugs in the lowlands, where both slugs and mice are more abundant.
Key results: Bait degradation rates were very different between the highlands and the lowlands, with bait in the highlands lasting about six times longer, despite bait pellets receiving more precipitation and the highlands being persistently under cloud. Bait availability in the lowlands dropped by >80% within a few days of the second and third bait application, down to critically low levels (~2 kg ha−1). Importantly, mouse activity was negligible by this time. Non-native slugs appeared to be the main cause of such a sudden drop in rodent bait availability.
Conclusions: The expected rainy weather was not a significant direct cause of bait degradation in the short term. In contrast, the unexpected slug interference, overlooked in earlier planning trials, resulted in major adjustments of the baiting strategy. Indeed, the rapid bait disappearance in the lowlands triggered the third bait application over this area, at a higher rate. This was not enough, as mice are still present.
Implications: This is the first report of slug interference during aerial rodent eradications. Our results illustrate how interference by non-target species could affect future pest eradications using baits and should, as far as possible, be assessed early during planning.
Keywords: Ambigolimax, bait availability, Deroceras, eradication planning, invasive rodents, invertebrates, Mus musculus, non-target species, restoration.
Introduction
Eradicating invasive species from islands is a proven conservation tool for recovering threatened species and ecosystem services (Jones et al. 2016; Russell and Broome 2016). Invasive rodents (Mus musculus, Rattus spp.) are among the most damaging and widespread invasive animals (Towns et al. 2006; Angel et al. 2009; Shiels et al. 2014). The most efficient approach to rodent eradication is aerial broadcast of a pelleted rodenticide product, typically containing brodifacoum (Howald et al. 2007; Broome et al. 2019). Applying highly palatable bait to all rodent territories, for long enough that every rodent encounters and consumes a lethal dose, is one of the core eradication principles (Keitt et al. 2015; Broome et al. 2019).
Invasive house mice (M. musculus) on Gough Island (hereafter Gough), present since at least 1888, provide one of the best examples of the pervasive impacts mice can cause when they are the only invasive predator (Angel and Cooper 2006). Known for their large size, mice on Gough prey on eggs and chicks and even adult birds. Up to 2 million individuals of numerous bird species are estimated to be predated by mice annually, including the Critically Endangered Tristan albatross (Diomedea dabbenena), and the Endangered MacGillivray’s prion (Pachyptila macgillivrayi) (Cuthbert and Hilton 2004; Wanless et al. 2007; Cuthbert et al. 2013; Davies et al. 2015; Dilley et al. 2015; Caravaggi et al. 2019). To avoid the collapse of seabird populations on this internationally significant island, the Royal Society for the Protection of Birds (RSPB) and partners worked for over a decade on the logistical, legal, and operational requirements to eradicate mice from Gough. Implemented in June–August 2021, the project was the largest undertaken globally to date that targeted only house mice.
Acknowledging the scale and complexity of the challenge, the operational plans were informed by numerous field studies. For example, trials assessing the efficacy of aerial baiting under Gough conditions found that mice utilising caves would encounter and consume bait spread outside caves (Cuthbert et al. 2011a), and that retention of bait on cliffs was adequate (Cuthbert et al. 2014). The risk to terrestrial birds of primary and secondary poisoning by rodenticides was assessed (Wanless et al. 2010). The palatability and toxicity of baits was tested on captive Gough mice (Cuthbert et al. 2011b). Moreover, bait consumption in the wild was also assessed, and it was found that mice readily consumed bait while consumption by non-target species was negligible (Wanless et al. 2008). Planning then used best practice application rates for temperate islands (8 kg ha−1 per application), which considerably reduced logistical requirements (e.g. flying time, fuel, and space on ship) given the large size of the island (McClelland 2021).
Weather was a major consideration in planning and implementing the baiting operation. Helicopters can only effectively spread bait on days with little rain, low winds, and high clouds. In addition, at least 3 days without significant rain after baiting is highly desirable, so that bait does not degrade too quickly and become unpalatable or unavailable for mice. Rainy and windy conditions are a feature of Gough in winter, particularly at high elevations and over rugged terrain (Wace 1961), thus much of the focus in the planning phase was on applying and maintaining bait in the highlands. Winter is typically the operational season for eradications on temperate islands, which is when many non-target species are absent and mouse numbers are at a lower point in their annual population cycle (Broome et al. 2019).
Here we report on our responses to the expected and unexpected challenges faced during the mouse eradication attempt on Gough, namely poor weather and rapid bait disappearance. Once on the island, we were limited in time and resources for research – the focus was the mouse eradication, and we had not anticipated carrying out research during the operation. Yet, a series of simple studies was set up to inform the Gough project as it progressed. The results and lessons learned are important to future eradication project planning for Gough and elsewhere, particularly for large and complex islands.
Materials and methods
Gough Island
Gough (ca 6500 ha; 40°21′S, 009°53′W), part of a natural World Heritage Site, lies in the central South Atlantic about 2700 km west-southwest of South Africa. It is a volcanic island that rises to 910 m, and for the most part is encircled with cliffs up to 300 m high (Fig. 1). The average rainfall is 3236 mm (range 2580–3745 mm), with appreciable rainfall (>10 mm) occurring on 89 days per year (range 67–105 days). Daily precipitation of >25 mm occurs on average 4 days per month between 1 June and 30 August as measured at the Gough Island Meteorological Station near the coast, although at higher altitudes it may be around 50% greater. Monthly rainfall during the eradication operation was 199 mm (June), 439 mm (July), and 199 mm (August), according to the Gough Island Meteorological Station (hereafter Base). The island is uninhabited except for the Base, which is occupied throughout the year by 5–10 people.
|
|
Wace (1961) distinguishes five types of vegetation on Gough across an altitudinal gradient (tussock grassland, fern bush, wet heath, peat bogs, and moorland and montane rock); however, for simplicity we distinguish between the highlands (≥400 m asl, with low vegetation) and the lowlands (<400 m asl, a mosaic of fern bush and shrubland with more varied structure) (Fig. 1). The vegetation in the highlands is mainly moss and lichen with large boggy flats; in the lowlands it is a mix of tall Spartina tussock, Scirpus (a grass-like sedge), ferns (primarily Histiopteris incisa and Blechnum palmiforme), and Phylica arborea trees. Although widespread, mice, the only invasive vertebrates and the only land mammals on Gough, are typically more abundant in the lowlands (Rowe-Rowe and Crafford 1992). Gough is internationally renowned for its birdlife (Dilley et al. 2015; Caravaggi et al. 2019). Breeding species include 22 species of seabirds, including the globally threatened Tristan albatross, MacGillivray’s prion and Atlantic petrel (Pterodroma incerta). There are also two endemic species of land bird, the Gough finch (Rowettia goughensis), locally known as Gough bunting, and the flightless Gough moorhen (Gallinula comeri).
Invertebrates are less studied. Molluscs (Mollusca: Gastropoda) on Gough are represented by five species of native snails (Balea tristensis, B. ventricosa, B. costellata, B. goughensis and Succinea flexilis); non-native molluscs include at least three species: one snail (Oxychilus alliarius) and two slugs (Ambigolimax valentianus and Deroceras reticulatum) (Preece 2001; Hänel et al. 2005). Here we focus on the slug A. valentianus, the widespread species that was collected for experiments and observed in the field (identification by G.M. Barker, Manaaki Whenua – Landcare Research based on photographs) (selected photos in Supplementary Material). Based on our observations, slugs appear to be more abundant in the lowlands. Moreover, climatic tolerances of other invasive slugs support this observation. For example, D. invadens (originally misidentified as D. panormitanum; Hutchinson et al. 2014) on subantarctic Marion Island (colder and with shorter vegetation than Gough) is rarely seen above 200 m altitude due to the freezing temperatures on higher areas, but in the lowlands abundance reaches 344 slugs m−2, albeit with patchy distribution (Lee et al. 2009).
Mouse eradication attempt
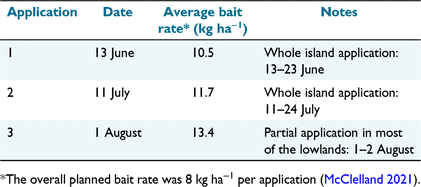
During the austral winter of 2021, Pestoff bait (2 g, 10-mm pellets with 20 ppm brodifacoum) made by Orillion (Whanganui, New Zealand) was spread across Gough, mainly by helicopter. Two bait applications were undertaken over the entire island between June and July 2021. The bait monitoring area (Fig. 1) was baited as part of the south block on 13 June and 11 July (Table 1). A third application was undertaken on 1 August over areas of concern, which included much of the lowlands (including the bait monitoring area) (Table 1). This followed the discovery of significant slug interference with bait pellets in this area. Having adequate contingency bait (23% of the order) allowed this additional bait application. The local bait application rates for the bait monitoring area (Table 1) were obtained through GIS analysis of the helicopter flight lines. Details of the wider aerial operation and the hand baiting of the Base will be published elsewhere.
|
Bait degradation
Originally, we planned to monitor bait degradation only after the second bait application, which was expected to be the last one around the Base given usually most mice die before the second application. However, given the unexpected high rates of bait disappearance (see Results) and the resulting changes in strategy, we repeated the work after the third bait application. Importantly, mouse activity (assessed with multiple detection devices including trail cameras) was undetectable by this time (Samaniego et al. in press).
Degradation Iteration 1 comprised 12 bait degradation plots, which were simultaneously set immediately after the second bait application (Table 2; Fig. 1). The goal was to monitor the condition of bait pellets over time, both in the lowlands and the highlands. We were particularly interested in the number of days that elapsed until no bait pellets remained. Locations were representative of the major vegetation types and micro-environmental conditions to which bait pellets were exposed (e.g. rain, wind, proximity to invertebrates). Bait consumption (by vertebrates or invertebrates) was considered a form of degradation. Each plot consisted of 12 bait pellets, ~10 cm apart, in two rows of six, loose on the ground. Bamboo skewers were used to mark the individual pellets and larger bamboo sticks to mark each plot. This arrangement was denser than the bait spread by helicopter and by hand, but we wanted to maximise the chances of some pellets remaining after mouse (or any vertebrate) consumption to be able to track the effects of rain or invertebrate take. Plots near the Base were checked every 2–3 days between 12 July and 10 August, but this changed to as much as 3 weeks when we left Gough and the smaller team had limited capacity for additional work from 10 August. Plots in the highlands were always checked opportunistically, but this did not hinder comparisons, given the rate of degradation (see Results). At each check the number of pellets remaining in the plot was recorded, plus information on condition, invertebrate presence, and mould. A pellet was recorded as present if any amount remained, even just a few crumbs.

|
Degradation Iteration 2 mostly repeated the work described above, starting immediately after the third bait application (Table 2). Lowland plots had been devoid of pellets for several days when Iteration 2 began. The exception was one plot, where we continued to monitor the remaining pellets.
Bait availability
Monitoring bait availability was not part of the operational plan. Earlier trials raised no concerns (Wanless et al. 2008; Cuthbert et al. 2011b), and the scale of the island made comprehensive monitoring during the eradication unfeasible. However, opportunistic observations were made while conducting other work around the island. On 27 June (2 weeks after the first bait application), a casual bait search about 1 km west from Base resulted in zero bait found. This was unexpected, particularly after a recent check around the highlands had shown good bait coverage after several days of heavy rain (140 mm). We accessed the highlands by helicopter, so only checked high-altitude (≥600 m) areas. The low vegetation in the highlands makes looking for bait easy because it sits out in the open. To better assess bait availability in the lowlands, which has taller and denser vegetation, a bait search was organised.
Bait Search 1 took place on 5 July (23 days after the first bait application). The objective was to confirm presence or absence of bait over a wide area in the lowlands (Fig. 1). Thorough bait searches (e.g. within grass clumps, under ferns and trees, at seabird burrow entrances) were conducted by nine observers, divided into groups of three. Each observer meticulously searched 1-m2 plots 5–10 m apart. A total of 157 plots (157 m2) was checked in 60 min. Given the surprising absence of bait (see Results), we planned for a similar exercise after the second bait application.
Bait Search 2 took place closer to the application of bait, on 16 July (5 days after the second bait application). The search area and methods remained the same, but the number of observers increased to 15. Pellets greater than two-thirds of a standard pellet size were counted as whole pellets but fragments were not counted, thus allowing rapid observer movement between plots. In total, 298 plots (298 m2) were thoroughly searched in 60 min. Results were again surprising (see Results), and triggered a third bait application in this area, so we used the opportunity to monitor bait availability in greater detail.
Bait Search 3 commenced shortly after the third bait application. This time we aimed to understand both bait availability and daily bait consumption by non-target species immediately after baiting. Daily searches were conducted during 2–5 August (1–4 days after the third bait application), by which time mouse activity had been undetectable for weeks according to our camera monitoring (Samaniego et al. in press). We designed a simple protocol using the same general area used for the previous searches (Fig. 1). Each day, 11 plots (1 × 11 m) were thoroughly searched for bait (Fig. 1), i.e. 121 m2 searched each day. Each plot was divided into 11 equal squares (1 × 1 m) using 1-m PVC poles, with one observer per square. The number of bait pellets per plot was recorded (either as 0.5 for fragments or 1 for larger pieces), then the actual pellets were collected and replaced with fresh pellets to avoid creating bait gaps. Collecting the pellets allowed us to quantify bait density more accurately (by weighing the pellets) and caused minimum interference with the subsequent monitoring because the plots did not overlap (Fig. 1). Due to logistical limitations, pellets were pooled at the end of each day (i.e. without separating per plot). Pellets were dried for 48–72 h; control pellets (2.0 g) maintained their weight under these conditions. Once dry, pellets were weighed individually to the nearest 0.1 g using a digital scale accurate to 0.01 g. We calculated bait density per plot based on the respective number of pellets and the average pellet weight for each day. Daily bait availability rates were then calculated. We estimated daily bait consumption based on initial local application rates (which slightly deviated from target rates), according to GIS data (Table 1).
Slug trials
After the second bait application, field observations of deformed pellets and slugs eating bait triggered slug trials. Trials were conducted in the bird laboratory at Base. A preliminary trial observing individual slugs showed that a single slug would only eat a small amount of bait (<5% of a pellet in 5 days); therefore, trials were set up with groups of slugs.
Slug Trial 1 aimed to assess bait consumption by small groups of slugs. About 300 slugs were caught around the helipad (Fig. 1), and the 200 largest were selected for this trial. Twenty boxes (sealed plastic mouse stations with fresh wet grass as bedding and natural food) were used to house groups of 10 slugs each (~30–60 mm). Half the boxes received one fresh dry pellet; the other half received one wet pellet (i.e. a fresh pellet that had been soaked in water for 2 h, simulating rainy conditions). All pellets were 2.0 g when dry. We checked the boxes daily for 5 days, then the experiment was terminated because consumption slowed down. All remaining pellets were dried for 48 h, then weighed to 0.1 g. To obtain a crude estimate of slug abundance in the field, we divided the mean daily bait loss from Bait Search 3 (0.26 g m−2) by the mean slug consumption/day from this trial (consumption per box with wet pellets (0.8 g)/4 days/10 slugs = 0.02 g/slug/day).
Slug Trial 2 aimed to assess bait consumption by a large group of slugs. Of the 100 slugs left after Slug Trial 1 was set up, the next 60 largest (~15–29 mm) were placed in a 3-L plastic container with wet grass as bedding. The smallest slugs were discarded. Two bait pellets were placed in the container: one fresh and dry, one wet and soft collected from outdoors that had been weathered for ~10 days (unlike the fresh wet pellet in the first trial). A timelapse video was recorded overnight. Both pellets were collected the following day to be dried and weighed, then the experiment was terminated.
Slug Trial 3 aimed to assess time elapsed for slug eggs to hatch. Rainy weather meant time between bait applications was taking longer than planned, so we wanted to confirm if an increase in slug abundance during the mouse eradication was a possibility. About 100 of the eggs found in the boxes used for the Slug Trial 2 were placed in two Petri dishes with a wet cotton dressing on 27 July. Collections were from random boxes and species could not be distinguished. Small holes were drilled in the covers of both petri dishes and then put on a shelf with dim light and at room temperature. We were unclear if these were favourable conditions for slug eggs to hatch, because limited internet access at Base hampered on-line research.
Results
Bait degradation
On average over Iterations 1 and 2, complete bait degradation in the lowlands (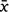 = 16.9 ± 7.2 days; range: 12–38 days) was evidently faster than in the highlands (
= 16.9 ± 7.2 days; range: 12–38 days) was evidently faster than in the highlands (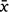 = 97 ± 50 days; range: 33–155 days). In lowland plots, pellets among thick ground-level vegetation (e.g. H. incisa ferns) degraded faster than pellets on more exposed conditions; indeed, bait persisted the longest on top of Scirpus, where it was exposed to drying wind and less accesible to invertebrates (Table 2, Fig. 2). Non-target invertebrate consumers, particularly millipedes and slugs, were consistently noted on pellets in the lowlands to varying degrees, but rarely in the highlands (i.e. only one small slug once).
= 97 ± 50 days; range: 33–155 days). In lowland plots, pellets among thick ground-level vegetation (e.g. H. incisa ferns) degraded faster than pellets on more exposed conditions; indeed, bait persisted the longest on top of Scirpus, where it was exposed to drying wind and less accesible to invertebrates (Table 2, Fig. 2). Non-target invertebrate consumers, particularly millipedes and slugs, were consistently noted on pellets in the lowlands to varying degrees, but rarely in the highlands (i.e. only one small slug once).
|
|
Bait availability
Bait Search 1 (23 days after the first bait application) recorded zero bait availability because no bait pellets nor fragments were found, meaning the 10.5 kg ha−1 had completely dissapeared. We also saw no bait pellets when walking between plots and to and from Base.
Bait Search 2 (5 days after the second bait application) resulted in low bait availability. Most plots (90.3%) had no bait at all; the average number of pellets was low (0.1 ± 0.4 pellets m−2; range 0–3). This translates to an average of 2 kg ha−1, with patchy distribution. Most pellets were found under Phylica trees, which is both the driest and most open microhabitat at ground level (i.e. probably less suitable for slugs). Based on a local bait application rate of 11.7 kg ha−1 (Table 1), the rate of bait disappearance (~10 kg ha−1 in five nights, a drop of 85%) was considerably higher than expected based on our experience with multiple rodent eradications. Again, observations made when we walked between plots confirmed the rapid rate of bait disappearance.
Bait Search 3 (1–4 days after the third bait application) was also surprising, with substantial declines in bait availability during the first 3 days (Fig. 3) despite using a slightly higher bait rate (13.4 kg ha−1). Bait consumption rates, after correcting for differences in initial local application rates (according to GIS), were 2.5 kg ha−1, 3.0 kg ha−1, 2.7 kg ha−1, and 2.3 kg ha−1 for days 1–4, respectively. Combined, about 10.5 kg ha−1 (~81%) of bait disappeared in four nights in the lowlands after the third bait application.
|
|
Slug trials
Slug Trial 1 (small groups)
At Days 1 and 2, consumption of wet pellets was evidently higher than of dry pellets. However, by Day 4 when the dry pellets were wet and soft, the difference was less obvious. All slugs (n = 200) turned green (presumably due to the green dye from the bait), and all boxes had abundant green faeces. Consumption of bait appeared to slow down at Day 4, and the following day (last day) there was no noticeable change in the amount of bait left. After 5 days, slugs had consumed about 40% of the wet pellets (final mean weight: 1.16 ± 0.22 g) and about 30% of the dry pellets (final mean weight: 1.39 ± 0.15 g). The shape of partially eaten pellets, particularly wet pellets, resembled the shape of degraded pellets commonly found outdoors (Fig. 4). The crude estimation of slug density (daily bait consumption in the field divided by daily consumption/slug) resulted in an estimate of 13 slugs m−2 in the lowlands of Gough. This is in line with observations made by the team collecting slugs in preparation for the Slug Trial 1.
|
|
Slug Trial 2 (large group)
The timelapse video (YouTube: slugs eating rodent bait; https://www.youtube.com/shorts/8VMyCyn4NoE) shows how abundant small slugs (n = 60) can quickly eat bait pellets, in this case 70% of the fresh dry pellet and 10% of the weathered pellet, in one night (Fig. 5).
|
|
Slug Trial 3 (slug eggs)
The eggs in Petri dishes hatched 26–34 days later, and hatching success was high (>90%).
Discussion
Island rodent eradications are increasing in size, complexity, and variety of habitats targeted (Howald et al. 2007; Martin and Richardson 2017; Samaniego et al. 2018; Broome et al. 2019). At 6500 ha the operation on Gough represents a major increase in mouse eradication attempts on islands where mice are the only introduced mammal species. Even larger projects targeting mice are being planned (e.g. Marion Island (29 000 ha); Preston et al. 2019). Learning from past successes and failures, while preparing for novel challenges, is crucial to maintain the astounding level of rodent eradication success achieved in recent years (Samaniego et al. 2021). The Gough Island mouse eradication is a good example of a challenging project that, despite years of preparation, faced unexpected challenges ‘on the day’ and benefited from having experienced practitioners on the ground to both identify and address the challenges. In hindsight, greater overlap between the teams planning for and implementing the mouse eradication would have also been beneficial.
Initially, for the Gough operation we were concerned about bait availability in the highlands because of the steep terrain and the exposed bait on short vegetation potentially receiving heavy rain. However, bait degradation trials and casual checks proved that, consumption aside, bait in the lowlands lasted for 2–5 weeks, compared with 9–18 weeks in the highlands, despite heavy rain (837 mm in June–August). The longevity of bait in the highlands may be due to the drying effect of near constant wind in higher altitudes, and the low abundance and/or activity of invertebrates and soil microorganisms.
Low bait consumption rates (by target and non-target species) were expected in the highlands because both mice and invertebrates are typically less abundant here (Rowe-Rowe and Crafford 1992; Jones et al. 2002). Indeed, a far greater number of invertebrates was observed on pellets in the lowlands than in the highlands, where only a single small slug and few millipedes were noted during checks of the bait degradation plots.
In contrast, the lower initial concern regarding bait availability in the lowlands switched to high concern shortly after the first bait application. Consumption by mice and mechanical breakdown by rain were the main expected contributors to bait disappearance, with bait consumption by non-target species considered negligible after earlier trials (Wanless et al. 2008). However, shortly after the first bait application we observed a marked difference between the highlands (abundant bait in good condition) and the lowlands (no bait). Such a difference in bait availability was unexpected and concerning, and well beyond the bait removal rate from the expected (and observed) higher mouse abundance in the lowlands. Based on prior experience with rodent eradications, we suspected there were other factors at play.
After the second bait application, about 10 kg ha−1 disappeared in 5 days in the lowlands. The low density (2 kg ha−1) and highly patchy distribution of the bait remaining on the ground was concerning, because it potentially challenged the principle of ensuring that every mouse had access to bait. Concurrent bait degradation plots were indicating that rain was not breaking down the bait at that rate. Conversely, our slug trials and field observations were indicating that slugs were a significant cause of bait disappearance. Mouse consumption was no longer a factor – monitoring (using cameras and flavoured detection devices) indicated the mouse population had dramatically declined and was close to zero density before the second bait application (Samaniego et al. in press).
Interestingly, after the third bait application the weather was much drier (no rain for the first 3 days after application) and bait consumption was higher. About 10.5 kg ha−1 of bait disappeared in 4 days in the lowlands, with only negligible precipitation, confirming that consumption by non-target species, rather than rain, was the main cause of bait disappearance. Indeed, our results demonstrate that non-native slugs were heavily involved in the rapid declines of bait availability throughout the mouse eradication attempt on Gough. No avian species was sufficiently abundant to account for the scale of bait uptake, nor frequented the habitat where most bait disappearance occurred. Several other species of invertebrates, of which non-native millipedes were by far the most commonly seen around bait, were also bait consumers. However, we strongly believe this was of secondary importance compared with consumption by slugs. Observations of pellets constantly covered by millipedes (Supplementary Material) indicate their rate of consumption was very slow. However, all bait consumption rates discussed here are only indicative and deserve further investigation.
Bait consumption by slugs was critically overlooked during planning trials, and this is an important lesson for future eradication projects. Had this been previously identified, it is likely to have altered the baiting strategy in the planning phase, i.e. the initial bait rate over the higher slug density areas would have been increased to allow for it. The third bait application in the lowlands was triggered by identification of slug interference. One reason for overlooking slugs is their mainly nocturnal behaviour. In addition, Harper et al. (2020) noted that, to the untrained eye, damage to bait by slugs can be confused with rat or mice damage. Yet, the way slugs eat and deform pellets (Supplementary Material) allows trained people to detect slug activity, which is how we first detected the issue on Gough.
From the slug trials we also learned that bait consumption is heavily influenced by slug abundance. Individual slugs eat little per day, but a small group of slugs can eat about 40% of a 2-g bait pellet in a few days. A larger group of small slugs was video recorded almost consuming a pellet overnight. Despite their nocturnal preferences (to prevent water loss), groups of up to eight small slugs were observed feeding on a single pellet in the field during rainy days. Spurr and Drew (1999) studied invertebrates feeding on baits and reported more invertebrate activity during the night. We had no time to assess slug density in the field, but our results suggest that slugs in the lowlands of Gough were abundant. Little is published about slug density and diversity on wild and protected areas (Moss and Hermanutz 2010). However, our observations while collecting slugs and our crude estimate of slug density of 13 slugs m−2 are over an order of magnitude lower than the slug density that has been reported on Marion Island (up to 344 slugs m−2) (Lee et al. 2009). This means that slug abundances that can go unnoticed could still lead to significant bait consumption, potentially jeopardising eradication efforts. In fact, at the time of submission (February 2022) mice had been recently recorded on Gough, indicating the eradication had been unsuccessful, and the causes are being investigated (A. Callender, pers. comm.). The relative rates at which mice and slugs consume bait (along with possible toxicant pathways through slugs) are poorly understood; therefore, more research regarding time required with bait on the ground to allow mice to consume a lethal dose is needed. The mouse monitoring conducted on Gough suggests most mice ate bait within days despite slug presence (Samaniego et al. in press). However, all the mouse, slug, and bait monitoring was conducted in an area (south-east part of Gough) with apparently only one slug species (A. valentianus). Whether this varies around the island or whether D. reticulatum, a major pest worldwide (CABI 2021) and reported for Gough, is in fact present (adding to slug abundance and in turn accelerating bait disappearance in other areas) is unclear.
The strong preference for the fresh pellet (drier, harder) over the weathered one (softer, older) during the second slug trial was surprising, given the preference for wet pellets during the first trial. The key difference between trials is the level of freshness of the wet pellet. It is likely that a fresh soaked pellet has a stronger smell than a pellet weathered outdoors for days, but the underlying reasons deserve further investigation. We also recommend further field and laboratory trials considering species, body sizes, and levels of food deprivation of the slugs. After all, our laboratory trials were conducted after slugs had been exposed to two aerial bait applications. Moreover, both laboratory and field results suggest a potential satiation effect after 4–5 days with ad libitum food. All our monitoring protocols can and should be improved. Our design was improvised and limited by time and resources.
Slug eggs were commonly observed in the laboratory and outdoors. Whether slug breeding was due to the season (rainy winter) or to the novel, abundant food in the form of rodent bait, or both, is uncertain. In any case, we recorded slug eggs hatching from 26 days and the mature slugs were evidently not susceptible to brodifacoum. An interval of 50 days between the first and third bait applications (longer than expected, due to poor weather) potentially allowed an increase in slug abundance, which would explain the higher bait consumption rate after the third application. In future, this should be considered when deciding bait application intervals for projects facing similar issues. Additional open questions with important implications are regularity of slug breeding cycles, and average and maximum slug densities across habitat types.
Molluscs, as for invertebrates in general, are considered to be of low susceptibility to brodifacoum (Brooke et al. 2011; Broome et al. 2017). However, acknowledging that molluscs are phylogenetically and ecophysiologically highly diverse, potential susceptibility of local fauna should be examined and caution exercised when planning rodent eradications (Parent et al. 2019).
Some slugs species have been found not to be preferred food by house mice (Smith et al. 2002), but slug species vary greatly in their defensive mucus physical and chemical properties (G.M. Barker, pers. comm.). More research is needed to understand if secondary poisoning of mice through slug (or mollusc) consumption could be at play on some islands. Palatability of slimy pellets to rodents also deserves further study, but our experience on Gough, where mice certainly ate pellets in areas of high slug abundance, suggests this may not be a significant issue for aerial bait applications.
Slugs of several species have been found to interfere with rodent bait in bait stations during eradication operations (Taylor et al. 2000; Bell et al. 2019; Main et al. 2019; Harper et al. 2020) and control programmes (Kawelo et al. 2012). However, because bait station operations require multiple checks there is opportunity to replenish bait and manage the issue, as evidenced by the eradication successes. We notice that mitigation measures like wrapping bait blocks in plastic (e.g. Taylor et al. 2000) or spreading slug bait outside bait stations (Harper et al. 2020) have been used in the final stages of some operations. Slugs (among other invertebrates) collected from baits on Langara Island tested positive for brodifacoum (Howald 1997). Slugs and snails were found to be responsible for the breakdown of the leftover bait after the aerial baiting on Motutapu Island in New Zealand, a process that took less than a month in grassland (where slugs are abundant), and up to 10 months in rocky areas with fewer invertebrates (Griffiths et al. 2013). On subantarctic Macquarie Island slugs are present (Houghton et al. 2019), but no interference with the aerial eradication was reported (Springer 2016). To our knowledge, this is the first report of slug interference during an aerial rodent eradication. Climate change may be facilitating slug number increases in areas that were previously too cold for them (Lee et al. 2009). Fortunately, slug repellents and control strategies for protected areas are being investigated (Bogardus et al. 2020; S. Joe, pers. comm.), given invasive slugs are also under-appreciated obstacles to rare plant restoration (e.g. Joe and Daehler 2008) and food production.
Conclusions
The unexpected bait consumption by slugs in fern bush habitat on Gough Island caused rapid decline in bait availability for mice and reduction of the size of toxic bait pellets, meaning that some mice may have had to encounter and eat more pellets to get a lethal dose. We addressed the issue on Gough thanks to the combination of our eradication experience and the generous allocation of both time and contingency bait for the operation. Nevertheless, our efforts with the resources at hand were insufficient – mice are still present on Gough. Whether slug interference and/or other factors were involved is being investigated, although it may never be confirmed. Our findings highlight the importance of understanding degradation factors and non-target consumers in developing a baiting strategy for future rodent eradications attempts on Gough and elsewhere. Practitioners should not assume slugs will be the primary invertebrate consumer, that bait availability will be constant across different vegetation types on the same island, or that bait will always persist longer in the highlands, even on similar islands to Gough. Instead, the planning process should ensure the particular conditions and risks on each specific island are understood and properly managed. The ecology of remote islands with a simple assemblage of species and a range of invasive species may be difficult to predict. Moreover, some issues may only become apparent on specific sites, seasons, or weather conditions. Having experienced eradication practitioners at all phases of planning and implementation, including the design, completion, and analysis of trials, is crucial.
Supplementary material
Supplementary material is available online.
Data availability
The data that support this study are available in the article and accompanying online supplementary material.
Conflicts of interest
This work was conducted during the implementation of the Gough Island mouse eradication, led by the Royal Society for the Protection of Birds. The authors formed the operational decision group of the eradication operation (Operations Manager: Pete McClelland; Technical Advisor: Araceli Samaniego; Operations Assistant: Wes Jolley).
Declaration of funding
The Royal Society for the Protection of Birds and its partners were funded mainly by the National Fish and Wildlife Foundation, The David & Lucile Packard Foundation, Ludwick Family Foundation, John Ellerman Foundation, and Garfield Weston Foundation to plan and implement the Gough Island Restoration Programme. This research was conducted as part of the programme. Additional funding for writing of the manuscript was provided by Manaaki Whenua – Landcare Research and Island Conservation.
Acknowledgements
We thank all the Gough Island Restoration Programme team members for their important field contributions and enthusiasm. The overwintering team, Kim Stevens, Vonica Perold, and Roelf Daling, completed the bait degradation monitoring when we left Gough, and kindly shared the limited laboratory spaces and equipment during our stay. Amy King watched over the slug eggs when we left Gough. Special thanks to Katie Milne for the fine maps, to Alexis Osborne, Craig Thomas, and George Lemann for their field and laboratory contributions, and to Sam Gandhi for video editing. The South African National Antarctic Programme (SANAP) and the Department of Forestry, Fisheries, and the Environment (DFFE) kindly allowed the use of their Base on Gough Island. We are grateful to Gary M. Barker for his species identification work and his valuable insights from the mollusc world. Steffen Oppel, Keith Springer, Gary M. Barker, and Thomas Buckley provided useful comments on an earlier draft. Anne Austin provided editorial input. We thank the two anonymous reviewers and editors for their useful feedback.
References
Angel A, Cooper J (2006) A review of the impacts of introduced rodents on the islands of Tristan da Cunha and Gough. RSPB Research Report No. 17. Royal Society for the Protection of Birds, Sandy, Bedfordshire, UK.Angel, A, Wanless, RM, and Cooper, J (2009). Review of impacts of the introduced house mouse on islands in the Southern Ocean: are mice equivalent to rats? Biological Invasions 11, 1743–1754.
| Review of impacts of the introduced house mouse on islands in the Southern Ocean: are mice equivalent to rats?Crossref | GoogleScholarGoogle Scholar |
Bell E, Floyd K, Boyle D, Pearson J, St Pierre P, Lock L, Buckley P, Mason S, McCarthy R, Garratt W, Sugar K, Pearce J (2019) The Isles of Scilly seabird restoration project: the eradication of brown rats (Rattus norvegicus) from the inhabited islands of St Agnes and Gugh, Isles of Scilly. In ‘Island Invasives: Scaling up to Meet the Challenge’. (Eds CR Veitch, MN Clout, AR Martin, JC Russell, CJ West) pp. 88–94. (IUCN: Gland, Switzerland)
Bogardus T, Joe SM, Shiels AB (2020) Development of a rodent bait with slug-repellent properties. In ‘Proceedings of the 29th Vertebrate Pest Conference’. (Ed. DM Woods) Paper no. 14. (University of California: Santa Barbara, CA, USA)
Brooke, MdL, Cuthbert, RJ, Mateo, R, and Taggart, MA (2011). An experimental test of the toxicity of cereal pellets containing brodifacoum to the snails of Henderson Island, South Pacific. Wildlife Research 38, 34–38.
| An experimental test of the toxicity of cereal pellets containing brodifacoum to the snails of Henderson Island, South Pacific.Crossref | GoogleScholarGoogle Scholar |
Broome KG, Fairweather AAC, Fisher P (2017) ‘Brodifacoum Pesticide Information Review. Version 2017/1.’ (New Zealand Department of Conservation: Hamilton, New Zealand)
Broome K, Brown D, Brown K, Murphy E, Birmingham C, Golding C, Corson P, Cox A, Griffiths R (2019) House mice on islands: management and lessons from New Zealand. In ‘Island Invasives: Scaling Up to Meet the Challenge’. (Eds CR Veitch, MN Clout, AR Martin, JC Russell, CJ West) pp. 100–107. (IUCN: Gland, Switzerland)
CABI (2021) Deroceras reticulatum (grey field slug). In ‘Invasive Species Compendium’. (CAB International: Wallingford, UK) Available at https://www.cabi.org/isc/datasheet/85752 [Verified June 2022]
Caravaggi, A, Cuthbert, RJ, Ryan, PG, Cooper, J, and Bond, AL (2019). The impacts of introduced house mice on the breeding success of nesting seabirds on Gough Island. Ibis 161, 648–661.
| The impacts of introduced house mice on the breeding success of nesting seabirds on Gough Island.Crossref | GoogleScholarGoogle Scholar |
Cuthbert, R, and Hilton, G (2004). Introduced house mice Mus musculus: a significant predator of threatened and endemic birds on Gough Island, South Atlantic Ocean? Biological Conservation 117, 483–489.
| Introduced house mice Mus musculus: a significant predator of threatened and endemic birds on Gough Island, South Atlantic Ocean?Crossref | GoogleScholarGoogle Scholar |
Cuthbert R, Visser P, Louw H, Rexer-Huber K, Parker G, Ryan P (2011a) Preparations for the eradication of mice from Gough Island: results of bait acceptance trials above ground and around cave systems. In ‘Island Invasives: Eradication and Management’. (Eds CR Veitch, MN Clout, DR Towns) pp. 47–50. (IUCN: Gland, Switzerland)
Cuthbert, RJ, Visser, P, Louw, H, and Ryan, PG (2011b). Palatability and efficacy of rodent baits for eradicating house mice (Mus musculus) from Gough Island, Tristan da Cunha. Wildlife Research 38, 196–203.
| Palatability and efficacy of rodent baits for eradicating house mice (Mus musculus) from Gough Island, Tristan da Cunha.Crossref | GoogleScholarGoogle Scholar |
Cuthbert, RJ, Louw, H, Lurling, J, Parker, G, Rexer-Huber, K, Sommer, E, Visser, P, and Ryan, PG (2013). Low burrow occupancy and breeding success of burrowing petrels at Gough Island: a consequence of mouse predation. Bird Conservation International 23, 113–124.
| Low burrow occupancy and breeding success of burrowing petrels at Gough Island: a consequence of mouse predation.Crossref | GoogleScholarGoogle Scholar |
Cuthbert, RJ, Broome, K, Bradley, J, and Ryan, PG (2014). Evaluating the effectiveness of aerial baiting operations for rodent eradications on cliffs on Gough Island, Tristan da Cunha. Conservation Evidence 11, 25–28.
Davies, D, Dilley, BJ, Bond, AL, Cuthbert, RJ, and Ryan, PG (2015). Trends and tactics of mouse predation on Tristan albatross Diomedea dabbenena chicks at Gough Island, South Atlantic Ocean. Avian Conservation and Ecology 10, 5.
| Trends and tactics of mouse predation on Tristan albatross Diomedea dabbenena chicks at Gough Island, South Atlantic Ocean.Crossref | GoogleScholarGoogle Scholar |
Dilley, BJ, Davies, D, Bond, AL, and Ryan, PG (2015). Effects of mouse predation on burrowing petrel chicks at Gough Island. Antarctic Science 27, 543–553.
| Effects of mouse predation on burrowing petrel chicks at Gough Island.Crossref | GoogleScholarGoogle Scholar |
Griffiths R, Broome K, Brown P, Buchanan F (2013) Multispecies invasive mammal eradication on Rangitoto and Motutapu Islands, Hauraki Gulf New Zealand. Department of Conservation Internal Report DOCDM-898404, Wellington, New Zealand.
Hänel C, Chown S, Gaston KJ (2005) ‘Gough Island: A Natural History.’ (SUN Press: Stellenbosch, South Africa)
Harper GA, Pahor S, Birch D (2020) The Lord Howe Island rodent eradication: lessons learnt from an inhabited island. In ‘Proceedings of the 29th Vertebrate Pest Conference’. (Ed. DM Woods) pp. 1–11. (University of California: Santa Barbara, CA, USA)
Houghton M, Terauds A, Shaw JD (2019) Methods for monitoring invertebrate response to vertebrate eradication. In ‘Island Invasives: Scaling Up to Meet the Challenge’. (Eds CR Veitch, MN Clout, AR Martin, JC Russell, CJ West) pp. 381–388. (IUCN: Gland, Switzerland)
Howald G (1997) ‘The Risk of Non-target Species Poisoning from Brodifacoum used to Eradicate Rats from Langara Island, British Columbia, Canada.’ (The University of Brithish Columbia: Vancouver, BC, Canada)
Howald, G, Donlan, CJ, Galván, JP, Russell, JC, Parkes, J, Samaniego, A, Wang, Y, Veitch, D, Genovesi, P, Pascal, M, Saunders, A, and Tershy, B (2007). Invasive rodent eradication on islands. Conservation Biology 21, 1258–1268.
| Invasive rodent eradication on islands.Crossref | GoogleScholarGoogle Scholar |
Hutchinson, J, Reise, H, and Robinson, D (2014). A biography of an invasive terrestrial slug: the spread, distribution and habitat of Deroceras invadens. NeoBiota 23, 17–64.
| A biography of an invasive terrestrial slug: the spread, distribution and habitat of Deroceras invadens.Crossref | GoogleScholarGoogle Scholar |
Joe, SM, and Daehler, CC (2008). Invasive slugs as under-appreciated obstacles to rare plant restoration: evidence from the Hawaiian Islands. Biological Invasions 10, 245–255.
| Invasive slugs as under-appreciated obstacles to rare plant restoration: evidence from the Hawaiian Islands.Crossref | GoogleScholarGoogle Scholar |
Jones, A, Chown, S, and Gaston, K (2002). Terrestrial invertebrates of Gough Island: an assemblage under threat? African Entomology 10, 83–91.
Jones, HP, Holmes, ND, Butchart, SHM, Tershy, BR, Kappes, PJ, Corkery, I, Aguirre-Muñoz, A, Armstrong, DP, Bonnaud, E, Burbidge, AA, Campbell, K, Courchamp, F, Cowan, PE, Cuthbert, RJ, Ebbert, S, Genovesi, P, Howald, GR, Keitt, BS, Kress, SW, Miskelly, CM, Oppel, S, Poncet, S, Rauzon, MJ, Rocamora, G, Russell, JC, Samaniego-Herrera, A, Seddon, PJ, Spatz, DR, Towns, DR, and Croll, DA (2016). Invasive mammal eradication on islands results in substantial conservation gains. Proceedings of the National Academy of Sciences of the United States of America 113, 4033–4038.
| Invasive mammal eradication on islands results in substantial conservation gains.Crossref | GoogleScholarGoogle Scholar |
Kawelo HK, Harbin SC, Joe SM, Keir MJ, Weisenberger L (2012) Unique reintroduction considerations in Hawaii: case studies from a decade of rare plant restoration at the Oahu army natural resource rare plant program. In ‘Plant Reintroduction in a Changing Climate: Promises and Perils’. (Eds J Maschinski, KE Haskins, PH Raven) pp. 209–226. (Island Press/Center for Resource Economics: Washington, DC, USA)
Keitt, B, Griffiths, R, Boudjelas, S, Broome, K, Cranwell, S, Millett, J, Pitt, W, and Samaniego-Herrera, A (2015). Best practice guidelines for rat eradication on tropical islands. Biological Conservation 185, 17–26.
| Best practice guidelines for rat eradication on tropical islands.Crossref | GoogleScholarGoogle Scholar |
Lee, JE, Janion, C, Marais, E, Jansen van Vuuren, B, and Chown, SL (2009). Physiological tolerances account for range limits and abundance structure in an invasive slug. Proceedings of the Royal Society B: Biological Sciences 276, 1459–1468.
| Physiological tolerances account for range limits and abundance structure in an invasive slug.Crossref | GoogleScholarGoogle Scholar |
Main CE, Bell E, Floyd K, Tayton J, Ibbotson J, Whittington W, Taylor PR, Reid R, Varnham K, Churchyard T, Bambini L (2019) Scaling down (cliffs) to meet the challenge: the Shiants’ black rat eradication. In ‘Island Invasives: Scaling Up to Meet the Challenge’. (Eds CR Veitch, MN Clout, AR Martin, JC Russell, CJ West) pp. 138–146. (IUCN: Gland, Switzerland)
Martin, AR, and Richardson, MG (2017). Rodent eradication scaled up: clearing rats and mice from South Georgia. Oryx 53, 27–35.
| Rodent eradication scaled up: clearing rats and mice from South Georgia.Crossref | GoogleScholarGoogle Scholar |
McClelland P (2021) ‘Gough Island Restoration Programme: Operational Plan for the Eradication of House Mice from Gough Island, Tristan da Cunha.’ (Royal Society for the Protection of Birds: Sandy, Bedfordshire, UK)
Moss, M, and Hermanutz, L (2010). Monitoring the small and slimy – protected areas should be monitoring native and non-native slugs (Mollusca: Gastropoda). Natural Areas Journal 30, 322–327.
| Monitoring the small and slimy – protected areas should be monitoring native and non-native slugs (Mollusca: Gastropoda).Crossref | GoogleScholarGoogle Scholar |
Parent CE, Fisher P, Jolley W, Alifano A, Campbell KJ (2019) Assessment of snail exposure to the anticoagulant rodenticide brodifacoum in the Galapagos Islands. In ‘Island Invasives: Scaling Up to Meet the Challenge’. (Eds CR Veitch, MN Clout, AR Martin, JC Russell, CJ West) pp. 394–399. (IUCN: Gland, Switzerland)
Preece, R (2001). Introduced land molluscs on the islands of the Tristan da Cunha-Gough group (South Atlantic). Journal of Conchology 37, 253–259.
Preston GR, Dilley BJ, Cooper J, Beaumont J, Chauke F, Chown SL, Devanunthan N, Dopolo M, Fikizolo L, Heine J, Henderson S, Jacobs CA, Johnson F, Kelly J, Makhado AB, Marais C, Maroga J, Mayekiso M, McClelland G, Mphepya J, Muir D, Ngcaba N, Ngcobo N, Parkes JP, Paulsen F, Schoombie S, Springer K, Stringer C, Valentine H, Wanless RM, Ryan PG (2019) South Africa works towards eradicating introduced house mice from sub-Antarctic Marion Island: the largest island yet attempted for mice. In ‘Island Invasives: Scaling Up to Meet the Challenge’. (Eds CR Veitch, MN Clout, AR Martin, JC Russell, CJ West) pp. 40–46. (IUCN: Gland, Switzerland)
Rowe-Rowe, DT, and Crafford, JE (1992). Density, body size, and reproduction of feral house mice on Gough Island. South African Journal of Zoology 27, 1–5.
| Density, body size, and reproduction of feral house mice on Gough Island.Crossref | GoogleScholarGoogle Scholar |
Russell, JC, and Broome, KG (2016). Fifty years of rodent eradications in New Zealand: another decade of advances. New Zealand Journal of Ecology 40, 197–204.
| Fifty years of rodent eradications in New Zealand: another decade of advances.Crossref | GoogleScholarGoogle Scholar |
Samaniego, A, Aguirre-Muñoz, A, Bedolla-Guzmán, Y, Cárdenas-Tapia, A, Félix-Lizárraga, M, Méndez-Sánchez, F, Reina-Ponce, O, Rojas-Mayoral, E, and Torres-García, F (2018). Eradicating invasive rodents from wet and dry tropical islands in Mexico. Oryx 52, 559–570.
| Eradicating invasive rodents from wet and dry tropical islands in Mexico.Crossref | GoogleScholarGoogle Scholar |
Samaniego, A, Kappes, P, Broome, K, Cranwell, S, Griffiths, R, Harper, G, McClelland, P, Palmer, R, Rocamora, G, Springer, K, Will, D, and Siers, S (2021). Factors leading to successful island rodent eradications following initial failure. Conservation Science and Practice 3, e404.
| Factors leading to successful island rodent eradications following initial failure.Crossref | GoogleScholarGoogle Scholar |
Samaniego, A, Stevens, K, Perold, V, Oppel, S, and McClelland, P (). Detections of house mice on Gough Island approach zero within days of aerial baiting. Wildlife Research , .
| Detections of house mice on Gough Island approach zero within days of aerial baiting.Crossref | GoogleScholarGoogle Scholar |
Shiels, AB, Pitt, WC, Sugihara, RT, and Witmer, GW (2014). Biology and impacts of Pacific island invasive species. 11. Rattus rattus, The black rat (Rodentia, Muridae). Pacific Science 68, 145–184.
| Biology and impacts of Pacific island invasive species. 11. Rattus rattus, The black rat (Rodentia, Muridae).Crossref | GoogleScholarGoogle Scholar |
Smith, VR, Avenant, NL, and Chown, SL (2002). The diet and impact of house mice on a sub-Antarctic island. Polar Biology 25, 703–715.
| The diet and impact of house mice on a sub-Antarctic island.Crossref | GoogleScholarGoogle Scholar |
Springer, K (2016). Methodology and challenges of a complex multi-species eradication in the sub-Antarctic and immediate effects of invasive species removal. New Zealand Journal of Ecology 40, 273–278.
| Methodology and challenges of a complex multi-species eradication in the sub-Antarctic and immediate effects of invasive species removal.Crossref | GoogleScholarGoogle Scholar |
Spurr, EB, and Drew, KW (1999). Invertebrates feeding on bait used for vertebrate pest control in New Zeland. New Zealand Journal of Ecology 23, 167–173.
Taylor, RH, Kaiser, GW, and Drever, MC (2000). Eradication of Norway rats for recovery of seabird habitat on Langara Island, British Columbia. Restoration Ecology 8, 151–160.
| Eradication of Norway rats for recovery of seabird habitat on Langara Island, British Columbia.Crossref | GoogleScholarGoogle Scholar |
Towns, DR, Atkinson, IAE, and Daugherty, CH (2006). Have the harmful effects of introduced rats on islands been exaggerated? Biological Invasions 8, 863–891.
| Have the harmful effects of introduced rats on islands been exaggerated?Crossref | GoogleScholarGoogle Scholar |
Wace, NM (1961). The vegetation of Gough Island. Ecological Monographs 31, 337–367.
| The vegetation of Gough Island.Crossref | GoogleScholarGoogle Scholar |
Wanless, RM, Angel, A, Cuthbert, RJ, Hilton, GM, and Ryan, PG (2007). Can predation by invasive mice drive seabird extinctions? Biology Letters 3, 241–244.
| Can predation by invasive mice drive seabird extinctions?Crossref | GoogleScholarGoogle Scholar |
Wanless, RM, Fisher, P, Cooper, J, Parkes, J, Ryan, PG, and Slabber, M (2008). Bait acceptance by house mice: an island field trial. Wildlife Research 35, 806–811.
| Bait acceptance by house mice: an island field trial.Crossref | GoogleScholarGoogle Scholar |
Wanless, RM, Cooper, J, Slabber, MJ, and Ryan, PG (2010). Risk assessment of birds foraging terrestrially at Marion and Gough Islands to primary and secondary poisoning by rodenticides. Wildlife Research 37, 524–530.
| Risk assessment of birds foraging terrestrially at Marion and Gough Islands to primary and secondary poisoning by rodenticides.Crossref | GoogleScholarGoogle Scholar |


