Repeated experimental removals unveil sex and age-specific dispersal strategies in a social passerine bird
Farzaneh Etezadifar A , Jacob A. T. Vickers B , Kristine French B , Paul G. McDonald A , Ahmad Barati A , Rose L. Andrew C and Richard E. Major B D *
B D *
A Animal Behaviour and Ecology Laboratory, Zoology, University of New England, Armidale, NSW, Australia.
B School of Earth, Atmospheric and Life Sciences, University of Wollongong, Wollongong, NSW, Australia.
C School of Environmental and Rural Science, University of New England, Armidale, NSW, Australia.
D Australian Museum Research Institute, Australian Museum, Sydney, NSW, Australia.
Wildlife Research 50(2) 141-151 https://doi.org/10.1071/WR21170
Submitted: 25 November 2021 Accepted: 23 July 2022 Published: 26 August 2022
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Sex and age are frequently proposed as drivers of a number of behavioural and demographic patterns that can have important consequences for population dynamics including access to mates, sexual selection, parental care and lifetime productivity. Sex and age might also be important in shaping the movement patterns and colonisation processes of social species moving into vacant habitat. Such information is critical for the management of strongly interacting species such as the noisy miner (Manorina melanocephala), which structure ecological communities through aggressive exclusion of other taxa from areas that they occupy.
Aims: In Manorina colonies, young females are usually dispersive, while males remain in the natal colony as the philopatric sex. Following removal of individuals from an area, we aimed to determine whether female-biased dispersal, particularly of young females, would result in a more equal sex ratio and a younger age structure in the recolonising population.
Methods: These predictions were tested by anatomically ageing and sexing 1856 noisy miners that had been experimentally culled in two regions of New South Wales, Australia, to reduce the aggressive impact of this species on other native species.
Key results: Prior to removal, noisy miner populations were significantly male-biased in both regions (57% and 60%); however sex ratios after each of two removal episodes no longer differed from parity. Immature birds were a dominant feature (65%) of recolonising populations in both regions, however, the age structure of recolonising populations was different in each region, mostly likely due to the respective timing of culls during the year. Furthermore, the culling response in terms of age-specific sex ratio varied between regions. After the final cull, the sex ratio of mature birds had fallen to parity in one region but had become even more male biased (68%) in the other region. There was no sex-ratio bias among immature birds before or after culling.
Conclusion: These results confirm the expectation that immature birds are more likely to be colonisers, but the expectation of greater female dispersal was equivocal.
Implications: The differences in response between regions may reflect variation in population density, landscape connectivity or seasonality, highlighting challenges when implementing culling programs for conservation management.
Keywords: age structure, dispersal, lethal management, Manorina melanocephala, noisy miner, recolonisation, sex ratio, superabundant species.
Introduction
Sex ratio variation is a fundamental component of wild population dynamics (Eberhart-Phillips et al. 2018) and has extensive implications for the ecology, behaviour and life history of populations (Székely et al. 2014). Adult sex ratios can determine the social structure of the population where mating system traits such as divorce, extra-pair copulations and paternal care can be altered due to a biased sex ratio in the population (Liker et al. 2014; Remeš et al. 2015; Eberhart-Phillips et al. 2018). Consequently, sex ratio is important for population productivity (Liker et al. 2014) and may result in decreased fitness of a particular sex (Grayson et al. 2014). Sex ratio and sex allocation are therefore important metrics for management and conservation practices (Komdeur 1994; Baumgardt et al. 2013; Reichard et al. 2014; Székely et al. 2014).
The overall sex ratio of a population is influenced by age structure, being dependant on the proportion of each age group in the population and the variation between adult and non-adult sex ratios (Székely et al. 2014). These population attributes may influence movement patterns and recolonisation of new territories (Kokko et al. 2006) through sex-specific and age-specific philopatry. For example, in most bird species males tend to stay in their natal home range, whereas first-year females seek new territories (Kokko et al. 2006). Therefore, different sexes provide different fitness payoffs for parents, and sex-related dispersal could be a major factor that determines how parents invest in each sex (Hatchwell 2009).
Sex-related parental investment can happen by facultatively adjusting sex ratio in favour of production of the beneficial sex (Ewen et al. 2003), or during the rearing stage through differential resource allocation (Ridley and Huyvaert 2007). This is particularly important in cooperatively breeding species, in which offspring receive care from other individuals within a group in addition to parents. In these species, sex-biased investment can be determined by group size, as parents and helpers that might benefit from a larger group size would increase their fitness by preferentially investing in the philopatric sex (Ewen et al. 2003; Ridley and Huyvaert 2007; but see McDonald et al. 2010). Although producing more young of the helping sex might generally be favoured, producing more of the dispersive sex may be favoured when territories are at capacity.
Dispersal strategies might also be influenced by the age structure of the populations. If young birds have to queue to get a breeding position, then they may disperse to new territories with a smaller queue, allowing them to become part of the breeding population sooner (Wiley and Rabenold 1984; Nelson-Flower et al. 2018). Through their role in dispersal, natality and mortality, population age and sex composition have considerable influence on future reproduction (Alexander 1958) and so it is particularly important to understand these population attributes when undertaking management intervention on strongly interacting species, i.e. species that have disproportionately large ecological effects given their abundances (Thomson et al. 2015).
The noisy miner (Manorina melanocephala), a strongly interacting Australian honeyeater, has been recognised in Key Threatening Process listings on account of its adverse impact on threatened bird species (NSWSC 2013; TSSC 2013). Noisy miners have been described as a ‘despotic species’ (Mac Nally et al. 2012) because they aggressively exclude almost all smaller birds from their territories (Maron et al. 2013). They are cooperative breeders and live in aggregations of up to several hundred birds organised into tribes or coteries of 10–25 individuals within a colony (Higgins et al. 2001). Colony members cooperate in various contexts, including the feeding of young (Higgins et al. 2001; Barati et al. 2018a), mobbing of predators (Arnold 2000) and defence of the colony’s territory from intra and interspecific intruders (Dow 1977). In natural, well established colonies there is a male-biased sex ratio (Barati et al. 2018a). It is thought that this is due to females dispersing from established colonies, with males being the philopatric sex, as occurs in the congeneric Bell Miner (Manorina melanophrys) (Dare et al. 2007, 2008).
Culling of noisy miners has been trialled as a management strategy to recover depleted populations of small woodland birds, but outcomes have been inconsistent and further research is required to understand the circumstances under which rapid recolonisation of noisy miner-vacated habitat does, and does not, occur (Melton et al. 2021). The key to this understanding is rooted in the drivers of dispersal, of which age and sex composition are fundamental considerations.
The aim of this study was to examine individuals removed in a large scale cull of noisy miners to determine how age structure and sex ratios varied between the initial population and subsequent recolonised populations. Specifically, we tested the following predictions based on the known social structure of noisy miner colonies: (1) Recolonised populations will have a significantly higher proportion of females than were present in the original population. This prediction is based on males being more philopatric in noisy miner social systems and thus less likely to seek out vacant habitat than females. (2) Recolonised populations will have a significantly higher percentage of immature birds than were present in the original population. This prediction is based on the queuing hypotheses whereby younger birds will have faster access to breeding opportunities through accessing new habitat than by waiting within an established colony.
Materials and methods
Study sites and sample collection
The study was conducted in two distinct regions of New South Wales, Australia (Fig. 1a), selected to capture variation in landscape cover of remnant eucalypt woodland, which is the primary habitat of noisy miners and may explain some of the variation in noisy miner behaviour (Melton et al. 2021). The Bundarra study region (Fig. 1b) is situated in the New England Tablelands Bioregion (Thackway and Cresswell 1995) and has 31% cover of eucalypt forest and woodland, compared with 13% cover in the Fifield study region (Fig. 1c), which is situated in the South Western Slopes Bioregion. The two regions also differ in annual rainfall which is higher at Bundarra than Fifield (Fig. 2). Rainfall in the year preceding sample collection was typical of long-term means.
|
|
|
|
Samples used in this study were collected as a consequence of a noisy miner removal experiment investigating the restoration of woodland bird communities (see Davitt et al. 2018 for details). In brief, a 12-gauge shotgun was used to systematically remove noisy miners from six separate linear strips of remnant eucalypt woodland in each region. All removal sites were at least 50 m in width, 2 km in length, and ranged between 16 and 50 hectares in area. Three removal episodes were implemented at each site (Fig. 2), with the first, second and third removals at Fifield occurring from 19–24 August 2015; 12–17 September 2015; and 12–19 April 2016 respectively. The Bundarra removal episodes occurred after the corresponding Fifield removals, from 14–20 November 2015; 4–10 December 2015; and 3–10 May 2016. These removals yielded three samples of birds from each region termed, hereafter, Initial, Recolonisation 1 and Recolonisation 2.
A trained, licensed shooter accompanied by two experienced ornithologists traversed the length of each site by foot, using broadcast of noisy miner calls at ∼50–100 m intervals to attract noisy miners to trees where they could easily be shot. The three personnel spread out across the width of each site when walking between playback points to detect, shoot and collect all noisy miners encountered. Sites were visited on at least two occasions during each removal period, until the vast majority of noisy miners had been removed from a site (fewer than 10 individuals remaining – Davitt et al. 2018). Carcasses were stored frozen until subsequent anatomical analysis to determine age and sex of collected birds.
Noisy miner density was significantly higher (F1,30 = 20.66, P < 0.001) in the Bundarra region than the Fifield region as determined by the number of birds shot per hectare per hour (Fig. 3). We analysed a sample of collected birds (1131 from Bundarra; 725 from Fifield). We attempted to balance sample sizes across all sites and removals, but this was not always possible because of the relatively small number of individuals culled in some sites in some periods (e.g. Recolonisation 1 at sites C45 and C52 – Supplementary materials Fig. S1b). Accordingly, sites within each region are pooled in our analysis of the difference in sex ratio and age structure of the initial and recolonising populations. Within each site × removal combination, birds were selected randomly. (All birds had been wrapped in paper towel and bagged, so they were not visible at the time of selection).
Ageing and sexing birds
Each noisy miner carcass was examined by dissection to determine its sex and age, although a small number of Fifield birds could not be assigned a sex due to extensive shotgun damage to the gonads. Most birds could be sexed by the presence of testes or ovary, but in some cases where gonads were particularly regressed, careful searching for the oviduct was necessary. The sexes of 130 Bundarra birds that were either extensively damaged or had inconclusive gonads were determined using a PCR-based method that focuses on two Chromo-Heli-case-DNA (CHD) binding genes that are found on avian sex chromosomes (Griffiths et al. 1998; Kopps et al. 2013; Etezadifar 2021).
Age determination was based upon the degree of skull pneumatisation (Pyle et al. 1987), assessed by peeling back the cranial skin after making an incision in the crown, and classified as ‘minimal’, ‘partial’, or ‘complete’. As it is not known at what age the skull of the noisy miner becomes completely pneumatised, an exact age cannot be determined from these characteristics. However, as passerines generally complete pneumatisation by 4–8 months of age (Serventy et al. 1967) all individuals with minimal or partial skull pneumatisation were classified as ‘immatures’ (Dow 1978), and all other individuals were classified as ‘mature’, recognising that this category may be over-represented as some immatures may consequently be classed as mature.
Analysis of demographic structure
Nominal logistic models (implemented in JMP® v.15 (JMP 2019)) were used to identify differences in sex ratio and age structure between the initial population and recolonising populations 1 month and 6–8 months after the initial removal. Region (Bundarra/Fifield) was also included in the model because there were fundamental differences in the two regions in terms of vegetation cover, climate, and the season in which the various culls occurred.
Where significant main effects and interactions were identified, pairwise nominal logistic models were used to determine which recolonisation events differed from the initial population in terms of age structure and sex ratio. Pairwise models were also used to identify differences in sex ratios from parity.
Ethics statement
This study used specimens collected from a separate study assisted by the New South Wales Government through the Environmental Trust. The initial study was authorised by scientific license S101522 under the NSW National Parks and Wildlife Act, with ethics approval granted by the Australian Museum Animal Care and Ethics under approval number 15/04.
Results
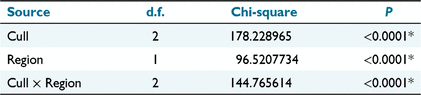
The overall sex ratio of noisy miners sampled in each cull ranged between 41% and 70% with mostly low variability among individual sites located within each region (Fig. S1). However, there was considerable variation between regions in the percentage of immature individuals in each sample, with particularly low numbers of immature birds in the initial population and first recolonisation of sites at Fifield (Fig. S2). The nominal logistic model of age structure identified a significant Cull × Region interaction necessitating consideration of separate demographic responses in each region (Table 1). In the Bundarra region 41% of birds in the initial population were immature, which was significantly lower than the percentage of immature birds in both the first and second recolonisation events (Fig. 4a, Table S1). In contrast, only 12% of birds in the initial Fifield population were immature, which was similar to the percentage of immature birds in the first recolonisation event, and significantly lower than the percentage in the second recolonisation event (Fig. 4b, Table S1).
|
The nominal logistic model of sex ratio (including Age in the model) identified a significant effect of Age, and also a significant Age × Cull × Region effect (Table 2). Accordingly, the variation in sex ratio between recolonisation events must be evaluated separately for each age group in each region (Fig. 5).
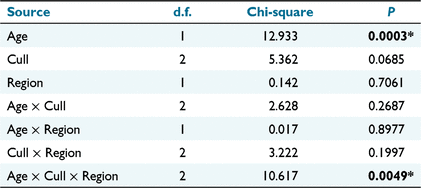
|
The sex ratio of mature birds in the second colonisation event at Bundarra was significantly less male biased than in the initial population (66% male). The sex ratio of the first recolonisation event was also less male biased, but intermediate between the two (Fig. 5a). Mature individuals displayed a significantly male biased sex ratio in the initial population (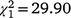 , N = 277, P < 0.0001) but the sex ratio did not differ from parity in the first (
, N = 277, P < 0.0001) but the sex ratio did not differ from parity in the first (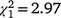 , N = 66, P = 0.084) or second recolonisation events (
, N = 66, P = 0.084) or second recolonisation events (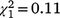 , N = 152, P = 0.740). For immature birds, there were no significant differences in the sex ratio among recolonisation events (Fig. 5b, Table S1) and none of the immature sex ratios differed from parity.
, N = 152, P = 0.740). For immature birds, there were no significant differences in the sex ratio among recolonisation events (Fig. 5b, Table S1) and none of the immature sex ratios differed from parity.
The sex ratio of recolonising birds at Fifield followed a different pattern from Bundarra. The sex ratio of adult birds (Fig. 5c, Table S1) was significantly lower in the initial and first recolonisation events than in the second recolonisation event. Mature individuals displayed a significantly male-biased sex ratio in the initial population (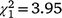 , N = 243, P = 0.047) and the second recolonisation event (
, N = 243, P = 0.047) and the second recolonisation event (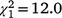 , N = 91, P < 0.001), but the sex ratio in the first recolonisation event was not significantly different from parity (
, N = 91, P < 0.001), but the sex ratio in the first recolonisation event was not significantly different from parity (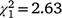 , N = 152, P = 0.105). However, the lack of a significant difference from parity is likely to be a consequence of statistical power, because this sex ratio was equal to that observed in the initial population which did differ significantly from parity and had a larger sample size. As was the case in the Bundarra region, there were no significant differences in the sex ratio of immature birds among recolonisation events (Fig. 5d, Table S1), and none of the immature sex ratios differed from parity.
, N = 152, P = 0.105). However, the lack of a significant difference from parity is likely to be a consequence of statistical power, because this sex ratio was equal to that observed in the initial population which did differ significantly from parity and had a larger sample size. As was the case in the Bundarra region, there were no significant differences in the sex ratio of immature birds among recolonisation events (Fig. 5d, Table S1), and none of the immature sex ratios differed from parity.
Discussion
Understanding variations in the age and sex ratio of wild populations is extremely challenging because these traits can vary seasonally, annually, according to spatial scale and also between the age and sex classes of a given population (Ellegren and Sheldon 1997). In this study, we detected changes in age and sex structure of wild populations in response to human intervention through removal of resident populations, but the response varied with location and could not be generalised.
Significant male biased sex ratios were observed in both study regions in pre-cull populations, but they did not differ from parity in recolonising populations. This equalisation in overall sex ratios appeared to be driven by an influx of immature birds that exhibited an unbiased sex ratio. Superficially, these results confirm our predictions that (1) recolonised populations will have a significantly higher proportion of females than were present in the original population, and (2) recolonised populations will have a significantly higher percentage of immatures than were present in the original population. However, the swamping of the overall demographic signal by immature birds concealed a differential response in the adult cohorts between study regions, which contradicts the mechanisms we proposed to explain these predictions. Specifically, while one population exhibited a significant reduction in male bias in the final population, the other exhibited a significant increase in male bias. The two study regions differed in several fundamental attributes, and although these attributes are confounded, it is important to consider their possible influence on recolonisation dynamics.
Timing of bird removal and the effect on age structure
Noisy miners breed year round, but most breeding occurs during the more productive months of spring (Higgins et al. 2001). Because culling at Bundarra took place in November and December, free flying immature birds were available in the landscape as potential colonists of the newly vacant habitat. This contrasted with the situation at Fifield where the August and September culls occurred prior to the fledging of new season birds. This explains the much younger age structure observed in the initial and first recolonisation samples at Bundarra compared with Fifield. By the following autumn, when the final culls occurred, peak breeding was completed and immature birds were fully represented in both regions.
Juvenile dispersal is typically the dominant dispersal phase for passerine birds (Greenwood and Harvey 1982; Donovan et al. 1995) and this is the simplest explanation for the high proportion of juveniles in recolonised populations of noisy miners. While an age structure comprising 65–67% immature birds (as observed in Bundarra first recolonisation and Fifield second recolonisation) strongly suggests recolonisation by disproportionate movement of immatures, the culling intervention did not have the necessary controls to draw this conclusion experimentally – it is conceivable that recolonists were simply a random representation of what was naturally a landscape wide young population structure. However, such young age structures are not generally the case among long lived, colonial Australian honeyeaters. The closely related and cooperatively breeding Bell Miner (M. melanophrys) provides the strongest comparison and, surveyed over 5 years, it exhibited an annual mean age structure of 20–45% immatures, which fluctuated between seasons (Clarke and Heathcote 1990). This is consistent with the pre-treatment age structures we observed of 41% and 12% immatures, at Bundarra and Fifield, respectively in our study. Moreover, the high percentage of immature birds in the second recolonisation events is likely to be an underestimate, because skull pneumatisation is expected to be complete in 4–8 months (Serventy et al. 1967) rendering birds that fledged in early spring unidentifiable as immatures. We are therefore confident that at the time of year when they were present (November–May), immature birds were disproportionately responsible for recolonisation.
Sex ratio of immature birds
Immature recolonists could originate from the surrounding landscape or as new recruits from on-site breeding. While some on site breeding recruitment was probable in the second (autumn) recolonisation event, it was unlikely in the first, because there was insufficient time for recolonists to fledge young in the short period 1 month before the second cull. Irrespective of any on-site breeding that occurred, there was no evidence of immature sex ratios deviating from parity. In both regions and all culls, immature sex ratios reflected the even primary sex ratios normally found in the species prior to the development of the male-bias that arises through the high occurrence of female displacement (Arnold et al. 2001; Higgins et al. 2001; Barati 2017). While it is unclear at what age female-biased displacement occurs, it is thought to occur as birds approach breeding age and begin to search for breeding positions, which could occur from 8–12 months of age (Higgins et al. 2001). This is known to be the situation in the Bell Miner, where the mean age of dispersal is 8 months, close to the minimum observed age of first breeding for both males (8.3 months) and females (9.7 months) (Clarke 1988; Clarke and Heathcote 1990).
In the present study, it seems that both male and female immatures chose to disperse from colonies at an earlier age, and at the same rate. Whilst females usually disperse more often than males due to mother–daughter breeding competition (Clarke and Heathcote 1990), male dispersal may also be substantial. The movement of young males might reflect an optimisation of the queuing strategy, where young birds seek access to better breeding resources in a new colony than they have in their existing space (Wiley and Rabenold 1984). For example, a reproductive queue forms in superb fairy-wrens (Malurus cyaneus) in which males queue to gain a breeding opportunity (Cockburn et al. 2008), with some males moving from their natal territory to minimise the wait for a breeding position. Male dispersal may result in greater fitness benefits, than remaining as a philopatric helper, in the absence of ecological constraint (Woolfenden 1989) which, in territorial species, is hypothesised to be the saturation of suitable habitat by sedentary established groups (Koenig 1981; Emlen 1982; Stacey and Ligon 1987). Following thorough removal of noisy miners in our study sites there was a sudden large scale release of productive habitat, and immatures of both sexes from nearby colonies may have perceived a benefit from early dispersal. The perception of vacant habitat could have arisen while performing social activities such as ‘long-flights’ (Dow 1975), or chasing intruders, and possibly during what would be the short term transient dispersals that act as a precursor to successful permanent dispersal, as seen in other cooperative breeders (Gaston 1978; Lewis 1982; Clarke and Heathcote 1990).
Sex ratio of mature birds
The unchanged sex ratio amongst matures in the first recolonisation event, which was evident in both regions, indicates that mature individuals of both sexes initially moved into suitable vacant habitat in proportion to their original respective abundances. The most likely explanation for this type of recolonisation is that nearby colonies shifted or extended their boundaries to incorporate the newly vacated habitat, rather than movement of subordinate non-breeding floaters (Smith 1978). Recolonisation by floaters that could not previously establish a breeding position appears unlikely because floaters would be expected to be female dominated, given the higher incidence of female displacement (Arnold et al. 2001; Higgins et al. 2001; Barati 2017).
The disproportionate representation of female colonists in the second recolonisation at Bundarra, resulting in sex-ratio parity among mature birds, is consistent with the female dispersal patterns suggested in this species (Dow 1977, 1978). Females establish activity spaces within a colony and aggressively exclude other female noisy miners from this area. Due to territory spaces being limited within the colony, young female birds are rarely able to establish their own activity space in their natal colony (Dow 1978). This favours females dispersing, while males remain philopatric and contribute to helping behaviour (Barati et al. 2018b). Females are thus expected to be disproportionately represented in recolonistion of vacant habitat, as observed.
However, the even higher represenation of mature male colonists in the second recolonisation at Fifield, resulting in an even greater male bias, cannot be explained by existing knowledge of this species (Arnold et al. 2001; Higgins et al. 2001; Barati 2017). Elevated immigration rates of mature males into the vacant habitat could lead to increased male bias, but such dispersal behaviour is inconsistent with previous studies of unmanipulated populations. A plausible mechanism for disproportionate male dispersal in our manipulated populations is that mature male helpers that are queuing for breeding positions may perceive an advantage in emigrating into newly vacant habitat that has been colonised predominantly by immatures. Such an advantage might be accrued from an increased likelihood of finding a mate on account of an even sex-ratio and the inferior competitiveness of less experienced males. However, if valid, this strategy should have been equally profitable in the Bundarra region, where it was not evident. An alternative mechanism is that as they matured, some immature females that had initially recolonised the vacant habitat emigrated a second time, perhaps because the overall density of birds became higher than the original population. Again, this hypothesis is weak, given that the same circumstances occurred at Bundarra but disproportionate male dispersal was not evident.
Drivers of regional variation in recolonisation response
So why were the recolonisation responses in the two regions different? The simplest explanation for differences in sex biased dispersal between regions is regional variation in background sex ratio. Previous studies on undisturbed noisy miner populations have found considerable variation in the magnitude of male bias, range between 2.1:1 and 4.7:1 (Dow 1978; Dow and Whitmore 1990; Arnold et al. 2001; Barati et al. 2018a), with no reports of female biased or sex ratio parity in adult populations. Interestingly, the initial sex ratios we observed of 1.5:1 (Bundarra) and 1.3:1 (Fifield) are the least skewed recorded to date, highlighting the possibility of different sampling biases between ‘catch and release’, and ‘cull and keep’ sex determination methods. But regardless of any sampling bias, the similarity of the background sex ratios between study regions does not provide a compelling explanation for the different regional responses we observed in recolonisation demography.
Noisy miner supply in the landscape is another important theoretical driver of recolonisation dynamics, and we had clear evidence of differences between regions. Culling rates were significantly higher in the Bundarra region, most likely reflecting greater noisy miner densities, although we cannot exclude the possibility that the more extensive landscape vegetation cover at Bundara (Fig. 1) facilitated dispersal across the agricultural landscape, increasing mid-cull recolonisation. This difference in landscape supply is also likely to be related to productivity (Montague-Drake et al. 2011), with Fifield situated closer to the western edge of the species’ range (Fig. 1.) and receiving lower rainfall than Bundarra (Fig. 2.). Regardless of the cause of the greater culling rate of noisy miners in Bundarra, elevated supply could conceivably explain the more frequent dispersal of females given that it should increase competition, and female/female competition is thought to drive female dispersal (Clarke and Heathcote 1990). In the more challenging environment of Fifield, it seems that larger groups or even coteries of miners move as a unit to recolonise an area. This may be due to better quality habitat being available elsewhere for coteries living in sub-optimal habitat within existing colonies, for example. Under this scenario, increased male sex ratio bias in the second recolonisation episode at Fifield, relative to pre-culling levels, may reflect larger groups of (predominately male) birds emigrating along with dispersing females to better quality habitat. Molecular analysis is required to unravel some of these more elaborative interactions between factors or other as yet unidentified variables that are driving the current patterns observed.
Conclusion
The detrimental impact of the noisy miner’s hyper-aggression on Australia’s woodland avifauna is recognised in multiple Key Threatening Process listings (NSWSC 2013; TSSC 2013). While habitat restoration is the most favoured recovery action, culling is potentially a rapid and cost effective method of releasing suitable habitat for woodland birds. Culling has produced an enduring benefit in some situations with negligible recolonisation over several years (Grey et al. 1997), but in other situations, such as the experiment producing the birds analysed in the present study, recolonisation has been rapid (Davitt et al. 2018). A recent meta-analysis of culling trials (Melton et al. 2021) was unable to explain the success or failure of culls in terms of noisy miner recolonisation, but a common observation amongst trials was the recovery of small birds, even in situations where noisy miners recolonised quickly. Our study helps explain both the variability in recolonisation response of noisy miners, and the recovery of small birds in the face of noisy miner recolonisation.
The timing of a cull is likely to influence its impact because the source of colonists varies strongly with season. Post-spring culls should primarily result in recolonisation by immature birds, most likely small groups moving in from different colonies. These recolonists would not have established social structures, which would be unlikely to form until after the following breeding season, because the majority of helping behaviour is provided by adult males that are close genetic relatives of the breeders – primarily retained offspring (Barati et al. 2018a). Given that the hyper-aggression of noisy miners is related to their strong co-operative behaviour, small birds dispersing into habitat recolonised by unstructured immature noisy miners are less likely to be excluded than in habitat where aggression is co-ordinated. This provides a plausible mechanism for the observation that small birds often show positive responses to noisy miner removal, even when sites are rapidly recolonised.
Similarly, decreased aggression and positive small bird response are expected in situations where the post-cull sex ratio becomes less male biased (e.g. Bundarra), because male birds contribute more than females to group mobbing behaviour and colony defence (Higgins et al. 2001). The information here is not sufficient to explore these impacts in a quantitative manner, but the timing of culls and the sex ratio of recolonists are clearly of ecological importance and must be considered in further experimental removals to refine noisy miner management practices. To this end it would be very instructive if regional removals involving multiple sites were implemented such that replicated randomised sites were sampled in different seasons. It would also be instructive to use genetic techniques to determine comparative levels of relatedness among colonists to determine their likely sources.
Supplementary material
Supplementary material is available online.
Data availability
The datasets analysed in this study are available at the University of New England library (RUNE) and are available on request from the senior author.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This study was completed through a PhD research scholarship funded by the School of Environment and Rural Science at University of New England (UNE) and the Australian Commonwealth Government through an RTP scholarship. This study has been supported by the New South Wales Government through its Environmental Trust, and by the Ecological Society of Australia through their Holsworth Wildlife Research Endowment grant.
Acknowledgements
This study used specimens collected from a separate study assisted by the New South Wales Government through the Environmental Trust. We are particularly grateful to Ian and Moira Sirett for their assistance in collecting noisy miner carcasses, and to Walter Boles for training and assistance in the anatomical assessment of age and sex in collected birds.
References
Alexander, MM (1958). The place of aging in wildlife management. American Scientist 46, 123–137.Arnold, KE (2000). Group mobbing behaviour and nest defence in a cooperatively breeding Australian bird. Ethology 106, 385–393.
Arnold, KE, Griffith, SC, and Goldizen, AW (2001). Sex-biased hatching sequences in the cooperatively breeding noisy miner. Journal of Avian Biology 32, 219–223.
| Sex-biased hatching sequences in the cooperatively breeding noisy miner.Crossref | GoogleScholarGoogle Scholar |
Barati A (2017) Cooperative breeding in the noisy miner (Manorina melanocephala): the role of genetic relatedness, sex, extra-pair paternity and acoustic signals. PhD thesis, University of New England, Armidale NSW.
Barati, A, Andrew, RL, Gorrell, JC, Etezadifar, F, and McDonald, PG (2018a). Genetic relatedness and sex predict helper provisioning effort in the cooperatively breeding noisy miner. Behavioral Ecology 29, 1380–1389.
| Genetic relatedness and sex predict helper provisioning effort in the cooperatively breeding noisy miner.Crossref | GoogleScholarGoogle Scholar |
Barati, A, Andrew, RL, Gorrell, JC, and McDonald, PG (2018b). Extra-pair paternity is not driven by inbreeding avoidance and does not affect provisioning rates in a cooperatively breeding bird, the noisy miner (Manorina melanocephala). Behavioral Ecology 29, 244–252.
| Extra-pair paternity is not driven by inbreeding avoidance and does not affect provisioning rates in a cooperatively breeding bird, the noisy miner (Manorina melanocephala).Crossref | GoogleScholarGoogle Scholar |
Baumgardt, JA, Goldberg, CS, Reese, KP, Connelly, JW, Musil, DD, Garton, EO, and Waits, LP (2013). A method for estimating population sex ratio for sage-grouse using noninvasive genetic samples. Molecular Ecology Resources 13, 393–402.
| A method for estimating population sex ratio for sage-grouse using noninvasive genetic samples.Crossref | GoogleScholarGoogle Scholar |
BirdLife International (2018) Manorina melanocephala. The IUCN red list of threatened species 2018: e.T22704433A132071923. Available at https://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22704433A132071923.en
Bureau of Meteorology (2021) Monthly climate statistics. Available at http://www.bom.gov.au/climate/averages/tables/cw_050037.shtml
Clarke, MF (1988). The reproductive behaviour of the bell miner Manorina melanophrys. Emu - Austral Ornithology 88, 88–100.
| The reproductive behaviour of the bell miner Manorina melanophrys.Crossref | GoogleScholarGoogle Scholar |
Clarke, MF, and Heathcote, CF (1990). Dispersal, survivorship and demography in the co-operatively-breeding bell miner Manorina melanophrys. Emu - Austral Ornithology 90, 15–23.
| Dispersal, survivorship and demography in the co-operatively-breeding bell miner Manorina melanophrys.Crossref | GoogleScholarGoogle Scholar |
Cockburn, A, Osmond, HL, Mulder, RA, Double, MC, and Green, DJ (2008). Demography of male reproductive queues in cooperatively breeding superb fairy-wrens (Malurus cyaneus). Journal of Animal Ecology 77, 297–304.
| Demography of male reproductive queues in cooperatively breeding superb fairy-wrens (Malurus cyaneus).Crossref | GoogleScholarGoogle Scholar |
Dare, AJ, McDonald, PG, and Clarke, MF (2007). The ecological context and consequences of colonisation of a site by bell miners (Manorina melanophrys). Wildlife Research 34, 616–623.
| The ecological context and consequences of colonisation of a site by bell miners (Manorina melanophrys).Crossref | GoogleScholarGoogle Scholar |
Dare, AJ, McDonald, PG, and Clarke, MF (2008). The social and behavioural dynamics of colony expansion in the Bell Miner (Manorina melanophrys). Emu - Austral Ornithology 108, 175–180.
| The social and behavioural dynamics of colony expansion in the Bell Miner (Manorina melanophrys).Crossref | GoogleScholarGoogle Scholar |
Davitt, G, Maute, K, Major, RE, McDonald, PG, and Maron, M (2018). Short-term response of a declining woodland bird assemblage to the removal of a despotic competitor. Ecology and Evolution 8, 4771–4780.
| Short-term response of a declining woodland bird assemblage to the removal of a despotic competitor.Crossref | GoogleScholarGoogle Scholar |
Department of the Environment (2012) Australia – present major vegetation groups – National Vegetation Information System Version 4.1 (Albers 100m analysis product). Bioregional Assessment Source Dataset. Available at https://data.gov.au/data/dataset/57c8ee5c-43e5-4e9c-9e41-fd5012536374
Donovan, TM, Lamberson, RH, Kimber, A, Thompson, FR, and Faaborg, J (1995). Modeling the effects of habitat fragmentation on source and sink demography of neotropical migrant birds. Conservation Biology 9, 1396–1407.
| Modeling the effects of habitat fragmentation on source and sink demography of neotropical migrant birds.Crossref | GoogleScholarGoogle Scholar |
Dow, DD (1975). Displays of the honeyeater Manorina melanocephala. Zeitschrift für Tierpsychologie 38, 70–96.
| Displays of the honeyeater Manorina melanocephala.Crossref | GoogleScholarGoogle Scholar |
Dow, DD (1977). Indiscriminate interspecific aggression leading to almost sole occupancy of space by a single species of bird. Emu - Austral Ornithology 77, 115–121.
| Indiscriminate interspecific aggression leading to almost sole occupancy of space by a single species of bird.Crossref | GoogleScholarGoogle Scholar |
Dow, DD (1978). Breeding biology and development of the young of Manorina melanocephala, a communally breeding honeyeater. Emu - Austral Ornithology 78, 207–222.
| Breeding biology and development of the young of Manorina melanocephala, a communally breeding honeyeater.Crossref | GoogleScholarGoogle Scholar |
Dow DD, Whitmore MJ (1990) Noisy miners: variations on the theme of communality. In ‘Cooperative breeding in birds’. (Eds PB Stacey, WD Koenig) pp. 559–592. (Cambridge University Press: Cambridge)
Eberhart-Phillips, LJ, Küpper, C, Carmona-Isunza, MC, et al. (2018). Demographic causes of adult sex ratio variation and their consequences for parental cooperation. Nature Communications 9, 1651.
| Demographic causes of adult sex ratio variation and their consequences for parental cooperation.Crossref | GoogleScholarGoogle Scholar |
Ellegren, H, and Sheldon, BC (1997). New tools for sex identification and the study of sex allocation in birds. Trends in Ecology & Evolution 12, 255–259.
| New tools for sex identification and the study of sex allocation in birds.Crossref | GoogleScholarGoogle Scholar |
Emlen, ST (1982). The evolution of helping. I. An ecological constraints model. The American Naturalist 119, 29–39.
| The evolution of helping. I. An ecological constraints model.Crossref | GoogleScholarGoogle Scholar |
Etezadifar F (2021) Re-colonisation dynamics of a highly social and aggressive bird, the noisy miner (Manorina melanocephala): variations in sex ratio, age composition and physiological condition. Ph.D. Thesis, University of New England.
Ewen, JG, Crozier, RH, Cassey, P, Ward-Smith, T, Painter, JN, Robertson, RJ, Jones, DA, and Clarke, MF (2003). Facultative control of offspring sex in the cooperatively breeding bell miner, Manorina melanophrys. Behavioral Ecology 14, 157–164.
| Facultative control of offspring sex in the cooperatively breeding bell miner, Manorina melanophrys.Crossref | GoogleScholarGoogle Scholar |
Gaston, AJ (1978). Demography of the jungle babbler, Turdoides striatus. Journal of Animal Ecology 47, 845–870.
| Demography of the jungle babbler, Turdoides striatus.Crossref | GoogleScholarGoogle Scholar |
Grayson, KL, Mitchell, NJ, Monks, JM, Keall, SN, Wilson, JN, and Nelson, NJ (2014). Sex ratio bias and extinction risk in an isolated population of Tuatara (Sphenodon punctatus). PLoS ONE 9, e94214.
| Sex ratio bias and extinction risk in an isolated population of Tuatara (Sphenodon punctatus).Crossref | GoogleScholarGoogle Scholar |
Greenwood, PJ, and Harvey, PH (1982). The natal and breeding dispersal of birds. Annual Review of Ecology and Systematics 13, 1–21.
| The natal and breeding dispersal of birds.Crossref | GoogleScholarGoogle Scholar |
Grey, MJ, Clarke, MF, and Loyn, RH (1997). Initial changes in the avian communities of remnant eucalypt woodlands following a reduction in the abundance of noisy miners, Manorina melanocephala. Wildlife Research 24, 631–648.
| Initial changes in the avian communities of remnant eucalypt woodlands following a reduction in the abundance of noisy miners, Manorina melanocephala.Crossref | GoogleScholarGoogle Scholar |
Griffiths, R, Double, MC, Orr, K, and Dawson, RJG (1998). A DNA test to sex most birds. Molecular Ecology 7, 1071–1075.
| A DNA test to sex most birds.Crossref | GoogleScholarGoogle Scholar |
Hatchwell, BJ (2009). The evolution of cooperative breeding in birds: kinship, dispersal and life history. Philosophical Transactions of the Royal Society B: Biological Sciences 364, 3217–3227.
| The evolution of cooperative breeding in birds: kinship, dispersal and life history.Crossref | GoogleScholarGoogle Scholar |
Higgins PJ, Peter JM, Steele WK (2001) ‘Handbook of Australian, New Zealand and Antarctic birds.’ (Oxford Univ. Press: Melbourne)
JMP (2019) ‘JMP® Version 15.’ (SAS Institute Inc.: Cary, NC)
Koenig, WD (1981). Reproductive success, group size, and the evolution of cooperative breeding in the acorn woodpecker. The American Naturalist 117, 421–443.
| Reproductive success, group size, and the evolution of cooperative breeding in the acorn woodpecker.Crossref | GoogleScholarGoogle Scholar |
Kokko, H, Gunnarsson, TG, Morrell, LJ, and Gill, JA (2006). Why do female migratory birds arrive later than males? Journal of Animal Ecology 75, 1293–1303.
| Why do female migratory birds arrive later than males?Crossref | GoogleScholarGoogle Scholar |
Komdeur, J (1994). Conserving the seychelles warbler Acrocephalus sechellensis by translocation from Cousin Island to the islands of Aride and Cousine. Biological Conservation 67, 143–152.
| Conserving the seychelles warbler Acrocephalus sechellensis by translocation from Cousin Island to the islands of Aride and Cousine.Crossref | GoogleScholarGoogle Scholar |
Kopps, AM, McDonald, P, and Rollins, LA (2013). Isolation and characterisation of polymorphic microsatellite loci for noisy miners Manorina melanocephala, with successful cross-amplification in bell miners M. melanophrys. Conservation Genetics Resources 5, 39–41.
| Isolation and characterisation of polymorphic microsatellite loci for noisy miners Manorina melanocephala, with successful cross-amplification in bell miners M. melanophrys.Crossref | GoogleScholarGoogle Scholar |
Lewis, DM (1982). Dispersal in a population of white-browed sparrow weavers. The Condor 84, 306–312.
| Dispersal in a population of white-browed sparrow weavers.Crossref | GoogleScholarGoogle Scholar |
Liker, A, Freckleton, RP, and Székely, T (2014). Divorce and infidelity are associated with skewed adult sex ratios in birds. Current Biology 24, 880–884.
| Divorce and infidelity are associated with skewed adult sex ratios in birds.Crossref | GoogleScholarGoogle Scholar |
Mac Nally, R, Bowen, M, Howes, A, McAlpine, CA, and Maron, M (2012). Despotic, high-impact species and the subcontinental scale control of avian assemblage structure. Ecology 93, 668–678.
| Despotic, high-impact species and the subcontinental scale control of avian assemblage structure.Crossref | GoogleScholarGoogle Scholar |
Maron, M, Grey, MJ, Catterall, CP, Major, RE, Oliver, DL, Clarke, MF, Loyn, RH, Mac Nally, R, Davidson, I, and Thomson, JR (2013). Avifaunal disarray due to a single despotic species. Diversity and Distributions 19, 1468–1479.
| Avifaunal disarray due to a single despotic species.Crossref | GoogleScholarGoogle Scholar |
McDonald, PG, Ewen, JG, and Wright, J (2010). Brood sex ratio does not affect helper effort in a cooperative bird, despite extreme sex-biased dispersal. Animal Behaviour 79, 243–250.
| Brood sex ratio does not affect helper effort in a cooperative bird, despite extreme sex-biased dispersal.Crossref | GoogleScholarGoogle Scholar |
Melton, CB, Reside, AE, Simmonds, JS, McDonald, PG, Major, RE, Crates, R, Catterall, CP, Clarke, MF, Grey, MJ, Davitt, G, Ingwersen, D, Robinson, D, and Maron, M (2021). Evaluating the evidence of culling a native species for conservation benefits. Conservation Science and Practice 3, e549.
| Evaluating the evidence of culling a native species for conservation benefits.Crossref | GoogleScholarGoogle Scholar |
Montague-Drake, RM, Lindenmayer, DB, Cunningham, RB, and Stein, JA (2011). A reverse keystone species affects the landscape distribution of woodland avifauna: a case study using the noisy miner (Manorina melanocephala) and other Australian birds. Landscape Ecology 26, 1383–1394.
| A reverse keystone species affects the landscape distribution of woodland avifauna: a case study using the noisy miner (Manorina melanocephala) and other Australian birds.Crossref | GoogleScholarGoogle Scholar |
Nelson-Flower, MJ, Wiley, EM, Flower, TP, and Ridley, AR (2018). Individual dispersal delays in a cooperative breeder: ecological constraints, the benefits of philopatry and the social queue for dominance. Journal of Animal Ecology 87, 1227–1238.
| Individual dispersal delays in a cooperative breeder: ecological constraints, the benefits of philopatry and the social queue for dominance.Crossref | GoogleScholarGoogle Scholar |
NSWSC (2013) ‘Final determination to list ‘Aggressive exclusion of birds from woodland and forest habitat by abundant noisy miners Manorina melanocephala (Latham 1802)’ as a key threatening process.’ (New South Wales Scientific Committee: Sydney)
Pyle P, Howell SNG, Yunick RP, DeSante DF (1987) ‘Identification guide to North American Passerines.’ (Slate Creek Press: Bolinas, California). p. 278.
Reichard, M, Polačik, M, Blažek, R, and Vrtílek, M (2014). Female bias in the adult sex ratio of African annual fishes: interspecific differences, seasonal trends and environmental predictors. Evolutionary Ecology 28, 1105–1120.
| Female bias in the adult sex ratio of African annual fishes: interspecific differences, seasonal trends and environmental predictors.Crossref | GoogleScholarGoogle Scholar |
Remeš, V, Freckleton, RP, Tökölyi, J, Liker, A, and Székely, T (2015). The evolution of parental cooperation in birds. Proceedings of the National Academy of Sciences 112, 13603–13608.
Ridley, AR, and Huyvaert, KP (2007). Sex-biased preferential care in the cooperatively breeding Arabian babbler. Journal of Evolutionary Biology 20, 1271–1276.
| Sex-biased preferential care in the cooperatively breeding Arabian babbler.Crossref | GoogleScholarGoogle Scholar |
Serventy, DL, Nicholls, CA, and Farner, DS (1967). Pneumatization of the cranium of the zebra finch Taeniopygia castanotis. Ibis 109, 570–578.
| Pneumatization of the cranium of the zebra finch Taeniopygia castanotis.Crossref | GoogleScholarGoogle Scholar |
Smith, SM (1978). The “underworld” in a territorial sparrow: adaptive strategy for floaters. The American Naturalist 112, 571–582.
| The “underworld” in a territorial sparrow: adaptive strategy for floaters.Crossref | GoogleScholarGoogle Scholar |
Stacey, PB, and Ligon, JD (1987). Territory quality and dispersal options in the acorn woodpecker, and a challenge to the habitat-saturation model of cooperative breeding. The American Naturalist 130, 654–676.
| Territory quality and dispersal options in the acorn woodpecker, and a challenge to the habitat-saturation model of cooperative breeding.Crossref | GoogleScholarGoogle Scholar |
Székely, T, Liker, A, Freckleton, RP, Fichtel, C, and Kappeler, PM (2014). Sex-biased survival predicts adult sex ratio variation in wild birds. Proceedings of the Royal Society B: Biological Sciences 281, 20140342.
| Sex-biased survival predicts adult sex ratio variation in wild birds.Crossref | GoogleScholarGoogle Scholar |
Thackway R, Cresswell ID (1995) ‘An interim biogeographic regionalisation for Australia: a framework for establishing the national system of reserves, Version 4.0.’ (Australian Nature Conservation Agency: Canberra)
Thomson, JR, Maron, M, Grey, MJ, Catterall, CP, Major, RE, Oliver, DL, Clarke, MF, Loyn, RH, Davidson, I, Ingwersen, D, Robinson, D, Kutt, A, MacDonald, MA, and Mac Nally, R (2015). Avifaunal disarray: quantifying models of the occurrence and ecological effects of a despotic bird species. Diversity and Distributions 21, 451–464.
| Avifaunal disarray: quantifying models of the occurrence and ecological effects of a despotic bird species.Crossref | GoogleScholarGoogle Scholar |
TSSC (2013) ‘Aggressive exclusion of birds from potential woodland and forest habitat by over-abundant noisy miners (Manorina melanocephala).’ (Threatened Species Scientific Committee, Department of the Environment: Canberra)
Wiley, RH, and Rabenold, RN (1984). The evolution of cooperative breeding by delayed reciprocity and queuing for favorable social positions. Evolution 38, 609–621.
| The evolution of cooperative breeding by delayed reciprocity and queuing for favorable social positions.Crossref | GoogleScholarGoogle Scholar |
Woolfenden, GE (1989). Population ecology of the cooperatively breeding acorn woodpecker. Evolution 43, 1129–1130.
| Population ecology of the cooperatively breeding acorn woodpecker.Crossref | GoogleScholarGoogle Scholar |


