A comparison of methods for monitoring a sparse population of the red fox (Vulpes vulpes) subject to lethal control using GPS telemetry, camera traps and sand plots
Andrew Carter A B * , Joanne M. Potts C , Joanne Stephens A and David A. Roshier
A B * , Joanne M. Potts C , Joanne Stephens A and David A. Roshier  A D
A D
A Australian Wildlife Conservancy, PO Box 8070, Subiaco East, WA 6008, Australia.
B Gulbali Institute of Agriculture, Water and Environment, Charles Sturt University, PO Box 789, Albury, NSW 2640, Australia.
C The Analytical Edge Pty Ltd, PO Box 47, Blackmans Bay, Tas. 7052, Australia.
D School of Animal and Veterinary Science, University of Adelaide, Roseworthy, SA 5371, Australia.
Wildlife Research 50(5) 366-380 https://doi.org/10.1071/WR22017
Submitted: 5 February 2022 Accepted: 23 August 2022 Published: 14 October 2022
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: The introduced red fox has driven the decline or extinction of numerous wildlife species in Australia, yet little information exists on the population densities of foxes in most ecosystems. Fox monitoring programs will differ widely depending on the goals of management, which, in turn, will determine whether the appropriate metric is a density estimate, or some proxy thereof, and the time and resources required.
Aims: This study aims to assist wildlife managers to design fit-for-purpose monitoring programs for foxes by providing a better understanding of the utility and precision of various monitoring methods.
Methods: We surveyed foxes monthly over four consecutive years in a semi-arid region of Australia by using sand plots, camera traps and GPS telemetry. The resultant data were used to produce population estimates from one count-based method, two spatially explicit methods, and two activity indices.
Key results: The incorporation of GPS-collar data into the spatial capture–recapture approaches greatly reduced uncertainty in estimates of abundance. Activity indices from sand plots were generally higher and more variable than were indices derived from camera traps, whereas estimates from N-mixture models appeared to be biased high.
Conclusions: Our study indicated that the Allen–Engeman index derived from camera-trap data provided an accurate reflection of change in the underlying fox density, even as density declined towards zero following introduction of lethal control. This method provides an efficient means to detect large shifts in abundance, whether up or down, which may trigger a change to more laborious, but precise, population monitoring methods. If accuracy is paramount (e.g. for reintroduction programs) spatially explicit methods augmented with GPS data provide robust estimates, albeit at a greater cost in resources and expertise than does an index.
Implications: Our study demonstrated that the shorter the survey period is, the greater is the likelihood that foxes are present but not detected. As such, if limited resources are available, longer monitoring periods conducted less frequently will provide a more accurate reflection of the underlying fox population than do shorter monitoring periods conducted more often.
Keywords: canid pest ejector, fox baiting, mark–resight, N-mixture model, population index, predator control, sodium fluoroacetate, spatially explicit.
Introduction
Since its introduction to Australia in the 1800s (Rolls 1984), the red fox (‘fox’, Vulpes vulpes) has become one of the country’s most destructive mammalian predators. The fox has driven the decline or extinction of numerous species of endemic terrestrial mammals (Woinarski et al. 2015; Doherty et al. 2016) and is also regarded as a major predator of livestock (Saunders et al. 1995; Saunders and McLeod 2007). The most recent economic figures have estimated that foxes cost the Australian economy approximately US$500 million annually (Bradshaw et al. 2021) through direct loss and damage, as well as for management interventions. Nevertheless, despite the impact of foxes in Australia, in most ecosystems, little information exists on their population densities.
The specific objectives of a fox monitoring program will differ widely depending on the goals of management, which, in turn, will influence the time and resources that monitoring efforts demand. For instance, a predator eradication program conducted inside a fenced reserve prior to the reintroduction of endangered wildlife species (e.g. Legge et al. 2018; Ruykys and Carter 2019) will demand greater resources than do short-term fox control programs (cf. Greentree et al. 2000; Gentle 2005). For the former, accuracy is imperative as density approaches zero, and elimination of predators from the control area, whereas for the latter, a trend model may suffice as evidence of the expected decline. Irrespective of the objectives, an underlying assumption of all population monitoring programs is that the methods used are a true reflection of the underlying population parameter of interest.
Direct estimates of population abundance and/or density are generally most desirable (Porteus et al. 2019), although they typically require more costly and labour-intensive field methods (Sadlier et al. 2004; Jones 2011) and high levels of precision may not be needed for the management objectives. As an alternative to direct population estimates, ecologists often make inferences about populations across space and time by using indices as proxies for population abundance and/or density (Schwarz and Seber 1999; Sollmann et al. 2013a; Stephens et al. 2015; Falcy et al. 2016). Indices can be any measure of animals, or their signs, that are expected to vary directly with population size (Caughley 1977). However, their use is contentious because the assumption of a direct and constant relationship between the index and true abundance/density is rarely verified (Pollock et al. 2002; Stephens et al. 2015). Consequently, there is long-standing discussion in the ecological literature about the use, and misuse, of indices (e.g. Edwards 1998; Anderson 2001; Johnson 2008; Gopalaswamy et al. 2015; Falcy et al. 2016). Yet, despite this considerable uncertainty, indices remain the most commonly implemented method for monitoring fox populations (e.g. Thompson and Fleming 1994; Thomson et al. 2000; Sharp et al. 2001; Gentle et al. 2004; Olsson et al. 2005; Moseby and Hill 2011; Bengsen 2014; Robley et al. 2014; Benshemesh et al. 2020). Noting that developments in scat genotyping is bridging the gap between the simplicity of using sign and the marking of individuals to estimate density (Piggott et al. 2008).
In this study, we compare two commonly applied activity indices and one count-based method with two spatially explicit methods for monitoring the abundance of red fox. This research was conducted in a semi-arid region of Australia over four consecutive years. Our primary objectives were to (1) determine whether activity indices show direct and/or constant correlations with fox population abundance estimates over time, and (2) evaluate the precision of the various methods for detecting change in fox abundance. Midway through the study, we introduced lethal control to reduce the abundance of foxes and we expected to observe a concomitant decline in the subsequent indices and population estimates. Our findings will assist wildlife managers to design fit-for-purpose fox monitoring programs to guide local decision-making and species-based management (sensu Jennelle et al. 2002), while also having broad application for monitoring other wildlife species with non-distinct pelage and/or similar behaviour.
Materials and methods
Study area
Our study was conducted at Scotia Wildlife Sanctuary, a 64 659 ha private conservation reserve in south-western New South Wales, Australia (−33.15°S, 141.06°E; Fig. 1), owned and managed by Australian Wildlife Conservancy. Approximately 8000 ha of the sanctuary is surrounded by an electrified conservation fence, to establish two adjacent 4000 ha exclosures, from which foxes (and feral cats) have been excluded since 2004 (Stage 1) and 2007 (Stage 2), and to which five threatened mammal species have been reintroduced successfully (Roshier et al. 2020). The climate is semi-arid with low and highly variable rainfall (spatially and temporally) that averages 230 mm per year with high evapotranspiration (1500 mm per year) and low relative humidity (average: 20%) (Australian Wildlife Conservancy, unpubl. data). Cool winters (average maximum temperature: 17°C) and hot summers (average maximum temperature: 30°C) characterise the site, with annual temperature extremes ranging from −6°C to 48°C. The landscape features stable east–west sand dunes of red sand and sandy solonised brown soil over clay (Westbrooke et al. 1998). Vegetation is predominantly multi-stemmed Eucalyptus spp. (‘mallee’) open-shrubland with a Triodia scariosa (‘spinifex’) understorey or mixed-shrub understorey (predominantly Senna, Dodonaea and Eremophila spp.), and Casuarina pauper woodland on the swales and open flats (Westbrooke et al. 1998). Red foxes are the largest predator present and their population in the study area was not subject to any form of population control during the 6 years prior to this study commencing.
|
|
Data collection
Camera traps
During the 4-year study, we monitored fox populations by using camera traps with passive-infrared sensors (HC600, Reconyx, Holmen, WI, USA) placed at 98 sites across 14 000 ha (Fig. 1). Sixty-three of these sites were located along roads and were monitored for the entirety of the study, except when 28 sites were removed during April and May 2016 to facilitate camera re-conditioning. An additional 35 sites were located away from roads and were operational for 7 months (July 2017–January 2018). All sites contained a single camera, except for nine road sites that had paired cameras (i.e. one camera either side of the road) to provide information for a related study. Detections from each camera pair were pooled together, so that data were comparable with single cameras (i.e. no detections were counted twice). Camera sites were spaced between 750 m and 2000 m apart. Further details on camera placement are provided in Carter et al. (2019). We conducted 48 camera-trapping sessions at monthly intervals, encompassing the period from 1 October 2015 until 25 September 2019. Each session consisted of 24 consecutive trapping occasions (i.e. 24-h periods from 09:00 hours to 08:59 hours), unless problems were noted with camera operability, whereby trap usage was accounted for in the analysis.
Cameras were attached to a galvanised steel post driven into the ground, with the sensor positioned 0.5 m above ground, aimed approximately 4.5 m away. Cameras recorded five consecutive images when triggered, with no time delay, and highest trigger sensitivity and image quality. Images were stamped with date, time and camera location. Cameras recorded monochromatic images at night and colour images during the day under ambient light. No lures or baits were used to attract predators to cameras.
Sand plots
A sand-plot monitoring program for foxes (and other species) had been in place at Scotia for 9 years prior to our study commencing (Australian Wildlife Conservancy, unpubl. data). Within our study area, this historical program included 60 sand plots (covering four roads) spaced approximately 500 m apart. For consistency, we maintained the historical survey methods in the current study; however, we considered the 500-m spacing to be too close, so we selected a subset of 17 antecedent sand plots spaced approximately 2000 m apart. This spacing was selected to (a) maximise the spatial coverage of the sand plots throughout the study area, and (b) increase the independence of each sand plot. Sand plots were positioned on the existing road network and monitored daily for a 4-day period each month, coinciding with camera-trapping sessions. Sand plots consisted of an area of sand (approximately 1.2 m wide) raked smooth across the entire width of the road (i.e. gutter to gutter). On the following morning, sand plots were inspected for the presence of fox footprints by the same experienced operator (J. S.) for the entirety of the study. Each plot was then smoothed clean in preparation for the following day. In the event that rain and/or wind obscured footprints on some plots, all data from that day were abandoned and all plots were re-surveyed the following day until 4 days of undisturbed data were collected. All sand plots were assumed to be independent and every set of fox tracks that entered and exited an individual plot were treated as independent detections. No lures were used and the same sand-plot locations were surveyed for the duration of the 4-year study (Fig. 1).
Fox identification
Because of their uniform pelage, individual red foxes cannot be identified reliably from photographs unless marked artificially (Guthlin et al. 2014). To identify individuals on camera-trap images, we fitted 28 foxes with GPS collars (Q4000E, Telemetry Solutions, Concord, CA, USA) over a 3-year period; seven foxes were caught and collared between October 2015 and March 2016, 10 foxes during July–December 2016, and 11 foxes during June–September 2017. Collars operated for approximately 4 months (before being programmed to detach from foxes automatically) and recorded location fixes at 20-min intervals between 17:00 hours and 09:00 hours and at 96-min intervals between 09:00 hours and 17:00 hours (for further details, see Roshier and Carter 2021). Individual foxes were identified in camera-trap images by comparing the image time stamp with all available GPS data (additional details are provided in Carter et al. 2019). Photographs of known individuals recorded ≤60 min apart at the same camera were not included in the analysis. Likewise, photographs of unmarked foxes recorded ≤60 min apart at the same camera were considered to be the same individual, and were not included in the analysis, unless body markings enabled clear distinction between individuals within this time period, or there was a second individual in a photograph. All camera-trap images were viewed and classified manually by the same operator (A. C.), without the use of machine learning or artificial intelligence software (e.g. Norouzzadeh et al. 2018; Tabak et al. 2019).
Fox management
During the first half of the study (October 2015–September 2017; Sessions 1–24) the fox population was not subject to any form of population control. Lethal fox control, subsequently, commenced in October 2017 (Session 25), and this was maintained constantly until the study ended in September 2019 (Session 48). Two control methods were implemented, namely, canid pest ejectors (hereafter ‘CPEs’) and fresh-meat baits (hereafter ‘baits’), both containing 3.0 mg of the toxicant sodium fluoroacetate (hereafter ‘1080’). Because of the nature of fox management, the exact number of foxes removed during the study remained unknown.
CPEs are an Australian derivative of the ‘M-44’ or ‘humane coyote getter’, as they are known elsewhere (Allen 2019). CPEs are mechanical devices that are buried partly in the ground, with a baited lure head, containing a sealed toxicant capsule, remaining exposed above ground level. When a fox attempts to remove the lure by pulling upward, the toxicant in the capsule is propelled into its mouth via a spring-loaded piston. An upward pull force of 1.6–2.7 kg is required to activate CPEs (Marks and Wilson 2005), which prevents many non-target species from accessing the toxicant. On 1 October 2017, we distributed 145 CPEs throughout the study area, placed at approximately 1-km intervals along the existing road network (Fig. 2). A variety of lures was used to create bespoke bait heads that contained a combination of dried liver, dried chicken, dried red meat, manufactured dog cubes, fish meal, and/or dried liver soaked in fish oil. These bait types were swapped every 2–3 months. For the first 4 months, CPE were inspected at 2–4-day intervals in an effort to identify triggers made by foxes fitted with GPS collars. Thereafter, inspections and servicing (lubricating and refreshing bait heads) occurred at approximately 1-month intervals. If CPEs had been triggered since the previous visit, the toxicant capsule was replaced and the lure head refreshed as necessary.
The use of meat-based baits (chicken wings, red meat) impregnated with 1080 is currently the most widespread and effective method of fox control in Australia (West and Saunders 2007; Mahon 2009). Hence, on 24 October 2017, we began supplementing CPEs with fresh chicken wings injected with 1080, distributed across 196 bait sites (Fig. 2). Nominally, baits were placed at 1-km intervals along the existing road network, although preference was given to placing baits (rather than CPEs) at road intersections; so, in some instances, bait spacing was at approximately 500 m. All sites were checked after 3 and 6 days and any baits taken were replaced. The final check was made after 8 days, when all remaining baits were removed. An additional nine baiting programs were implemented (using a combination of chicken wings, fresh beef meat, and chicken eggs) throughout the remainder of the study, with baits checked at 3-day intervals and remaining in place for a maximum of 10 days before being removed. When all control sites were combined, most roads across the study area contained alternating CPEs and baits at 500-m intervals.
Data analysis
Activity indices
Sand-plot and camera-trap data were analysed to produce two separate indices:
-
the Allen–Engeman (AI) index: the average number of tracks per sand plot, per day (or photographs per camera, per day). Variance of the AI was estimated following Engeman (2005). First, a linear mixed model was fitted to the data of the form:
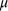 is the mean number per sand plot/camera site per day, Si is the random effect owing to ith sand-plot/camera site, Dj is the random effect owing to the day on which the observation was made, and eij is the random error term that is assumed to be independent and identically distributed, with a mean 0 and variance
is the mean number per sand plot/camera site per day, Si is the random effect owing to ith sand-plot/camera site, Dj is the random effect owing to the day on which the observation was made, and eij is the random error term that is assumed to be independent and identically distributed, with a mean 0 and variance 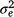 . Variance estimation of the AI index, when all sample sizes were equal, was calculated as follows:
. Variance estimation of the AI index, when all sample sizes were equal, was calculated as follows: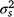 ,
, 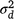 and
and 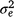 are, respectively, plot-to-plot/site-to-site variability, daily variability and random observational variability associated with each plot/trap each day (Engeman 2005).
are, respectively, plot-to-plot/site-to-site variability, daily variability and random observational variability associated with each plot/trap each day (Engeman 2005). -
the Catling index: the percentage of sand-plot nights with fox tracks (or camera-trap nights with independent sets of fox photos; following Catling and Burt 1994).
N-mixture modelling
N-mixture models are a commonly used method to estimate abundance by using spatially replicated count data, without requiring individual animals to be uniquely identifiable. Counts of animals at Site i and Time j (Yij) are assumed to be conditionally independent binomial random variables, based on the number of individuals actually present at Site i and Time j (Nij) during the survey, detected with probability pij. That is, 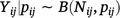 .
.
N-mixture models were run using the pcountOpen function in the unmarked package (v. 0.13.1, Fiske and Chandler 2011) in R (v. 3.6.1, R Core Team 2018). Given the 4-year duration of this study, populations were assumed to be closed within the primary survey periods (i.e. monthly sessions), and open between the primary periods. The Akaike information criterion (AIC, Akaike 1974; Buckland et al. 1997) was used to select the mixture distribution (Poisson, zero-inflated Poisson or negative binomial), and conduct model selection. In addition, we explored the influence of one site-level covariate on detectability (whether the trap was set on a road), while all other parameters were kept constant. Values of K, a parameter required to bound the integration, were explored to ensure that they were set large enough, such that model estimates were not affected.
Spatial mark–resight modelling (SMR–MLE)
Spatial capture–recapture (SCR) and spatial mark–resight (SMR) methods provide density estimates of marked (or partially marked) populations, using the spatial pattern of recapture events of marked animals (Borchers and Efford 2008; Royle et al. 2014). Even though SCR models can be fitted to populations without any marked individuals, marking a subset of the population is recommended where possible (Chandler and Royle 2013). SCR and SMR utilise heterogeneity in capture probability caused by the proximity of animals’ home-range centres to traps. SMR values of fox density at Scotia have been estimated previously within a maximum-likelihood framework (i.e. package ‘secr’, Efford 2019) in Carter et al. (2019). For brevity, we do not repeat the analysis here, but do convert these estimated fox densities to abundance in each of the primary survey periods, on the basis of all camera-trap data and an effective survey area of approximately 57 000 ha. This area was created using a 4000-m buffer around the trap locations in each survey, with inaccessible habitat removed (i.e. an 8000-ha fenced region that excludes foxes; Fig. 1). The choice of a 4000-m buffer was based on GPS location data (cf. Roshier and Carter 2021) that indicated that foxes rarely moved beyond this distance.
Spatial mark–resight modelling augmented with GPS-collar information (SMR–Bayes + GPS)
Spatial mark–resight analyses augmented with GPS-collar data were conducted within a Bayesian framework, following the approach of Sollmann et al. (2013b) and Whittington et al. (2018). In the context of spatial mark–resight analyses, telemetry and GPS-collar data provide additional information on individual location and movement. Sollmann et al. (2013b) extended spatial mark–resight models to utilise the spatial information from GPS-collared racoons to inform model parameters on spatial location. We adapted their approach, using the GPS-collar data from 28 collared foxes. Because GPS collars were not deployed on foxes in every session, estimates were available for only a portion of the sessions surveyed. We ran two Markov-chain Monte Carlo chains of the algorithm, with 100 000 iterations each, discarding the first 10 000 iterations as burn-in. To check for chain convergence, we calculated the Gelman–Rubin statistic R-hat (Gelman and Rubin 1992) by using the R package coda (v. 0.19-3, Plummer et al. 2006). Values >1 indicate lack of convergence.
Ethics statement
All work relating to this study was conducted as approved by and in accordance with the Animal Care and Ethics Committee of the Secretary of NSW Trade and Investment (Approval Numbers 13/1344), Animal Care and Ethics Committee of the Secretary of NSW Industry, Skills and Regional Development (Approval Numbers 13/1344#5, 16/1354, 16/1354#1) and the Office of Environment and Heritage, NSW National Parks and Wildlife Service (Scientific License number SL100473).
Results
Fox monitoring and management
More than 110 000 camera-trap nights were conducted in this study, across the 98 camera-trap sites and 48 primary (monthly) sampling sessions. The maximum number of trap nights available at a single site was 1152 (i.e. 48 primary sessions × 24 secondary occasions), and there were, on average, 955 (±233 s.d.) trap nights at each site (35 off-road sites were trapped for seven primary sessions only). Across the duration of the 4-year study, foxes were detected by cameras in every survey session except one (Session 42, March 2019; Supplementary material Table S1), and 90 of 98 individual sites had at least one fox detection.
Sand-plot surveys occurred in 44 of the 48 primary sampling sessions, with foxes being detected in 41 of these sessions. In 14 sessions, wind and/or rain caused the abandonment of a day’s surveys, which required the addition of one extra survey day to obtain data from 4 days.
The time spent conducting camera-trap surveys and processing the resultant data in preparation for analyses varied depending on accessibility (Table 1). When on-road and off-road cameras were combined, each camera site required approximately 16 min work each month (1545 min/98 camera-trap sites). For sand plots, a standard monthly 4-day survey required an average of 7 h, 35 min. (±1 h, 10 min s.d.) of person hours in the field to prepare the sand and record tracks. That equates to approximately 27 min per sand plot per month (455 min/17 plots). However, >31% of monthly sand-plot surveys (14/44) required an extra day of field work (mean effort 1 h, 53 min, ±17 min s.d.) because a day’s data were ruined by inclement weather.

|
During the first 24 sessions when there was no fox control (October 2015–September 2017), the mean number of independent fox detections on cameras per session was 116 (±68 s.d.), with detections at 64% (±16 s.d.) of sites, on average. During the final 24 sessions when fox control was implemented (October 2017–September 2019), the mean number of fox detections on cameras per session was 22 (±18 s.d.), with detections at 22% (±13 s.d.) of sites, on average. During the final session prior to commencement of lethal fox control (Session 24, September 2017), there were 90 fox detections on cameras. Two months later (Session 26, November 2017), fox detections on cameras had fallen by >50%, and by the following September (Session 36, September 2018), camera detections were down by almost 85% (Table S1). During the final year of our study (Sessions 37–48, October 2018–September 2019), total camera detections in several sessions were in single figures and down >90% from total detections in Session 24 (September 2017; Table S1).
Detections on sand plots also fell following the commencement of fox control. During the 2 years prior to control, there was an average of 10 (±6 s.d.) fox detections per session, with detections occurring at 36% (±18% s.d.) of the 17 sites, on average. During the 2 years of lethal control, there was an average of 2 (±2 s.d.) fox detections per session, with detections at 12% (±9% s.d.) of the 17 sites, on average. During the final year of monitoring, in most sessions, fox prints were recorded at one or two sand plots only (Table S1).
Nine of the eleven foxes fitted with GPS collars in June–September 2017 were within the study area at the commencement of lethal fox control on 1 October 2017. For the first 3 weeks of lethal control, only CPEs were deployed; none of which was triggered, and no kills of collared or unmarked foxes were recorded. Fresh-meat baits were first deployed on 24 October, and across the 196 bait sites, 24 baits were removed after 3 days, 20 baits were removed during Days 4–6, whereas another 21 baits were taken from Days 7–8 (Fig. 3). Examining the GPS data from collared foxes indicated that three of nine collared foxes took baits and died on the third day of the baiting program, one fox took a bait and died on Day 7, and three other collared foxes died in the same period but their precise movements in the day prior to their death could not be deduced because the GPS units in their collars malfunctioned. Another collared fox is likely to have triggered a CPE and died on 31 October 2017, 30 days after CPEs were first activated. Only one of nine collared foxes remained alive at the end of October 2017, which equates to an 89% reduction in the collared population during the first month of baiting. The one collared fox that survived (5.4 kg ♂) had access to 10 CPEs and 13 fresh-bait stations within its home range (fox MF366 in Roshier and Carter 2021). This individual remained active throughout the study area until 24 November 2017 when its collar drop-off mechanism activated. CPEs continued to be deployed until the end of the study in September 2019, and fresh-meat baits were deployed in each month (except in December 2017) until February 2018, and at 3-month intervals thereafter. It was not possible to determine whether non-target species removed baits or triggered CPEs. No carcasses of unmarked foxes (or non-target species) were found in the 2 years of lethal control.
|
|
Estimates of activity from sand plots and camera traps
The Allen–Engeman and Catling indices were near identical for each session (R2 = 0.97) and the Catling index is not considered further in this analysis because it provides no means to generate confidence limits. In comparing activity indices from camera traps and sand plots, the metric from sand plots was generally higher and more variable than that derived from camera traps (Fig. 4). Although both indices recorded a decline in fox activity following introduction of lethal control, the confidence limits for the camera-trap data were much narrower in absolute terms than for the sand-plot data.
Estimates of abundance from camera-trap data
The number of marked foxes in a session ranged from 0 to 14, so SMR analyses were not possible in every session. Also, during Session 7 (April 2016), no marked foxes were detected on any cameras, so abundance estimates during that session could not be obtained. Following the commencement of lethal control in Session 25 (October 2017), there were too few individually marked foxes remaining in the survey region to use SMR methods. In sessions with high congruence in the estimates between SMR–MLE and SMR–Bayes + GPS (i.e. October 2016, November 2016), the MLE approach is more uncertain and has wider confidence limits (Fig. 5). For the SMR–Bayes + GPS analysis, the Gelman–Rubin statistic R-hat for all sessions was ≤1, indicating that the chains converged for the 10 sessions for which this method could be applied. Across these 10 sessions, the SMR–Bayes + GPS method produced abundance estimates of ~50 animals (range 35–78) in the study area (Fig. 5). This compared with an estimate of ~80 animals (range 27–230) for the same sessions using SMR–MLE. The confidence limits associated with both SMR methods in this analysis were improved (i.e. uncertainty was reduced) by including marked animals (pID) that were known to be on the grid in any particular session but were not detected.
Both the SMR–MLE and SMR–Bayes + GPS methods produced similar estimates when there were sufficient marked foxes on the grid to be detected (Fig. 5, also see Carter et al. 2019). As the number of marked foxes detected on cameras declined, SMR–Bayes + GPS models failed to converge, and the point estimates and confidence limits inflated for SMR–MLE models, or failed if no marked foxes were detected, as in Session 7 (April 2016).
The best-fitting N-mixture model (i.e. that with the lowest AIC) using the camera-trap data was a Poisson mixture, which had no covariates on the detection probability. As expected, since our camera-trap sites were not independent, estimates from N-mixture models appear biased high (i.e. higher than SMR-methods). In comparing the camera-trap N-mixture with the camera-trap Allen–Engeman index, which is the only other method we applied that does not require marked animals, the confidence intervals are much narrower for the Allen–Engeman index method (Fig. 6). We were unable to generate a plausible N-mixture model using the sand-plot data because the models failed to converge (see Discussion).
|
|
Discussion
The primary goal of our study was to assist wildlife managers design fit-for-purpose monitoring programs by providing a better understanding of the utility of various methods available for monitoring foxes. Because a subset of our fox population was fitted with GPS collars, we had a unique opportunity to compare a statistical method that is considered ‘best-practice’ for estimating population density (i.e. spatial capture–recapture using both MLE and Bayesian frameworks) with methods that are less arduous in terms of data collection, processing and analysis (i.e. simple counts of detection events expressed as the Allen–Engeman index and N-mixture models). Our comparison of these methods was made in terms of uncertainty and utility.
Abundance estimate methods
We found that the incorporation of GPS-collar data into the spatial capture–recapture approaches (i.e. SMR–Bayes + GPS vs SMR–MLE) greatly reduced uncertainty in estimates of abundance (Fig. 5). This was expected, given that the Bayesian approach can incorporate data from GPS collars to better estimate the distribution of individual activity centres (see Gerber and Parmenter 2015), whereas the maximum-likelihood approach cannot. In general, these two SMR approaches provided similar estimates of abundance, except in situations where marked detection events were sparse (e.g. Sessions 1–3, October–December 2015). Further, in sessions where there were few marked animals resighted, the SMR–Bayes + GPS models did not converge, and although SMR–MLE models could provide estimates in these instances, they were unrealistic and had wide confidence limits (e.g. Sessions 18–20, March–May 2017).
We acknowledge that obtaining uniquely marked animals is not often feasible in studies of fox populations and that additional methods not implemented here have been used successfully to measure fox abundance elsewhere. For instance, several studies have used non-invasive genetic sampling to demonstrate changes in fox abundance following lethal control programs (e.g. Piggott et al. 2008; Berry et al. 2012, 2013), but inclusion of such methods was beyond the scope of our study. Instead, in addition to SMR analyses, we also analysed our data using count-based indices and N-mixture modelling. N-mixture models typically assume that the population is demographically closed (but extensions exist), individuals are not counted at more than one site, and all individuals have the same probability of being detected (Royle 2004). Our results showed that N-mixture estimates were comparable to those from SMR methods but the uncertainty was much greater (compare Figs 5 and 6). Here, camera traps were specifically placed to ensure that SMR methodology could be used (i.e. individually marked animals detected across multiple sites), violating an assumption of N-mixtures. Given that sites were not independent, although the total number of animals using each site remains unbiased, abundance estimates for the survey region are biased positively because animals moving between sites are double counted (Keever et al. 2017). In addition, in many situations, N-mixture models did not converge. Barker et al. (2018) found that when p is low or highly variable, N can be unidentifiable (Barker et al. 2018; Link et al. 2018). In our study, estimates of p were very small (mean = 0.006, var = 0.003) and this is likely to explain why many sessions did not converge. In recent times, the density of unmarked populations has been estimated elsewhere with numerous other statistical approaches (e.g. FMP Formula, Stephens et al. 2006; REM, Rowcliffe et al. 2008; SPA Model, Ramsey et al. 2015; REST Model, Nakashima et al. 2018; see also SPIM Model, Augustine et al. 2018; Royle et al. 2014, Chapter 18; Chandler and Royle 2013) and these may warrant further investigation for estimating fox abundance in future studies.
Following the introduction of lethal control, foxes fitted with GPS collars did not survive long enough to enable density estimates to be produced using spatial capture–recapture approaches. Nevertheless, during the first month of baiting, we observed an 89% reduction in the collared-fox population, and, as anticipated, we observed considerable declines in the subsequent indices and abundance estimates using count methods. Values for the Allen–Engeman index from camera-trap data were 85% lower, on average, during the 2 years of lethal control than the pre-control values, whereas the same metric using sand-plot data was 75% lower, on average, during the lethal control period. Estimates of fox abundance using N-mixture modelling from camera-trap data were likewise lower, ranging from 101 to 258 foxes per session, pre-control, compared with 43–130 foxes per session, when fox control was implemented. We were unable to generate abundance estimates using N-mixture modelling from sand-plot data because models would not converge (as discussed above). Combined, these results align with studies published previously that have demonstrated that broad-scale 1080 baiting can be effective at reducing fox abundance (e.g. Thomson et al. 2000; Berry et al. 2012, 2013; Marlow et al. 2015, 2016), and contrast some studies that concluded that baiting did not produce a clear decline in fox activity or abundance (e.g. Towerton et al. 2011; Bengsen 2014).
Broadly speaking, when undertaking population monitoring, we advocate for obtaining some marked animals, at least for a subset of sessions. If robust estimates of abundance are available at more than one point in time, they can be used to scale an index of activity if it changes in the same direction as the underlying population density. In this study, prior to the introduction of lethal control, changes in abundance were detected by using methods based on robust methods of density estimation (i.e. spatial mark–resight modelling augmented with GPS-collar information (SMR–Bayes + GPS)) and simple data collection and analyses (i.e. Allen–Engeman index; Fig. 7). Scaling both data sets showed a measure of agreement and we are confident, that after the introduction of lethal control, zero on the index was at or near zero population density.
Little published information exists on the time demands of camera trapping and sand-plot surveys. In our study, when the two methods were compared on a time-per-unit basis, an individual camera trap (on- and off-road combined) required approximately 60% of the person-hours each month of those that a sand plot did. This is noteworthy given the richness of data that camera traps can provide. Compared with sand plots, cameras can collect meaningful data in all types of weather, they are less dependent on observer skill (Ruykys and Carter 2019), they can operate for extended periods (months) with no human intervention, and they can facilitate estimation of population density (cf. Rovero and Zimmermann 2016). Moreover, in our study, all camera-trap images were viewed and classified manually. As machine learning and artificial intelligence software become more readily available (e.g. Norouzzadeh et al. 2018; Tabak et al. 2019), the time required to review large quantities of camera-trap images will decrease considerably, leading to further increases in the efficiency of camera-trap surveys.
Fox control
During the first 23 days of our lethal control program, we deployed only canid pest ejectors (CPEs), at an average density of 1.03 devices per km2 (Fig. 2). During this period, each of the nine foxes fitted with GPS collars had access to multiple CPEs within their home range (range = 4–17), yet no CPEs were triggered. This prompted us to introduce buried fresh-meat baits (mean density = 1.4 baits per km2), which delivered an immediate impact, with four of the nine collared foxes succumbing to baits within 7 days of baiting commencing. A further three collared foxes died in the same period and we suspect they too consumed fresh baits, but GPS-collar malfunction prevented confirmation. The high mortality rate of radio-collared foxes during the baiting program supports the findings of two previous studies. In coastal New South Wales, 100% of collared foxes (n = 6) died within 10 days of baiting commencing (Dexter and Meek 1998), whereas in semi-arid Western Australia, mortality rates were 60% (of 45 collared foxes) within 3 days and 100% within 44 days following 1080 baiting (Thomson et al. 2000). Conflicting results were obtained from two other Australian studies that found that most foxes collared survived 1080 baiting programs in mixed farmland (Carter et al. 2011; Bengsen 2014). In our study, one collared fox survived 54 days of the CPE program and a 9-day baiting campaign (at which point its collar released).
Results from field-based trials on the effectiveness of CPEs against foxes in Australia are limited. Of the published data that are available, the success rate of CPEs on foxes (i.e. percentage of ‘CPE nights’ with triggers) is relatively low, ranging from 0 (from 810 nights; Kreplins et al. 2018) to 2.3% (Gil-Fernández et al. 2021). Our study is, by far, the largest published study at present and delivered a success rate of 0.01% (i.e. 15 triggers from 105 705 CPE nights) across a 2-year period. Our results follow those of Moseby and Read (2014) in semi-arid South Australia, who also found CPEs to be less efficacious than 1080 baits. The low success rate of CPEs may stem from the fox’s neophobic behaviour, which is supported by observational studies at CPE sites (Moseby and Read 2014; Gil-Fernández et al. 2021). Considerable interference with CPEs by non-target species has also been noted (see Kreplins et al. 2018) and this too may influence the low success rate by limiting access to, or reducing the appeal of, attractants. The choice of attractants will also certainly influence the efficacy of CPEs, and further investigation into this issue is required, as are evaluations into the overall usefulness of CPEs as a method of fox control (Kreplins et al. 2018).
Management implications
Despite the vast impact of foxes in Australia, the development and implementation of fit-for-purpose monitoring programs over large areas remain a challenge (Marlow et al. 2015) and, as such, little information exists on fox population densities or abundance in most ecosystems. The results from our study showed that the Allen–Engeman index derived from camera-trap data appears to provide an accurate reflection of change in the underlying fox density. This method provides land managers with an efficient means to detect large shifts in abundance, whether up or down, which may trigger a shift to more precise population monitoring methods. If accuracy is paramount (e.g. for reintroduction programs), then SMR–GPS using a Bayesian framework provides robust estimates, noting that this method is computationally demanding and requires more expertise and resources than an index. Alternatively, within a similar analytical framework, rapidly developing genotyping methods mentioned above can provide density estimates without the need for managers or researchers to have expertise in genetics.
Regardless of the method chosen, our study has provided empirical data to demonstrate that the shorter the survey period is, the greater the likelihood of false absences (i.e. the target species is present, but not detected; cf. Field et al. 2005). From an operational standpoint, if short-term surveys are used to inform assessments of predation pressure, for instance, this could result in adverse outcomes (e.g. Hayward et al. 2012). If limited resources are available, we encourage land managers to prioritise survey duration over survey frequency. In other words, longer surveys conducted less frequently will provide a more accurate reflection of the underlying population than do shorter surveys conducted more often. These findings will help wildlife managers design fit-for-purpose monitoring programs for red foxes and other species with non-distinct pelage or similar behaviour patterns.
Supplementary material
Supplementary material is available online.
Data availability
The data are archived at the Movebank Data Repository (https://www.datarepository.movebank.org) as ‘Carter and Roshier (2019) Red fox (Vulpes vulpes) – Scotia, NSW Australia’. https://doi.org/10.5441/001/1.72hh609t.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This study was an initiative of the Australian Wildlife Conservancy’s science program. Funding was provided by supporters of AWC with additional support from the Australian Government’s National Environmental Science Program through the Threatened Species Recovery Hub.
Acknowledgements
We thank J. Kanowski for providing comments that improved the paper, and AWC staff and volunteers for fieldwork assistance.
References
Akaike, H (1974). A new look at the statistical model identification. IEEE Transactions on Automatic Control 19, 716–723.| A new look at the statistical model identification.Crossref | GoogleScholarGoogle Scholar |
Allen, BL (2019). Para-aminopropiophenone (PAPP) in canid pest ejectors (CPEs) kills wild dogs and European red foxes quickly and humanely. Environmental Science and Pollution Research 26, 14494–14501.
| Para-aminopropiophenone (PAPP) in canid pest ejectors (CPEs) kills wild dogs and European red foxes quickly and humanely.Crossref | GoogleScholarGoogle Scholar |
Anderson, DR (2001). The need to get the basics right in wildlife field studies. Wildlife Society Bulletin 19, 1294–1297.
Augustine, BC, Royle, JA, Kelly, MJ, Satter, CB, Alonso, RS, Boydston, EE, and Crooks, KR (2018). Spatial capture–recapture with partial identity: an application to camera traps. The Annals of Applied Statistics 12, 67–95.
| Spatial capture–recapture with partial identity: an application to camera traps.Crossref | GoogleScholarGoogle Scholar |
Barker, RJ, Schofield, MR, Link, WA, and Sauer, JR (2018). On the reliability of N-mixture models for count data. Biometrics 74, 369–377.
| On the reliability of N-mixture models for count data.Crossref | GoogleScholarGoogle Scholar |
Bengsen, A (2014). Effects of coordinated poison-baiting programs on survival and abundance in two red fox populations. Wildlife Research 41, 194–202.
| Effects of coordinated poison-baiting programs on survival and abundance in two red fox populations.Crossref | GoogleScholarGoogle Scholar |
Benshemesh, J, Southwell, D, Barker, R, and McCarthy, M (2020). Citizen scientists reveal nationwide trends and drivers in the breeding activity of a threatened bird, the malleefowl (Leipoa ocellata). Biological Conservation 246, 108573.
| Citizen scientists reveal nationwide trends and drivers in the breeding activity of a threatened bird, the malleefowl (Leipoa ocellata).Crossref | GoogleScholarGoogle Scholar |
Berry, O, Algar, D, Angus, J, Hamilton, N, Hilmer, S, and Sutherland, D (2012). Genetic tagging reveals a significant impact of poison baiting on an invasive species. The Journal of Wildlife Management 76, 729–739.
| Genetic tagging reveals a significant impact of poison baiting on an invasive species.Crossref | GoogleScholarGoogle Scholar |
Berry, O, Tatler, J, Hamilton, N, Hilmer, S, Hitchen, Y, and Algar, D (2013). Slow recruitment in a red-fox population following poison baiting: a non-invasive mark–recapture analysis. Wildlife Research 40, 615–623.
| Slow recruitment in a red-fox population following poison baiting: a non-invasive mark–recapture analysis.Crossref | GoogleScholarGoogle Scholar |
Borchers, DL, and Efford, MG (2008). Spatially explicit maximum likelihood methods for capture–recapture studies. Biometrics 64, 377–385.
| Spatially explicit maximum likelihood methods for capture–recapture studies.Crossref | GoogleScholarGoogle Scholar |
Bradshaw, CJA, Hoskins, AJ, Haubrock, PJ, Cuthbert, RN, Diagne, C, Leroy, B, Andrews, L, Page, B, Cassey, P, Sheppard, AW, and Courchamp, F (2021). Detailed assessment of the reported economic costs of invasive species in Australia. NeoBiota 67, 511–550.
| Detailed assessment of the reported economic costs of invasive species in Australia.Crossref | GoogleScholarGoogle Scholar |
Buckland, ST, Burnham, KP, and Augustin, NH (1997). Model selection: an integral part of inference. Biometrics 53, 603–618.
| Model selection: an integral part of inference.Crossref | GoogleScholarGoogle Scholar |
Carter, A, Luck, GW, and McDonald, SP (2011). Fox-baiting in agricultural landscapes in south-eastern Australia: a case-study appraisal and suggestions for improvement. Ecological Management & Restoration 12, 214–223.
| Fox-baiting in agricultural landscapes in south-eastern Australia: a case-study appraisal and suggestions for improvement.Crossref | GoogleScholarGoogle Scholar |
Carter, A, Potts, JM, and Roshier, DA (2019). Toward reliable population density estimates of partially marked populations using spatially explicit mark–resight methods. Ecology and Evolution 9, 2131–2141.
| Toward reliable population density estimates of partially marked populations using spatially explicit mark–resight methods.Crossref | GoogleScholarGoogle Scholar |
Catling, PC, and Burt, RJ (1994). Studies of the ground-dwelling mammals of eucalypt forests in south-eastern New South Wales: the species, their abundance and distribution. Wildlife Research 21, 219–239.
| Studies of the ground-dwelling mammals of eucalypt forests in south-eastern New South Wales: the species, their abundance and distribution.Crossref | GoogleScholarGoogle Scholar |
Caughley G (1977) ‘Analysis of vertebrate populations.’ (Wiley: New York, NY, USA)
Chandler, RB, and Royle, JA (2013). Spatially explicit models for inference about density in unmarked or partially marked populations. The Annals of Applied Statistics 7, 936–954.
| Spatially explicit models for inference about density in unmarked or partially marked populations.Crossref | GoogleScholarGoogle Scholar |
Dexter, N, and Meek, P (1998). An analysis of bait-take and non-target impacts during a fox-control exercise. Wildlife Research 25, 147–155.
| An analysis of bait-take and non-target impacts during a fox-control exercise.Crossref | GoogleScholarGoogle Scholar |
Doherty, TS, Glen, AS, Nimmo, DG, Ritchie, EG, and Dickman, CR (2016). Invasive predators and global biodiversity loss. Proceedings of the National Academy of Sciences 113, 11261–11265.
| Invasive predators and global biodiversity loss.Crossref | GoogleScholarGoogle Scholar |
Edwards, D (1998). Issues and themes for natural resources trend and change detection. Ecological Applications 8, 323–325.
Efford, MG (2019). Non-circular home ranges and the estimation of population density. Ecology 100, e02580.
| Non-circular home ranges and the estimation of population density.Crossref | GoogleScholarGoogle Scholar |
Engeman, RM (2005). Indexing principles and a widely applicable paradigm for indexing animal populations. Wildlife Research 32, 203–210.
| Indexing principles and a widely applicable paradigm for indexing animal populations.Crossref | GoogleScholarGoogle Scholar |
Falcy, MR, McCormick, JL, and Miller, SA (2016). Proxies in practice: calibration and validation of multiple indices of animal abundance. Journal of Fish and Wildlife Management 7, 117–128.
| Proxies in practice: calibration and validation of multiple indices of animal abundance.Crossref | GoogleScholarGoogle Scholar |
Field, SA, Tyre, AJ, Thorn, KH, O’Connor, PJ, and Possingham, HP (2005). Improving the efficiency of wildlife monitoring by estimating detectability: a case study of foxes (Vulpes vulpes) on the Eyre Peninsula, South Australia. Wildlife Research 32, 253–258.
| Improving the efficiency of wildlife monitoring by estimating detectability: a case study of foxes (Vulpes vulpes) on the Eyre Peninsula, South Australia.Crossref | GoogleScholarGoogle Scholar |
Fiske, I, and Chandler, R (2011). unmarked: an R package for fitting hierarchical models of wildlife occurrence and abundance. Journal of Statistical Software 43, 1–23.
| unmarked: an R package for fitting hierarchical models of wildlife occurrence and abundance.Crossref | GoogleScholarGoogle Scholar |
Gelman, A, and Rubin, DB (1992). Inference from iterative simulation using multiple sequences. Statistical Science 7, 457–472.
| Inference from iterative simulation using multiple sequences.Crossref | GoogleScholarGoogle Scholar |
Gentle MN (2005) Factors affecting the efficiency of Fox (Vulpes vulpes) baiting practices on the Central Tablelands of New South Wales. PhD Thesis, University of Sydney, NSW, Australia.
Gentle, M, Massei, G, and Saunders, G (2004). Levamisole can reduce bait monopolization in wild red foxes Vulpes vulpes. Mammal Review 34, 325–330.
| Levamisole can reduce bait monopolization in wild red foxes Vulpes vulpes.Crossref | GoogleScholarGoogle Scholar |
Gerber, BD, and Parmenter, RR (2015). Spatial capture–recapture model performance with known small-mammal densities. Ecological Applications 25, 695–705.
| Spatial capture–recapture model performance with known small-mammal densities.Crossref | GoogleScholarGoogle Scholar |
Gil-Fernández, M, Harcourt, R, Towerton, A, Newsome, T, Milner, HA, Sriram, S, Gray, N, Escobar-Lasso, S, Gonzalez-Cardoso, VH, and Carthey, A (2021). The canid pest ejector challenge: controlling urban foxes while keeping domestic dogs safe. Wildlife Research 48, 314–322.
| The canid pest ejector challenge: controlling urban foxes while keeping domestic dogs safe.Crossref | GoogleScholarGoogle Scholar |
Gopalaswamy, AM, Delampady, M, Karanth, KU, Kumar, NS, Macdonald, DW, and Yoccoz, N (2015). An examination of index-calibration experiments: counting tigers at macroecological scales. Methods in Ecology and Evolution 6, 1055–1066.
| An examination of index-calibration experiments: counting tigers at macroecological scales.Crossref | GoogleScholarGoogle Scholar |
Greentree, C, Saunders, G, McLeod, L, and Hone, J (2000). Lamb predation and fox control in south-eastern Australia. Journal of Applied Ecology 37, 935–943.
| Lamb predation and fox control in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Guthlin, D, Storch, I, and Küchenhoff, H (2014). Is it possible to individually identify red foxes from photographs? Wildlife Society Bulletin 38, 205–210.
| Is it possible to individually identify red foxes from photographs?Crossref | GoogleScholarGoogle Scholar |
Hayward, MW, L’Hotellier, F, O’connor, T, Ward-Fear, G, Cathcart, J, Cathcart, T, Stephens, J, Stephens, J, Herman, K, and Legge, S (2012). Reintroduction of bridled nailtail wallabies beyond fences at Scotia Sanctuary – Phase 1. Proceedings of the Linnean Society of New South Wales 134, A27–A37.
Jennelle, CS, Runge, MC, and MacKenzie, DI (2002). The use of photographic rates to estimate densities of tigers and other cryptic mammals: a comment on misleading conclusions. Animal Conservation 5, 119–120.
| The use of photographic rates to estimate densities of tigers and other cryptic mammals: a comment on misleading conclusions.Crossref | GoogleScholarGoogle Scholar |
Johnson, DH (2008). In defense of indices: the case of bird surveys. Journal of Wildlife Management 72, 857–868.
| In defense of indices: the case of bird surveys.Crossref | GoogleScholarGoogle Scholar |
Jones, JPG (2011). Monitoring species abundance and distribution at the landscape scale. Journal of Applied Ecology 48, 9–13.
| Monitoring species abundance and distribution at the landscape scale.Crossref | GoogleScholarGoogle Scholar |
Keever, AC, McGowan, CP, Ditchkoff, SS, Acker, PK, Grand, JB, and Newbolt, CH (2017). Efficacy of N-mixture models for surveying and monitoring white-tailed deer populations. Mammal Research 62, 413–422.
| Efficacy of N-mixture models for surveying and monitoring white-tailed deer populations.Crossref | GoogleScholarGoogle Scholar |
Kreplins, TL, Kennedy, MS, Dundas, SJ, Adams, PJ, Bateman, PW, and Fleming, PA (2018). Corvid interference with canid pest ejectors in the southern rangelands of Western Australia. Ecological Management & Restoration 19, 169–172.
| Corvid interference with canid pest ejectors in the southern rangelands of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Legge, S, Woinarski, JCZ, Burbidge, AA, Palmer, R, Ringma, J, Radford, JQ, Mitchell, N, Bode, M, Wintle, B, Baseler, M, Bentley, J, Copley, P, Dexter, N, Dickman, CR, Gillespie, GR, Hill, B, Johnson, CN, Latch, P, Letnic, M, Manning, A, McCreless, EE, Menkhorst, P, Morris, K, Moseby, K, Page, M, Pannell, D, and Tuft, K (2018). Havens for threatened Australian mammals: the contributions of fenced areas and offshore islands to the protection of mammal species susceptible to introduced predators. Wildlife Research 45, 627–644.
| Havens for threatened Australian mammals: the contributions of fenced areas and offshore islands to the protection of mammal species susceptible to introduced predators.Crossref | GoogleScholarGoogle Scholar |
Link, WA, Schofield, MR, Barker, RJ, and Sauer, JR (2018). On the robustness of N-mixture models. Ecology 99, 1547–1551.
| On the robustness of N-mixture models.Crossref | GoogleScholarGoogle Scholar |
Mahon, PS (2009). Targeted control of widespread exotic species for biodiversity conservation: the Red Fox (Vulpes vulpes) in New South Wales, Australia. Ecological Management & Restoration 10, S59–S69.
| Targeted control of widespread exotic species for biodiversity conservation: the Red Fox (Vulpes vulpes) in New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Marks, CA, and Wilson, R (2005). Predicting mammalian target-specificity of the M-44 ejector in south-eastern Australia. Wildlife Research 32, 151–156.
| Predicting mammalian target-specificity of the M-44 ejector in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Marlow, NJ, Thomas, ND, Williams, AAE, Macmahon, B, Lawson, J, Hitchen, Y, Angus, J, and Berry, O (2015). Lethal 1080 baiting continues to reduce European Red Fox (Vulpes vulpes) abundance after more than 25 years of continuous use in south-west Western Australia. Ecological Management & Restoration 16, 131–141.
| Lethal 1080 baiting continues to reduce European Red Fox (Vulpes vulpes) abundance after more than 25 years of continuous use in south-west Western Australia.Crossref | GoogleScholarGoogle Scholar |
Marlow, N, Thomson, P, Rose, K, and Kok, N (2016). Compensatory responses by a fox population to artificial density reduction in a rangeland area in Western Australia. Conservation Science Western Austalia 10, 1–10.
Moseby, KE, and Hill, BM (2011). The use of poison baits to control feral cats and red foxes in arid South Australia I. Aerial baiting trials. Wildlife Research 38, 338–349.
| The use of poison baits to control feral cats and red foxes in arid South Australia I. Aerial baiting trials.Crossref | GoogleScholarGoogle Scholar |
Moseby KE, Read JL (2014) Using camera traps to compare poison bait uptake by invasive predators and non-target species. In ‘Camera trapping: wildlife management and research’. (Eds P Meek, P Fleming) pp. 131–139. (CSIRO Publishing: Melbourne, Vic., Australia)
Nakashima, Y, Fukasawa, K, and Samejima, H (2018). Estimating animal density without individual recognition using information derivable exclusively from camera traps. Journal of Applied Ecology 55, 735–744.
| Estimating animal density without individual recognition using information derivable exclusively from camera traps.Crossref | GoogleScholarGoogle Scholar |
Norouzzadeh, MS, Nguyen, A, Kosmala, M, Swanson, A, Palmer, MS, Packer, C, and Clune, J (2018). Automatically identifying, counting, and describing wild animals in camera-trap images with deep learning. Proceedings of the National Academy of Sciences 115, E5716–E5725.
| Automatically identifying, counting, and describing wild animals in camera-trap images with deep learning.Crossref | GoogleScholarGoogle Scholar |
Olsson, M, Wapstra, E, Swan, G, Snaith, E, Clarke, R, and Madsen, T (2005). Effects of long-term fox baiting on species composition and abundance in an Australian lizard community. Austral Ecology 30, 899–905.
| Effects of long-term fox baiting on species composition and abundance in an Australian lizard community.Crossref | GoogleScholarGoogle Scholar |
Piggott, MP, Wilson, R, Banks, SC, Marks, CA, Gigliotti, F, and Taylor, AC (2008). Evaluating exotic predator control programs using non-invasive genetic tagging. Wildlife Research 35, 617–624.
| Evaluating exotic predator control programs using non-invasive genetic tagging.Crossref | GoogleScholarGoogle Scholar |
Plummer, M, Best, N, Cowles, K, and Vines, K (2006). CODA: convergence diagnosis and output analysis for MCMC. R News 6, 7–11.
Pollock, KH, Nichols, JD, Simons, TR, Farnsworth, GL, Bailey, LL, and Sauer, JR (2002). Large scale wildlife monitoring studies: statistical methods for design and analysis. Environmetrics 13, 105–119.
| Large scale wildlife monitoring studies: statistical methods for design and analysis.Crossref | GoogleScholarGoogle Scholar |
Porteus, TA, Reynolds, JC, and McAllister, MK (2019). Modelling the rate of successful search of red foxes during population control. Wildlife Research 46, 285–295.
| Modelling the rate of successful search of red foxes during population control.Crossref | GoogleScholarGoogle Scholar |
Ramsey, DSL, Caley, PA, and Robley, A (2015). Estimating population density from presence–absence data using a spatially explicit model. The Journal of Wildlife Management 79, 491–499.
| Estimating population density from presence–absence data using a spatially explicit model.Crossref | GoogleScholarGoogle Scholar |
R Core Team (2018) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at https://www.R-project.org/
Robley, A, Gormley, AM, Forsyth, DM, and Triggs, B (2014). Long-term and large-scale control of the introduced red fox increases native mammal occupancy in Australian forests. Biological Conservation 180, 262–269.
| Long-term and large-scale control of the introduced red fox increases native mammal occupancy in Australian forests.Crossref | GoogleScholarGoogle Scholar |
Rolls EC (1984) ‘They all ran wild: the animals and plants that plague Australia.’ Revised edn. (Angus & Robertson: Sydney, NSW, Australia)
Roshier, DA, and Carter, A (2021). Space use and interactions of two introduced mesopredators, European red fox and feral cat, in an arid landscape. Ecosphere 12, e03628.
| Space use and interactions of two introduced mesopredators, European red fox and feral cat, in an arid landscape.Crossref | GoogleScholarGoogle Scholar |
Roshier, DA, Hotellier, FL, Carter, A, Kemp, L, Potts, J, Hayward, MW, and Legge, SM (2020). Long-term benefits and short-term costs: small vertebrate responses to predator exclusion and native mammal reintroductions in south-western New South Wales, Australia. Wildlife Research 47, 570–579.
| Long-term benefits and short-term costs: small vertebrate responses to predator exclusion and native mammal reintroductions in south-western New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Rovero F, Zimmermann F (2016) ‘Camera trapping for wildlife research.’ (Pelagic Publishing: Exeter, UK)
Rowcliffe, JM, Field, J, Turvey, ST, and Carbone, C (2008). Estimating animal density using camera traps without the need for individual recognition. Journal of Applied Ecology 45, 1228–1236.
| Estimating animal density using camera traps without the need for individual recognition.Crossref | GoogleScholarGoogle Scholar |
Royle, JA (2004). N-Mixture models for estimating population size from spatially replicated counts. Biometrics 60, 108–115.
| N-Mixture models for estimating population size from spatially replicated counts.Crossref | GoogleScholarGoogle Scholar |
Royle JA, Chandler RB, Sollmann R, Gardner B (Eds) (2014) ‘Spatial capture–recapture.’ (Academic Press: Boston, MA, USA)
Ruykys, L, and Carter, A (2019). Removal and eradication of introduced species in a fenced reserve: quantifying effort, costs and results. Ecological Management & Restoration 20, 239–249.
| Removal and eradication of introduced species in a fenced reserve: quantifying effort, costs and results.Crossref | GoogleScholarGoogle Scholar |
Sadlier, LMJ, Webbon, CC, Baker, PJ, and Harris, S (2004). Methods of monitoring red foxes Vulpes vulpes and badgers Meles meles: are field signs the answer? Mammal Review 34, 75–98.
| Methods of monitoring red foxes Vulpes vulpes and badgers Meles meles: are field signs the answer?Crossref | GoogleScholarGoogle Scholar |
Saunders G, McLeod L (2007) ‘Improving fox management strategies in Australia.’ (Bureau of Rural Sciences: Canberra, ACT, Australia)
Saunders G, Coman B, Kinnear J, Braysher M (1995) ‘Managing vertebrate pests: foxes.’ (Australian Government Publishing Service: Canberra, ACT, Australia)
Schwarz, CJ, and Seber, GAF (1999). Estimating animal abundance: review III. Statistical Science 14, 427–456.
| Estimating animal abundance: review III.Crossref | GoogleScholarGoogle Scholar |
Sharp, A, Norton, M, Marks, A, and Holmes, K (2001). An evaluation of two indices of red fox (Vulpes vulpes) abundance in an arid environment. Wildlife Research 28, 419–424.
| An evaluation of two indices of red fox (Vulpes vulpes) abundance in an arid environment.Crossref | GoogleScholarGoogle Scholar |
Sollmann, R, Mohamed, A, Samejima, H, and Wilting, A (2013a). Risky business or simple solution – relative abundance indices from camera-trapping. Biological Conservation 159, 405–412.
| Risky business or simple solution – relative abundance indices from camera-trapping.Crossref | GoogleScholarGoogle Scholar |
Sollmann, R, Gardner, B, Parsons, AW, Stocking, JJ, McClintock, BT, Simons, TR, Pollock, KH, and O’Connell, AF (2013b). A spatial mark–resight model augmented with telemetry data. Ecology 94, 553–559.
| A spatial mark–resight model augmented with telemetry data.Crossref | GoogleScholarGoogle Scholar |
Stephens, PA, Zaumyslova, OY, Miquelle, DG, Myslenkov, AI, and Hayward, GD (2006). Estimating population density from indirect sign: track counts and the Formozov–Malyshev–Pereleshin formula. Animal Conservation 9, 339–348.
| Estimating population density from indirect sign: track counts and the Formozov–Malyshev–Pereleshin formula.Crossref | GoogleScholarGoogle Scholar |
Stephens, PA, Pettorelli, N, Barlow, J, Whittingham, MJ, and Cadotte, MW (2015). Management by proxy? The use of indices in applied ecology. Journal of Applied Ecology 52, 1–6.
| Management by proxy? The use of indices in applied ecology.Crossref | GoogleScholarGoogle Scholar |
Tabak, MA, Norouzzadeh, MS, Wolfson, DW, Sweeney, SJ, Vercauteren, KC, Snow, NP, Halseth, JM, Di Salvo, PA, Lewis, JS, White, MD, Teton, B, Beasley, JC, Schlichting, PE, Boughton, RK, Wight, B, Newkirk, ES, Ivan, JS, Odell, EA, Brook, RK, Lukacs, PM, Moeller, AK, Mandeville, EG, Clune, J, and Miller, RS (2019). Machine learning to classify animal species in camera trap images: applications in ecology. Methods in Ecology and Evolution 10, 585–590.
| Machine learning to classify animal species in camera trap images: applications in ecology.Crossref | GoogleScholarGoogle Scholar |
Thompson, JA, and Fleming, PJS (1994). Evaluation of the efficacy of 1080 poisoning of red foxes using visitation to non-toxic baits as an index of fox abundance. Wildlife Research 21, 27–39.
| Evaluation of the efficacy of 1080 poisoning of red foxes using visitation to non-toxic baits as an index of fox abundance.Crossref | GoogleScholarGoogle Scholar |
Thomson, PC, Marlow, NJ, Rose, K, and Kok, NE (2000). The effectiveness of a large-scale baiting campaign and an evaluation of a buffer zone strategy for fox control. Wildlife Research 27, 465–472.
| The effectiveness of a large-scale baiting campaign and an evaluation of a buffer zone strategy for fox control.Crossref | GoogleScholarGoogle Scholar |
Towerton, AL, Penman, TD, Kavanagh, RP, and Dickman, CR (2011). Detecting pest and prey responses to fox control across the landscape using remote cameras. Wildlife Research 38, 208–220.
| Detecting pest and prey responses to fox control across the landscape using remote cameras.Crossref | GoogleScholarGoogle Scholar |
West P, Saunders G (2007) ‘Pest animal survey: a review of the distribution, impacts and control of invasive animals throughout NSW and the ACT.’ (NSW Department of Primary Industries: Orange, NSW, Australia)
Westbrooke, ME, Miller, JD, and Kerr, MKC (1998). The vegetation of the Scotia 1:100 000 map sheet, western New South Wales. Cunninghamia 5, 665–684.
Whittington, J, Hebblewhite, M, Chandler, RB, and Lentini, P (2018). Generalized spatial mark–resight models with an application to grizzly bears. Journal of Applied Ecology 55, 157–168.
| Generalized spatial mark–resight models with an application to grizzly bears.Crossref | GoogleScholarGoogle Scholar |
Woinarski, JCZ, Burbidge, AA, and Harrison, PL (2015). Ongoing unraveling of a continental fauna: decline and extinction of Australian mammals since European settlement. Proceedings of the National Academy of Sciences 112, 4531–4540.
| Ongoing unraveling of a continental fauna: decline and extinction of Australian mammals since European settlement.Crossref | GoogleScholarGoogle Scholar |


