Capture predicates corticosterone responses and a low recapture likelihood in a varanid lizard
Tim S. Jessop A *
A *
A Centre for Integrative Ecology, School of Life and Environmental Sciences, Deakin University, Waurn Ponds, Vic. 3216, Australia.
Wildlife Research 50(7) 517-525 https://doi.org/10.1071/WR22013
Submitted: 27 January 2022 Accepted: 13 June 2022 Published: 11 July 2022
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Understanding both the short- and long-term consequences of live animal capture is desirable to limit potential data biases or compromise animal welfare. The short-term elevation of glucocorticoid hormones in animals is an expected short-term stress consequence of trapping and restraint experiences. However, because these hormones also influence behaviour and memory, they may provide a physiological basis through which individuals vary in their recapture responses to subsequent trapping episodes.
Aims: This objective of this study was to evaluate the interplay among trapping method, corticosterone responsiveness and recapture likelihood in a lizard, the lace monitor (Varanus varius). The first aim compared how different capture methods and associated restraint durations influenced plasma corticosterone of lace monitors. The second aim evaluated the relationship between capture methodology, corticosterone response and annual recapture frequency. The third aim measured yearly estimates for the probability of lace monitor recapture.
Methods: Lace monitors were cage-trapped or noose-captured at 76 sampling sites across three annual sampling periods to measure capture experience, obtain blood samples and estimate recapture probabilities.
Results: As expected, an increased restraint time and exposure to different capture methods significantly influenced corticosterone concentrations in lace monitors. Lace monitor recapture rates were meagre, suggesting that irrespective of the capture method, restraint duration and corticosterone levels, any form of initial capture experience typically leads to long-lasting aversive behaviour.
Conclusions: Although plasma corticosterone concentrations may be tell-tale of a lace monitor’s duration to short-term capture and restraint, they were not associated with the recapture likelihood in subsequent trapping events.
Implications: Rapid and seemingly long-lasting trap aversion has apparent implications for the design of population monitoring programs used to study lace monitor population ecology.
Keywords: adrenocortical responsiveness, capture stressor, lace monitor, learning, live animal trapping, memory, reptile, restraint.
Introduction
The capture of individuals through trapping and short-term restraint is often needed to gain essential knowledge of wild animals (Williams et al. 2002; Laver et al. 2012; Purwandana et al. 2014; Molyneux et al. 2017). Such practices may provide the only means to gain insights into an individual’s health or phenotypic condition and, through recapture of marked animals, permit longer-term estimates of individual, population or community-level processes (Andrews 1982; Madsen and Shine 1996; Pike et al. 2008; Bickford et al. 2010; Nimmo et al. 2012; Stokeld et al. 2018). Given that methods of trapping and restraint vary considerably among individuals and species, they can potentially offer different regimes of physiological or psychological stress (Wingfield and Romero 2001; Moore and Jessop 2003; Jessop and Hamann 2005; Langkilde and Shine 2006; Anson et al. 2013). The duration of time spent in traps or restraint means that individuals can face exposure to the elements, be deprived of food and water, and endure fear, anxiety or physiological stress (Broom et al. 1993). Because animals are often exposed to novel stressors during the capture process, they can face short- and potentially longer-term consequences (Lynn et al. 2003; Wikelski and Michael Romero 2003; Arnemo et al. 2006; Breed et al. 2019).
Short-term effects associated with the capture and restraint of animals typically include inducing a physiological-stress response (Wingfield and Romero 2001; Romero 2004). In response to stressors, reptiles activate the hypothalamic–pituitary–adrenal/interrenal (HPA) axis. This neuroendocrine pathway results in the release of glucocorticoids (corticosterone) from the inter-renal tissue (Moore and Jessop 2003). Glucocorticoids have system-wide effects on behaviour and physiology; therefore, trapping methods that elevate glucocorticoids could lead to short- and long-term phenotypic changes. For example, glucocorticoids have well known effects on intermediary metabolism (Romero 2004). However, they are also expected to influence cognition and memory consolidation and affect fear conditioning associated with noxious stimuli (i.e. trapping and restraint; Roozendaal 2000; Rodrigues et al. 2009). In general, glucocorticoids act on long-term fear memory consolidation in a dose–response relationship (de Kloet et al. 1999). In mammals such as humans and rodents, glucocorticoid concentrations appear to influence memory consolidation and learning in a concave up relationship (i.e. memory is most affected at low and high hormone concentrations; de Kloet et al. 1999; Diamond et al. 2007; Salehi et al. 2010). In particular, at low and high plasma concentrations, glucocorticoids have inhibitory effects on memory consolidation, whereas moderate doses produce positive effects. Mammalian studies indicate that glucocorticoids modulate memory consolidation via binding to receptors in multiple brain regions, especially the amygdala, a temporal brain structure associated with emotion and fear (reviewed in McGaugh 2004; Diamond et al. 2007; Schwabe et al. 2012). Indeed, evidence from the neurobiological literature suggests that trapping animals (a procedure that is likely to elicit both a stress and fear response) could have sustained effects on learning and memory and affect an individual’s responses to a repeated exposure to adverse stimuli (Schwabe et al. 2012). Furthermore, correlations between stress responsiveness and personality type may influence how individuals experience and learn from novel stressors. For example, individuals with bold personalities are expected to have low-glucocorticoid stress responsiveness that promotes engagement with novel stimuli and reduces aversive learning if such stimuli are generally considered threatening (Cockrem 2007, 2013).
If trapping-induced stress and fear influence an individual’s learning and memory to affect their recapture likelihood in future trapping events, it can have significant implications for field studies of wild animals. Whereby differences in HPA axis output promote aversive learning as a result of the initial trapping experience, then sampling biases may arise if only animals with lower stress responses are recaptured (Biro and Dingemanse 2009; Camacho et al. 2017). For example, in willow warblers (Phylloscopus trochilus) captured by mist-netting, researchers found that the male birds could memorise the first capture event conditions, which caused them to avoid a subsequent recapture by recognising similar conditions and modifying their behaviour (Linhart et al. 2012). Thus, addressing the relationship between capture-induced stress and behaviour is essential to avoid confounding data in longer-term field studies (Fowler et al. 2013). Indeed, the assumption that individuals demonstrate similar capture probabilities underpins many analytical or statistical techniques used in wildlife ecology (Williams et al. 2002; Zuur et al. 2009). Thus, the propensity for animals to develop trap-wariness can make it difficult to reliably estimate population parameters (e.g. abundance, dispersal or survival) in long-term wildlife studies (Williams et al. 2002; Goñi et al. 2003; Purwandana et al. 2015; Jessop et al. 2018).
Although there may be potential for corticosterone to mediate individual heterogeneity in subsequent capture-related behaviour, there is broad recognition that animals may develop more general patterns of recapture behaviour or other responses (Cubaynes et al. 2010; Pradel and Sanz-Aguilar 2012). For example, many studies have reported that wild animal populations, via learning, can develop neophillic (i.e. trappy happy) or neophobic responses (e.g. aversion/trap shyness; Brehm and Mortelliti 2018). These broad-scale responses demonstrate that many animals within a population can rapidly learn to adjust their behaviour to future trapping events (Stryjek et al. 2019). Additionally, exposure to trapping may lead aminals to suffer from capture-related mortality. For example, individuals of some species suffer from rapid capture-related mortality (e.g. capture myopathy; Breed et al. 2019). Here, if individuals undertake intense physical activity associated with the capture process, they may induce metabolic acidosis that causes myocyte necrosis, which can be lethal if sufficiently severe (Paterson 2007).
The objective of this study was to address three aims. The first aim evaluated the effect of noose and cage trap-related capture methods and associated restraint times on plasma corticosterone concentrations in a large monitor lizard, the lace monitor (Varanus varius).
Like most reptiles, it would be predicted that both the method of capture and the associated duration of restraint time would increase plasma corticosterone concentrations of lace monitors (Moore and Jessop 2003; Hamann et al. 2007; Lancaster et al. 2010; Payne et al. 2012; Jessop et al. 2015).
For the second aim, I evaluated the associations between an individual’s first capture experience and corticosterone response at release to see whether it was related to their recapture likelihood in subsequent trapping events. Here, individuals with the most stressful capture experiences and associated higher corticosterone responses are expected to increase aversive learning and memory consolidation and limit an individual’s recapture in subsequent trapping events (Thaker et al. 2010). Hence, those individuals who produce greater corticosterone responses at the initial capture are predicted to have a lower likelihood of recapture than have lace monitors that have smaller responses.
For the third aim, in recognition that wild animals may develop distinct patterns of recapture behaviour different from those predicted by their corticosterone responses alone (Cubaynes et al. 2010; Pradel and Sanz-Aguilar 2012; Stryjek et al. 2019), I assessed the ratio of recaptured to newly captured lace monitors across three annual trapping events to estimate the yearly recapture probability. If the proportion of recaptured to newly captured lace monitor remains low in the second and third trapping events, it could suggest that lace monitors develop aversion (e.g. neophobia or trap shyness) to subsequent annual trapping events. Alternatively, if the proportion of recaptured to newly captured lace monitors disproportionately increases in the second and third yearly trapping events, it could suggest that lace monitors develop a neophilic response to subsequent capture events.
Methods
Study species
The lace monitor is a semi-arboreal diurnal reptile that hunts on the ground and in trees (Jessop et al. 2010). It is widely distributed in non-arid areas of eastern Australia, and weighing up to 14 kg, it functions as a large native predator (Weavers 1988; Guarino 2001; Smissen et al. 2013). It is a generalist predator, and, in southern Australia, its activity is seasonal, with it being predominantly active in warmer months and inactive in winter (Guarino 2002; Jessop et al. 2012; Jessop et al. 2013a).
Study area
The study was conducted in the Cape Conran Coastal Park and adjacent Murrungowar State Forest in East Gippsland, Victoria, Australia (37°48′S, 148°52′E). The study area comprised coastal forests covering 42 000 ha of two common vegetation types, namely (1) coastal woodland dominated by Banksia serrata and B. integrifolia; and (2) lowland forest dominated by Eucalyptus sieberi and E. globoidea. Here, 76 fixed trapping sites were located within the study area, and a 2-km interval separated each trapping locality. The capture of lace monitors was conducted over three annual trapping events in the summers of December 2007–January 2008, December 2008–January 2009 and December 2009–January 2010. These summer periods of capture coincided with higher seasonal temperatures favourable to optimising the trapping effort of lace monitors (Jessop et al. 2013a).
Lace monitor capture methods
Lace monitors were captured either by using traps or noose capture. These capture methods are described as below.
Traps
Aluminium box traps (2 m × 0.3 m × 0.3 m) were used to capture lace monitors (N = 17; mean SVL = 56.67 cm; mean body mass = 3.37 kg; 12 males, 5 females). These traps were purpose-built for lace monitor capture, and the trap design was down-sized from that used for Komodo dragons (Ariefiandy et al. 2014). The trap had solid walls of aluminium sheeting, and a single row of ventilation holes was present on each wall of the trap. This design provided a trapped lace monitor with a largely enclosed and darkened internal space. There were no other structures (e.g. hide) inside the trap to provide additional shelter. These traps were positioned randomly within each of the 76 sites. Traps were baited with beef infused with tuna emulsion oil and located 50–100 m off the adjacent forest management track. Traps were always placed in shaded areas (e.g. under tree cover) to limit the risk of overheating to lace monitors. For lace monitors captured in traps, it was impossible to measure the specific capture duration. However, the duration that individuals were exposed to this procedure is likely hours (up to ~4 h) because traps were opened during daylight hours (08:00 am to 06:00 pm) and checked twice daily in the mid- to late morning (09:00 am to 11:45 am) and again mid- to late afternoon (03:00 pm to 06:00 pm). Lace monitors were not confined and could move about inside the trap. Again after capture, each lace monitor was removed from a trap and restrained with electrical tape to close the mouth shut and secure the fore and hind limbs. Post-restraint, trap-captured lace monitors were then immediately blood-sampled, had morphological measures taken and released at their points of capture. The effective trapping effort for each annual survey amounted to ~760 trapping hours per year.
Noose capture
During the twice-daily checking of traps, lace monitors were detected using visual search methods along forest management tracks (i.e. used to travel between trap localities) and when walking off the management track to inspect each trap. Once sighted, I approached and attempted to capture each lizard by using a long pole fitted with a noose rope. The noose was placed over the lizard’s head and pulled taut to secure the animal. Restraint was initiated by removing each lizard from the noose and binding the mouth and limbs with electrical tape. The combined time for approaching, capturing, and restraining individuals ranged between 90 and 150 s. In the first annual capture event, noose-caught lace monitors (N = 36 individuals; mean SVL = 55.46 cm mean body mass = 3.22 kg; 25 males, 11 females) were then held for three randomly assigned restraint periods consisting of 3 (N = 12), 45 (N = 12) or 90 (N = 12) min durations to enable comparison of the effect of capture and different restraint periods on plasma corticosterone responses. Lace monitors assigned to the 3 min restraint period were manually restrained, whereas individuals assigned for the 45 and 90 min restraint periods were secured inside an individual hessian sack.
Lace monitor blood sampling
In the first year of study (December 2008–January 2009), each lace monitor (N = 53) after its designated trap-capture or post-noose restraint period had a ~1 mL blood sample taken from its ventral caudal vein with a 21-gauge needle and a 3 mL syringe. Blood samples were then placed into individually labelled lithium heparin containers (BD Microtainer™ Tubes, BD Vacutainer Systems, Franklin Lakes, NJ, USA) and stored on ice. Once back at the camp (~3 h), samples were centrifuged (2100g for 5 min at room temperature) and the plasma was removed and stored at −20°C until the corticosterone assay was performed (Jessop et al. 2012; Smissen et al. 2013). These first-year captures provided an initial sample of marked individuals to evaluate the effect of an individual’s corticosterone concentration on their recapture likelihood in the two subsequent annual trapping events.
Lace monitor identification, measurements and sex determination
Across the three annual trapping events, all lace monitors were individually identified with a passive integrated transponder to identify individuals beyond their first capture (Scheelings and Jessop 2011; Jessop et al. 2012). A sterile needle and applicator were used to insert the PIT tag laterally between the dermis and the muscle of each individual’s upper left hind leg. The PIT-tag insertion site was sealed with surgical glue (Vetbond Tissue Adhesive, 3M, USA). In addition, I recorded each individual’s snout to vent length (SVL), measured as the distance between the ventral tip of the snout and the most posterior opening of the cloacal slit. The body mass of each individual was recorded using a digital scale. The sex of individuals was subsequently determined using polymerase chain reaction (PCR) primers that amplified sex-specific alleles from genomic DNA obtained from blood (Jessop et al. 2012). On completion of the different capture and restraint durations, lace monitors were released at their capture point.
Corticosterone radioimmunoassay
Total corticosterone in lace monitor plasma was measured using radioimmunoassay (RIA) techniques identical to those used elsewhere (Jessop et al. 2015). Plasma samples (100 μL) were extracted for corticosterone concentrations by using a Corticosterone 3H Kit (MP Biomedicals, LLC). Final steroid concentrations were calculated from standard curves and corrected for individual sample recovery, individual plasma volume and the addition of tritiated steroid. Average (±s.e.m.) sample recovery was 75.7% ±0.028, with an intra-assay CV of 7.6% and an inter-assay CV of 13.04%. As reported by the manufacturer, the antibody had 100% cross-reactivity with corticosterone, 11% with 11-dehydrocorticosterone, 7% with 11-deoxycorticosterone, and <1% with the following steroids: progesterone, cortisol, aldosterone, testosterone, pregnenolone and 5α-DHT.
Data analyses
For Aim 1, a generalised linear mixed model (GLMM) was used to evaluate the effects of different capture methods and their associated restraint time (and blood sampling) on lizard plasma corticosterone concentrations. I also included the effects of sex and body size (SVL) in the model to test whether these predictor variables were associated with significant differences in plasma corticosterone concentrations. The location of an individual’s capture was used as a random effect in the model to account for any spatial autocorrelation in plasma corticosterone concentrations related to the proximity of individuals. This model was fitted with a Gaussian distribution and an identity canonical link. For Aim 2, a GLM (with a Poisson distribution and logit link) was used to test for the effects of the capture method, corticosterone and their interaction on the number of captures acquired for each tagged lizard across the study. Post hoc tests were conducted to determine where statistical significance was obtained within the main effects. Statistics were performed using SPSS V.22 (IBM).
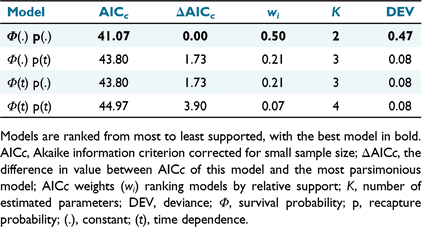
For Aim 3, I used a Cormack–Jolly–Seber (CJS) model in the program MARK to estimate the annual lace monitor probability of capture (i.e. the proportion of recaptures within the total samples of lace monitors captured in the second and third yearly trapping events; Brownie et al. 1993; Nichols and Kendall 1995). This model simultaneously estimates apparent survival (Φ) and capture probability (P) for marked individuals (White and Burnham 1999). A candidate set of four models was assessed to evaluate capture probability in lace monitors. These models considered temporal and non-temporal variation in Φ and P. Models were ranked using the AICc value. The individual model weights (wi) were estimated to measure relative model support further (Burnham and Anderson 2003).
Ethical standards
This research abided by the journal’s guidelines on ethical standards. The study was conducted under the Department of Sustainability and Environment and a National Parks Act (1975) research permit 10005037, and a University of Melbourne Ethics Permit 0911328.
Results
Effects of trapping method and restraint duration on plasma corticosterone
Capture method and restraint duration significantly influenced lace monitor plasma corticosterone concentrations (GLMM, F3,53 = 8.99, P < 0.001; Fig. 1). Plasma corticosterone increased across the three post-noosing restraint intervals (i.e. 3, 45 and 90 min). Pairwise post hoc tests for individuals captured by noosing indicated that the plasma corticosterone concentrations measured after 90 min of restraint were significantly higher than those reported after 3 (P < 0.001) and 45 (P = 0.044) min of restraint. Lizards captured in traps and held for multiple hours before blood sampling also exhibited high corticosterone concentrations. These were significantly greater than those exposed to the 3 (P < 0.001) and 45 (P = 0.035) min restraint periods after noosing. However, there was no significant (P = 0.717) difference between the plasma corticosterone concentrations of individuals captured in traps and those of individuals exposed to 90 min of restraint post-noosing. There was no significant effect of sex (GLMM, F1,54 = 0.17, P = 0.685) or body size (GLMM, F1,54 = 0.13, P = 0.72) on lace monitor plasma corticosterone concentrations.
Effects of capture and plasma corticosterone on lace monitor recapture rates
Variation in the number of recaptures for each lizard across the 3-year study duration was not significantly influenced by an individual’s corticosterone concentration on release at first capture (GLM, Wald χ2 = 0.126, P = 0.723). This non-significant relationship between an individual’s corticosterone concentration and its number of captures was defined by a nearly flat slope (β = 0.003 ± 0.002) and a low coefficient of variation (R2 = 0.01). A power test estimated that a minimum R2 of 0.23 would be needed with the current sample size. Similarly, neither trapping method (GLM, Wald χ2 = 0.024, P = 0.952) nor the interaction between these two effects (GLM, Wald χ2 = 0.012, P = 0.915; Fig. 2) influenced the number of lace monitor recaptures. These results reflected that just 3 of the 53 individuals captured in the first year of the study were recaptured again in the two subsequent annual sampling events (mean captures = 1.08 ± 0.001 per individual). Two of the three recaptured individuals comprised two large adult males (SVL = 61.45 and 69.55 cm) noosed at their first capture event and caught by trap in the second year of study. The third recapture was a subadult female (SVL = 48.90) captured by a trap in her first capture event and then recaptured by a noose in the third year of study.
Annual estimates of lace monitor recapture probability
I captured 152 lace monitors, including 53, 48, and 51 individuals in the summers of December 2007–January 2008, December 2008–January 2009 and December 2009–January 2010 respectively. Four recaptures were present among the total number of captures, with two tagged individuals captured in the second and third annual trapping events (Fig. 3). The four recaptured lace monitors included the three aforementioned individuals recaptured from the first trapping event. The fourth recapture was an adult male (SVL = 64.58 cm), first captured by a trap in the second trapping event and then retrapped in the third trapping event. The annual recapture probability estimates of previously tagged lace monitors relative to new captures for the second and third annual capture events were 0.039 ± 0.027 and 0.019 ± 0.013% respectively (Fig. 3). That top-ranked Cormack–Jolly–Seber model indicated that there was not sufficient evidence of annual differences in the lace monitor probability of recapture for tagged individuals (Table 1).
|
|
|
Discussion
Lace monitors responded to variation in capture method and the associated restraint duration by increasing plasma corticosterone concentration. This observation validates that field trapping methods activated a physiological stress response in this species (Scheelings and Jessop 2011). Typically, the HPA/I axis can rapidly up-regulate to stressors, causing increased glucocorticoid secretion into the blood plasma within 3–5 min (Romero and Reed 2005). The plasma corticosterone response increased over multiple hours of capture or restraint for lace monitors. Similar, slowly induced capture-related corticosterone responses are consistent with results found in other large reptiles (Lance and Elsey 1986; Jessop 2001; Moore and Jessop 2003; Jessop et al. 2004, 2013c). The duration of capture and restraint, as stressful stimuli, are expected to trigger a commensurate increase in glucocorticoids to enable individuals to activate physiological and behavioural responses to cope with capture experiences (Wingfield et al. 1998; Romero 2004).
Further research that can better assess the actual duration of lace monitor restraint within cage traps is needed to discount the effect of the trapping method on this species’ corticosterone responses. Other studies comparing different trapping methods have suggested that capture methodology can lead to differences in individuals’ acute stress response at similar restraint durations (Lakušić et al. 2020). Importantly, for individual lace monitors, given the short duration of the overall trapping event (i.e. acute exposure), it would be expected that any increase in plasma corticosterone would confer short-term benefits rather than pathological or other negative fitness-related consequences typical of either exposure to pervasive acute or chronic stress responses (Jessop et al. 2013b; Narayan et al. 2015).
The second aim evaluated the correlation between a lace monitor’s corticosterone concentration measured at their first capture and the number of recaptures in subsequent annual trapping events. It was evident that differences in trapping method, restraint time, and post-release corticosterone concentration did not influence an individual subsequent recapture rate. This result arose because almost all lace monitors captured in the first year were never recaptured again. However, there is also mixed evidence that experimentally increased plasma corticosterone concentrations can elevate the propensity for aversive, neophobia or fear-related learning behaviours in wild animals (Thaker et al. 2010; de Bruijn and Romero 2020). Additionally, other endocrine processes, besides glucocorticoids, are well recognised to influence aversive learning and memory consolidation in vertebrates exposed to stress (Joëls et al. 2006; Schwabe et al. 2012). Of course, stressful stimuli will also activate the autonomic nervous system to increase catecholamine production, such as norepinephrine (NE), which will rapidly bind to adrenoreceptors on vagal afferents to stimulate the prefrontal cortex, amygdala and hippocampus (Schwabe et al. 2012). Similar to glucocorticoids, the stimulatory actions of NE can enhance the encoding and processing of stressful stimuli to consolidate memories, to allow learning of aversive stimuli (Joëls et al. 2006). Thus, catecholamines or other stress-related neurotransmitters (e.g. CRF) may help explain the physiological basis to why lace monitors demonstrate extensive recapture aversion (de Bruijn and Romero 2020). Further study is now required to identify physiological mechanisms, so as to explain what causes this response but also to explain why rapid-onset capture-induced aversion appears so conserved within this lace monitor population.
The third aim confirmed that the annual recapture probability of lace monitors was low (i.e. <5% per year) and similar between the second and third trapping events. This result contrasts with the expectation that increased trapping effort and ongoing tagging of individuals will increase the proportion of recaptures to new captures over time, unless there are mitigating circumstances (Williams et al. 2002). For example, high annual recapture probabilities are obtained using similar methods in other lace monitor populations and the congeneric Komodo dragon (Varanus komodoensis). Indeed populations of Komodo dragons can obtain annual recapture probabilities greater than 70% after three-yearly trapping events (Laver et al. 2012; Ariefiandy et al. 2013, 2014; Purwandana et al. 2014; Lei and Booth 2018). The extremely low annual lace monitor recapture rate suggests that when this population is exposed to different capture or restraint protocols, they predominantly develop long-term aversive behaviour unrelated to plasma corticosterone responses. Other lizards have shown similar broad-scale and rapid aversion responses to capture or novel stressors (Marcellini and Jenssen 1991; Thaker et al. 2010).
Could low annual recapture rates indicate acute or longer-term capture-related consequences such as deferred capture-related mortality? Several reasons suggest this outcome to be unlikely. First, capture myopathy, a potential cause of acute mortality, typically arises when individuals exhibit intense physical activity during capture, confinement or restraint associated with the trapping procedure (Breed et al. 2019). Common clinical symptoms of capture myopathy preceding death include lethargy, loss of coordination and impaired movement (Paterson 2007). All lace monitors released in this study exhibited typical escape behaviour (i.e. fleeing or tree climbing) independent of restraint time, capture methods or corticosterone concentrations, suggesting no apparent short-term pathological consequences. Second, in a concurrent study using externally marked lace monitors also captured via trap and noose (i.e. from the same population), who exhibited restricted movement owing to their reliance on anthropogenic food subsidies, it was evident that through repeated resightings, these individuals survived over several weeks, suggesting an absence of long-term lethal trapping consequences (Jessop et al. 2012). Thus, most tagged individuals would be expected to persist throughout the study duration to permit recapture. Similarly, the concurrent use of non-invasive trapping methods (e.g. camera-traps and track counts) to measure population indices in lace monitors over the study duration indicated no evidence of rapid population decline to imply broad-scale mortality as a result of trapping or other environmental processes (Anson et al. 2014; Hu et al. 2019).
In conclusion, despite the vast amount of literature that posits that increased glucocorticoid concentrations following exposure to stressors can exert a wide variety of behavioural and physiological effects that influence animal performance and fitness (Wingfield and Romero 2001; Romero 2004), the present study indicated that even with increased exposures to lizard-trapping protocols that lead to proportional increases in the plasma glucocorticoid responses, it has little influence on lizard recapture behaviour. Thus, because capture and restraint are often perceived by animals as stressful, using plasma corticosterone as a putative physiological measure of an individual’s capture exposure may not correlate well with many short- or longer-term stress-related consequences.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The author declares no conflicts of interest.
Declaration of funding
Support was provided by University of Melbourne Early Career Research Grant to the author.
Acknowledgements
I thank Tim Lockwood, Dr Franciscus Scheelings and Dr Jen Anson for assistance in sample collection or laboratory processing. I also acknowledge David Booth and another anonymous reviewer for providing constructive comments that improved the paper.
References
Andrews, RM (1982). Patterns of growth in reptiles. Biology of the Reptilia 13, 273–320.Anson, JR, Dickman, CR, Boonstra, R, and Jessop, TS (2013). Stress triangle: do introduced predators exert indirect costs on native predators and prey? PLoS ONE 8, e60916.
| Stress triangle: do introduced predators exert indirect costs on native predators and prey?Crossref | GoogleScholarGoogle Scholar | 23585861PubMed |
Anson, JR, Dickman, CR, Handasyde, K, and Jessop, TS (2014). Effects of multiple disturbance processes on arboreal vertebrates in eastern Australia: implications for management. Ecography 37, 357–366.
| Effects of multiple disturbance processes on arboreal vertebrates in eastern Australia: implications for management.Crossref | GoogleScholarGoogle Scholar |
Ariefiandy, A, Purwandana, D, Seno, A, Ciofi, C, and Jessop, TS (2013). Can camera traps monitor Komodo dragons a large ectothermic predator? PLoS ONE 8, e58800.
| Can camera traps monitor Komodo dragons a large ectothermic predator?Crossref | GoogleScholarGoogle Scholar | 23527027PubMed |
Ariefiandy, A, Purwandana, D, Seno, A, Chrismiawati, M, Ciofi, C, and Jessop, TS (2014). Evaluation of three field monitoring-density estimation protocols and their relevance to Komodo dragon conservation. Biodiversity and Conservation 23, 2473–2490.
| Evaluation of three field monitoring-density estimation protocols and their relevance to Komodo dragon conservation.Crossref | GoogleScholarGoogle Scholar |
Arnemo, JM, Ahlqvist, P, Andersen, R, Berntsen, F, Ericsson, G, Odden, J, Brunberg, S, Segerström, P, and Swenson, JE (2006). Risk of capture-related mortality in large free-ranging mammals: experiences from Scandinavia. Wildlife Biology 12, 109–113.
| Risk of capture-related mortality in large free-ranging mammals: experiences from Scandinavia.Crossref | GoogleScholarGoogle Scholar |
Bickford, D, Howard, SD, Ng, DJJ, and Sheridan, JA (2010). Impacts of climate change on the amphibians and reptiles of Southeast Asia. Biodiversity and Conservation 19, 1043–1062.
| Impacts of climate change on the amphibians and reptiles of Southeast Asia.Crossref | GoogleScholarGoogle Scholar |
Biro, PA, and Dingemanse, NJ (2009). Sampling bias resulting from animal personality. Trends in Ecology & Evolution 24, 66–67.
| Sampling bias resulting from animal personality.Crossref | GoogleScholarGoogle Scholar |
Breed, D, Meyer, LCR, Steyl, JCA, Goddard, A, Burroughs, R, and Kohn, TA (2019). Conserving wildlife in a changing world: understanding capture myopathy—a malignant outcome of stress during capture and translocation. Conservation Physiology 7, coz027.
| Conserving wildlife in a changing world: understanding capture myopathy—a malignant outcome of stress during capture and translocation.Crossref | GoogleScholarGoogle Scholar | 31304016PubMed |
Brehm, AM, and Mortelliti, A (2018). Mind the trap: large-scale field experiment shows that trappability is not a proxy for personality. Animal Behaviour 142, 101–112.
| Mind the trap: large-scale field experiment shows that trappability is not a proxy for personality.Crossref | GoogleScholarGoogle Scholar |
Broom DM, Johnson KG, Broom DM (1993) ‘Stress and animal welfare.’ (Springer)
Brownie, C, Hines, JE, Nichols, JD, Pollock, KH, and Hestbeck, JB (1993). Capture–recapture studies for multiple strata including non-Markovian transitions. Biometrics 49, 1173–1187.
| Capture–recapture studies for multiple strata including non-Markovian transitions.Crossref | GoogleScholarGoogle Scholar |
Burnham KP, Anderson DR (2003) ‘Model selection and multimodel inference: a practical information-theoretic approach.’ (Springer Science & Business Media)
Camacho, C, Canal, D, and Potti, J (2017). Lifelong effects of trapping experience lead to age-biased sampling: lessons from a wild bird population. Animal Behaviour 130, 133–139.
| Lifelong effects of trapping experience lead to age-biased sampling: lessons from a wild bird population.Crossref | GoogleScholarGoogle Scholar |
Cockrem, JF (2007). Stress, corticosterone responses and avian personalities. Journal of Ornithology 148, 169–178.
| Stress, corticosterone responses and avian personalities.Crossref | GoogleScholarGoogle Scholar |
Cockrem, JF (2013). Individual variation in glucocorticoid stress responses in animals. General and Comparative Endocrinology 181, 45–58.
| Individual variation in glucocorticoid stress responses in animals.Crossref | GoogleScholarGoogle Scholar | 23298571PubMed |
Cubaynes, S, Pradel, R, Choquet, R, Duchamp, C, Gaillard, J-M, Lebreton, J-D, Marboutin, E, Miquel, C, Reboulet, A-M, Poillot, C, Taberlet, P, and Gimenez, O (2010). Importance of accounting for detection heterogeneity when estimating abundance: the case of french wolves. Conservation Biology 24, 621–626.
| Importance of accounting for detection heterogeneity when estimating abundance: the case of french wolves.Crossref | GoogleScholarGoogle Scholar | 20105205PubMed |
de Bruijn, R, and Romero, LM (2020). Prior restraint stress inhibits habituation to novel objects in the European starlings (Sturnus vulgaris). Journal of Experimental Zoology Part A: Ecological and Integrative Physiology 333, 88–95.
| Prior restraint stress inhibits habituation to novel objects in the European starlings (Sturnus vulgaris).Crossref | GoogleScholarGoogle Scholar |
de Kloet, ER, Oitzl, MS, and Joëls, M (1999). Stress and cognition: are corticosteroids good or bad guys? Trends in Neurosciences 22, 422–426.
| Stress and cognition: are corticosteroids good or bad guys?Crossref | GoogleScholarGoogle Scholar | 10481183PubMed |
Diamond, DM, Campbell, AM, Park, CR, Halonen, J, and Zoladz, PR (2007). The temporal dynamics model of emotional memory processing: a synthesis on the neurobiological basis of stress-induced amnesia, flashbulb and traumatic memories, and the Yerkes–Dodson law. Neural Plasticity 2007, 60803.
| The temporal dynamics model of emotional memory processing: a synthesis on the neurobiological basis of stress-induced amnesia, flashbulb and traumatic memories, and the Yerkes–Dodson law.Crossref | GoogleScholarGoogle Scholar | 17641736PubMed |
Fowler J, Cohen L, Jarvis P (2013) ‘Practical statistics for field biology.’ (John Wiley & Sons)
Goñi, R, Quetglas, A, and Reñones, O (2003). Differential catchability of male and female European spiny lobster Palinurus elephas (Fabricius, 1787) in traps and trammelnets. Fisheries Research 65, 295–307.
| Differential catchability of male and female European spiny lobster Palinurus elephas (Fabricius, 1787) in traps and trammelnets.Crossref | GoogleScholarGoogle Scholar |
Guarino, F (2001). Diet of a large carnivorous lizard, Varanus varius. Wildlife Research 28, 627–630.
| Diet of a large carnivorous lizard, Varanus varius.Crossref | GoogleScholarGoogle Scholar |
Guarino, F (2002). Spatial ecology of a large carnivorous lizard, Varanus varius (Squamata : Varanidae). Journal of Zoology 258, 449–457.
| Spatial ecology of a large carnivorous lizard, Varanus varius (Squamata : Varanidae).Crossref | GoogleScholarGoogle Scholar |
Hamann, M, Jessop, TS, and Schäuble, CS (2007). Fuel use and corticosterone dynamics in hatchling green sea turtles (Chelonia mydas) during natal dispersal. Journal of Experimental Marine Biology and Ecology 353, 13–21.
| Fuel use and corticosterone dynamics in hatchling green sea turtles (Chelonia mydas) during natal dispersal.Crossref | GoogleScholarGoogle Scholar |
Hu, Y, Gillespie, G, and Jessop, TS (2019). Variable reptile responses to introduced predator control in southern Australia. Wildlife Research 46, 64–75.
| Variable reptile responses to introduced predator control in southern Australia.Crossref | GoogleScholarGoogle Scholar |
Jessop, TS (2001). Modulation of the adrenocortical stress response in marine turtles (Cheloniidae): evidence for a hormonal tactic maximizing maternal reproductive investment. Journal of Zoology 254, 57–65.
| Modulation of the adrenocortical stress response in marine turtles (Cheloniidae): evidence for a hormonal tactic maximizing maternal reproductive investment.Crossref | GoogleScholarGoogle Scholar |
Jessop, TS, and Hamann, M (2005). Interplay between age class, sex and stress response in green turtles (Chelonia mydas). Australian Journal of Zoology 53, 131–136.
| Interplay between age class, sex and stress response in green turtles (Chelonia mydas).Crossref | GoogleScholarGoogle Scholar |
Jessop, T, Sumner, J, Lance, V, and Limpus, C (2004). Reproduction in shark-attacked sea turtles is supported by stress-reduction mechanisms. Proceedings of the Royal Society of London. Series B: Biological Sciences 271, S91–S94.
| Reproduction in shark-attacked sea turtles is supported by stress-reduction mechanisms.Crossref | GoogleScholarGoogle Scholar | 15101429PubMed |
Jessop, TS, Urlus, J, Lockwood, T, and Gillespie, G (2010). Preying possum: assessment of the diet of lace monitors (Varanus varius) from coastal forests in southeastern Victoria. Biawak 4, 59–66.
Jessop, TS, Smissen, P, Scheelings, F, and Dempster, T (2012). Demographic and phenotypic effects of human mediated trophic subsidy on a large Australian lizard (Varanus varius): meal ticket or last supper? PLoS ONE 7, e34069.
| Demographic and phenotypic effects of human mediated trophic subsidy on a large Australian lizard (Varanus varius): meal ticket or last supper?Crossref | GoogleScholarGoogle Scholar | 22509271PubMed |
Jessop, TS, Kearney, MR, Moore, JL, Lockwood, T, and Johnston, M (2013a). Evaluating and predicting risk to a large reptile (Varanus varius) from feral cat baiting protocols. Biological Invasions 15, 1653–1663.
| Evaluating and predicting risk to a large reptile (Varanus varius) from feral cat baiting protocols.Crossref | GoogleScholarGoogle Scholar |
Jessop, TS, Letnic, M, Webb, JK, and Dempster, T (2013b). Adrenocortical stress responses influence an invasive vertebrate’s fitness in an extreme environment. Proceedings of the Royal Society B: Biological Sciences 280, 20131444.
| Adrenocortical stress responses influence an invasive vertebrate’s fitness in an extreme environment.Crossref | GoogleScholarGoogle Scholar | 23945686PubMed |
Jessop, TS, Anson, JR, Narayan, E, and Lockwood, T (2015). An introduced competitor elevates corticosterone responses of a native lizard (Varanus varius). Physiological and Biochemical Zoology 88, 237–245.
| An introduced competitor elevates corticosterone responses of a native lizard (Varanus varius).Crossref | GoogleScholarGoogle Scholar | 25860823PubMed |
Jessop, TS, Woodford, R, and Symonds, MRE (2013c). Macrostress: do large-scale ecological patterns exist in the glucocorticoid stress response of vertebrates? Functional Ecology 27, 120–130.
| Macrostress: do large-scale ecological patterns exist in the glucocorticoid stress response of vertebrates?Crossref | GoogleScholarGoogle Scholar |
Jessop, TS, Ariefiandy, A, Purwandana, D, Ciofi, C, Imansyah, J, Benu, YJ, Fordham, DA, Forsyth, DM, Mulder, RA, and Phillips, BL (2018). Exploring mechanisms and origins of reduced dispersal in island Komodo dragons. Proceedings of the Royal Society B: Biological Sciences 285, 20181829.
| Exploring mechanisms and origins of reduced dispersal in island Komodo dragons.Crossref | GoogleScholarGoogle Scholar | 30429305PubMed |
Joëls, M, Pu, Z, Wiegert, O, Oitzl, MS, and Krugers, HJ (2006). Learning under stress: how does it work? Trends in Cognitive Sciences 10, 152–158.
| Learning under stress: how does it work?Crossref | GoogleScholarGoogle Scholar | 16513410PubMed |
Lakušić, M, Billy, G, Bjelica, V, Golubović, A, Anđelković, M, and Bonnet, X (2020). Effect of capture, phenotype, and physiological status on blood glucose and plasma corticosterone levels in free-ranging dice snakes. Physiological and Biochemical Zoology 93, 477–487.
| Effect of capture, phenotype, and physiological status on blood glucose and plasma corticosterone levels in free-ranging dice snakes.Crossref | GoogleScholarGoogle Scholar | 33164670PubMed |
Lancaster, P, Jessop, TS, and Stuart-Fox, D (2010). Testing the independent effects of population and shelter density on behavioural and corticosterone responses of tree skinks. Australian Journal of Zoology 58, 295–302.
| Testing the independent effects of population and shelter density on behavioural and corticosterone responses of tree skinks.Crossref | GoogleScholarGoogle Scholar |
Lance, VA, and Elsey, RM (1986). Stress-induced suppression of testosterone secretion in male alligators. Journal of Experimental Zoology 239, 241–246.
| Stress-induced suppression of testosterone secretion in male alligators.Crossref | GoogleScholarGoogle Scholar | 3746233PubMed |
Langkilde, T, and Shine, R (2006). How much stress do researchers inflict on their study animals? A case study using a scincid lizard, Eulamprus heatwolei. Journal of Experimental Biology 209, 1035–1043.
| How much stress do researchers inflict on their study animals? A case study using a scincid lizard, Eulamprus heatwolei.Crossref | GoogleScholarGoogle Scholar | 16513929PubMed |
Laver, RJ, Purwandana, D, Ariefiandy, A, Imansyah, J, Forsyth, D, Ciofi, C, and Jessop, TS (2012). Life-history and spatial determinants of somatic growth dynamics in komodo dragon populations. PLoS ONE 7, e45398.
| Life-history and spatial determinants of somatic growth dynamics in komodo dragon populations.Crossref | GoogleScholarGoogle Scholar | 23028983PubMed |
Lei, J, and Booth, DT (2018). Intraspecific variation in space use of a coastal population of lace monitors (Varanus varius). Australian Journal of Zoology 65, 398–407.
| Intraspecific variation in space use of a coastal population of lace monitors (Varanus varius).Crossref | GoogleScholarGoogle Scholar |
Linhart, P, Fuchs, R, Poláková, S, and Slabbekoorn, H (2012). Once bitten twice shy: long-term behavioural changes caused by trapping experience in willow warblers Phylloscopus trochilus. Journal of Avian Biology 43, 186–192.
| Once bitten twice shy: long-term behavioural changes caused by trapping experience in willow warblers Phylloscopus trochilus.Crossref | GoogleScholarGoogle Scholar |
Lynn, SE, Breuner, CW, and Wingfield, JC (2003). Short-term fasting affects locomotor activity, corticosterone, and corticosterone binding globulin in a migratory songbird. Hormones and Behavior 43, 150–157.
| Short-term fasting affects locomotor activity, corticosterone, and corticosterone binding globulin in a migratory songbird.Crossref | GoogleScholarGoogle Scholar | 12614645PubMed |
Madsen, T, and Shine, R (1996). Seasonal migration of predators and prey: a study of pythons and rats in tropical Australia. Ecology 77, 149–156.
| Seasonal migration of predators and prey: a study of pythons and rats in tropical Australia.Crossref | GoogleScholarGoogle Scholar |
Marcellini, DL, and Jenssen, TA (1991). Avoidance learning by the curly-tailed lizard, Leiocephalus schreibersi: implications for anti-predator behavior. Journal of Herpetology 25, 238–241.
| Avoidance learning by the curly-tailed lizard, Leiocephalus schreibersi: implications for anti-predator behavior.Crossref | GoogleScholarGoogle Scholar |
McGaugh, JL (2004). The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annual Review of Neuroscience 27, 1–28.
| The amygdala modulates the consolidation of memories of emotionally arousing experiences.Crossref | GoogleScholarGoogle Scholar | 15217324PubMed |
Molyneux, J, Pavey, CR, James, AI, and Carthew, SM (2017). The efficacy of monitoring techniques for detecting small mammals and reptiles in arid environments. Wildlife Research 44, 534–545.
| The efficacy of monitoring techniques for detecting small mammals and reptiles in arid environments.Crossref | GoogleScholarGoogle Scholar |
Moore, IT, and Jessop, TS (2003). Stress, reproduction, and adrenocortical modulation in amphibians and reptiles. Hormones and Behavior 43, 39–47.
| Stress, reproduction, and adrenocortical modulation in amphibians and reptiles.Crossref | GoogleScholarGoogle Scholar | 12614633PubMed |
Narayan, EJ, Jessop, TS, and Hero, J-M (2015). Invasive cane toad triggers chronic physiological stress and decreased reproductive success in an island endemic. Functional Ecology 29, 1435–1444.
| Invasive cane toad triggers chronic physiological stress and decreased reproductive success in an island endemic.Crossref | GoogleScholarGoogle Scholar |
Nichols, JD, and Kendall, WL (1995). The use of multi-state capture–recapture models to address questions in evolutionary ecology. Journal of Applied Statistics 22, 835–846.
| The use of multi-state capture–recapture models to address questions in evolutionary ecology.Crossref | GoogleScholarGoogle Scholar |
Nimmo, DG, Kelly, LT, Spence-Bailey, LM, Watson, SJ, Haslem, A, White, JG, Clarke, MF, and Bennett, AF (2012). Predicting the century-long post-fire responses of reptiles. Global Ecology and Biogeography 21, 1062–1073.
| Predicting the century-long post-fire responses of reptiles.Crossref | GoogleScholarGoogle Scholar |
Paterson J (2007) Capture myopathy. In ‘Zoo animal and wildlife immobilization and anesthesia’. (Eds G West, D Heard, N Caulket) pp. 171–179. (John Wiley & Sons, Ltd) https://doi.org/10.1002/9781118792919.ch12
Payne, CJ, Jessop, TS, Guay, P-J, Johnstone, M, Feore, M, and Mulder, RA (2012). Population, behavioural and physiological responses of an urban population of black swans to an intense annual noise event. PLoS ONE 7, e45014.
| Population, behavioural and physiological responses of an urban population of black swans to an intense annual noise event.Crossref | GoogleScholarGoogle Scholar | 23024783PubMed |
Pike, DA, Pizzatto, L, Pike, BA, and Shine, R (2008). Estimating survival rates of uncatchable animals: the myth of high juvenile mortality in reptiles. Ecology 89, 607–611.
| Estimating survival rates of uncatchable animals: the myth of high juvenile mortality in reptiles.Crossref | GoogleScholarGoogle Scholar | 18459324PubMed |
Pradel, R, and Sanz-Aguilar, A (2012). Modeling trap-awareness and related phenomena in capture–recapture studies. PLoS ONE 7, e32666.
| Modeling trap-awareness and related phenomena in capture–recapture studies.Crossref | GoogleScholarGoogle Scholar | 22396787PubMed |
Purwandana, D, Ariefiandy, A, Imansyah, MJ, Rudiharto, H, Seno, A, Ciofi, C, Fordham, DA, and Jessop, TS (2014). Demographic status of Komodo dragons populations in Komodo National Park. Biological Conservation 171, 29–35.
| Demographic status of Komodo dragons populations in Komodo National Park.Crossref | GoogleScholarGoogle Scholar |
Purwandana, D, Ariefiandy, A, Imansyah, MJ, Ciofi, C, Forsyth, DM, Gormley, AM, Rudiharto, H, Seno, A, Fordham, DA, Gillespie, G, and Jessop, TS (2015). Evaluating environmental, demographic and genetic effects on population-level survival in an island endemic. Ecography 38, 1060–1070.
| Evaluating environmental, demographic and genetic effects on population-level survival in an island endemic.Crossref | GoogleScholarGoogle Scholar |
Rodrigues, SM, LeDoux, JE, and Sapolsky, RM (2009). The influence of stress hormones on fear circuitry. Annual Review of Neuroscience 32, 289–313.
| The influence of stress hormones on fear circuitry.Crossref | GoogleScholarGoogle Scholar | 19400714PubMed |
Romero, LM (2004). Physiological stress in ecology: lessons from biomedical research. Trends in Ecology & Evolution 19, 249–255.
| Physiological stress in ecology: lessons from biomedical research.Crossref | GoogleScholarGoogle Scholar |
Romero, LM, and Reed, JM (2005). Collecting baseline corticosterone samples in the field: is under 3 min good enough? Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 140, 73–79.
| Collecting baseline corticosterone samples in the field: is under 3 min good enough?Crossref | GoogleScholarGoogle Scholar |
Roozendaal, B (2000). Glucocorticoids and the regulation of memory consolidation. Psychoneuroendocrinology 25, 213–238.
| Glucocorticoids and the regulation of memory consolidation.Crossref | GoogleScholarGoogle Scholar | 10737694PubMed |
Salehi, B, Cordero, MI, and Sandi, C (2010). Learning under stress: the inverted-U-shape function revisited. Learning & Memory 17, 522–530.
| Learning under stress: the inverted-U-shape function revisited.Crossref | GoogleScholarGoogle Scholar |
Scheelings, T, and Jessop, T (2011). Influence of capture method, habitat quality and individual traits on blood parameters of free-ranging lace monitors (Varanus varius). Australian Veterinary Journal 89, 360–365.
| Influence of capture method, habitat quality and individual traits on blood parameters of free-ranging lace monitors (Varanus varius).Crossref | GoogleScholarGoogle Scholar | 21864309PubMed |
Schwabe, L, Joëls, M, Roozendaal, B, Wolf, OT, and Oitzl, MS (2012). Stress effects on memory: an update and integration. Neuroscience & Biobehavioral Reviews 36, 1740–1749.
| Stress effects on memory: an update and integration.Crossref | GoogleScholarGoogle Scholar |
Smissen, PJ, Melville, J, Sumner, J, and Jessop, TS (2013). Mountain barriers and river conduits: phylogeographical structure in a large, mobile lizard (Varanidae: Varanus varius) from eastern Australia. Journal of Biogeography 40, 1729–1740.
| Mountain barriers and river conduits: phylogeographical structure in a large, mobile lizard (Varanidae: Varanus varius) from eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Stokeld, D, Fisher, A, Gentles, T, Hill, BM, Woinarski, JCZ, Young, S, and Gillespie, GR (2018). Rapid increase of Australian tropical savanna reptile abundance following exclusion of feral cats. Biological Conservation 225, 213–221.
| Rapid increase of Australian tropical savanna reptile abundance following exclusion of feral cats.Crossref | GoogleScholarGoogle Scholar |
Stryjek, R, Kalinowski, A, and Parsons, MH (2019). Unbiased sampling for rodents and other small mammals: how to overcome neophobia through use of an electronic-triggered live trap: a preliminary test. Frontiers in Ecology and Evolution 7, 11.
| Unbiased sampling for rodents and other small mammals: how to overcome neophobia through use of an electronic-triggered live trap: a preliminary test.Crossref | GoogleScholarGoogle Scholar |
Thaker, M, Vanak, AT, Lima, SL, and Hews, DK (2010). Stress and aversive learning in a wild vertebrate: the role of corticosterone in mediating escape from a novel stressor. The American Naturalist 175, 50–60.
| Stress and aversive learning in a wild vertebrate: the role of corticosterone in mediating escape from a novel stressor.Crossref | GoogleScholarGoogle Scholar | 19922261PubMed |
Weavers, BW (1988). Vital statistics of the lace monitor lizard (Varanus varius) in south-eastern Australia. Victorian Naturalist 105, 142–145.
White, GC, and Burnham, KP (1999). Program MARK: survival estimation from populations of marked animals. Bird Study 46, S120–S139.
| Program MARK: survival estimation from populations of marked animals.Crossref | GoogleScholarGoogle Scholar |
Wikelski, M, and Michael Romero, L (2003). Body size, performance and fitness in galapagos marine iguanas. Integrative and Comparative Biology 43, 376–386.
| Body size, performance and fitness in galapagos marine iguanas.Crossref | GoogleScholarGoogle Scholar | 21680446PubMed |
Williams BK, Nichols JD, Conroy MJ (2002) ‘Analysis and management of animal populations.’ (Academic Press)
Wingfield JC, Romero LM (2001) Adrenocortical responses to stress and their modulation in free-living vertebrates. In ‘Comprehensive physiology’. (Ed. R Terjung) pp. 211–234. (John Wiley & Sons, Ltd) https://doi.org/10.1002/cphy.cp070411
Wingfield, JC, Maney, DL, Breuner, CW, Jacobs, JD, Lynn, S, Ramenofsky, M, and Richardson, RD (1998). Ecological bases of hormone—behavior interactions: the “emergency life history stage”. American Zoologist 38, 191–206.
| Ecological bases of hormone—behavior interactions: the “emergency life history stage”.Crossref | GoogleScholarGoogle Scholar |
Zuur A, Ieno EN, Walker N, Saveliev AA, Smith GM (2009) ‘Mixed effects models and extensions in ecology with R.’ (Springer Science & Business Media)


