Seasonal and daily activity of non-native sambar deer in and around high-elevation peatlands, south-eastern Australia
Sebastien Comte A * , Elaine Thomas B , Andrew J. Bengsen
A * , Elaine Thomas B , Andrew J. Bengsen  A , Ami Bennett
A , Ami Bennett  C , Naomi E. Davis
C , Naomi E. Davis  C D , Sean Freney A G , Stephen M. Jackson
C D , Sean Freney A G , Stephen M. Jackson  A H , Matt White E , David M. Forsyth
A H , Matt White E , David M. Forsyth  A and Daniel Brown F
A and Daniel Brown F
A Vertebrate Pest Research Unit, NSW Department of Primary Industries, 1447 Forest Road, Orange, NSW 2800, Australia.
B Parks Victoria, Mt Beauty, Vic. 3699, Australia.
C School of BioSciences, The University of Melbourne, Melbourne, Vic. 3010, Australia.
D Parks Victoria, 535 Bourke Street, Melbourne, Vic. 3000, Australia.
E Department of Environment, Land, Water and Planning, Arthur Rylah Institute for Environmental Research, 123 Brown Street, Heidelberg, Vic. 3084, Australia.
F Parks Victoria, Bright, Vic. 3741, Australia.
G Present address: North Coast Local Land Services, 24–26 Mulgi Drive, South Grafton, NSW 2460, Australia.
H Present address: Australian Museum Research Institute, 1 William Street, Sydney, NSW 2010, Australia.
Wildlife Research 49(7) 659-672 https://doi.org/10.1071/WR21147
Submitted: 15 October 2021 Accepted: 7 March 2022 Published: 26 May 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Of the six species of non-native deer present in Australia, the sambar deer is the largest and has been identified as a major threat to high-elevation peatlands in south-eastern Australia. However, little is known about sambar deer activity in high-elevation peatlands.
Aims: The aims of this study were to quantify sambar deer activity (including wallowing) seasonally and daily in response to biotic and abiotic variables, and how activity was impacted by ground-based shooting.
Methods: To estimate sambar deer activity, camera traps were continuously deployed for 4 years in two ~4300-ha areas in Alpine National Park, Victoria, south-eastern Australia. One area was subject to management operations using ground-based shooting to target deer and the other was not. Monthly activity of sambar deer was modelled using biotic (woody vegetation cover), abiotic (snow depth, aspect, slope, distance to water, road and peatland) and management (treatment versus non-treatment) covariates. Additional camera traps were deployed to monitor sambar deer activity at wallows.
Key results: Sambar deer activity decreased when snow depth increased (between July and September), and was highest in easterly and northerly aspects with dense woody vegetation close to high-elevation peatlands and roads. During our 4-year study, sambar deer activity decreased in the treatment area but increased in the non-treatment area. Sambar deer exhibited a crepuscular diel cycle, with greatest activity around sunset. Only male sambar deer were observed to wallow, with most wallowing occurring in the afternoon during October–June.
Conclusions: Sambar deer utilised high-elevation peatlands during October–June. Daily activity was crepuscular and was greatest in dense tree cover close to roads. Ground-based shooting reduced sambar deer activity in and around high-elevation peatlands.
Implications: Control operations targeting sambar deer at high elevations in south-eastern Australia should be conducted during October–June. Outside this period sambar deer appear to use lower-elevation habitats. The effectiveness of ground-based shooting could be improved by focusing this control action around sunset (when sambar deer are most active) and in places with dense vegetation close to roads and high-elevation peatlands.
Keywords: Alpine National Park, biological invasions, camera trap, Cervus unicolor, diel cycle, invasive species, population dynamics, ungulates, wallowing.
Introduction
Introduced species can be important drivers of ecological change (Didham et al. 2005), and significant resources are spent managing their impacts (Diagne et al. 2021). This is particularly the case for deer (Family: Cervidae), which have been widely introduced around the world (Long 2003) and can significantly alter ecosystems, primary industries and human health (Wardle et al. 2001; Côté et al. 2004). Effective management of introduced species such as deer requires an understanding of their biology and the mechanisms by which their impacts occur (Simberloff et al. 2005) along with consideration for the local socio-economic context (Dolman and Wäber 2008). Factors that can be important predictors of deer activity and impacts in temperate ecosystems include aspect, tree cover, proximity to water and, at high elevations, snow depth (White et al. 2009; Allen et al. 2015; Coe et al. 2018). The presence of anthropogenic structures, such as roads, farmlands and habitations can also affect the activity and impacts of deer (Forman and Deblinger 2000; Menichetti et al. 2019; Pfeiffer et al. 2020).
Of the six deer species that were deliberately and successfully introduced into Australia, the sambar deer (Cervus unicolor1) is the largest; adult males and females weigh 220 and 140 kg, respectively (Bentley 1998; Moriarty 2004). Since their introduction in the Australian state of Victoria in 1860, sambar deer have colonised a wide range of habitats in south-eastern Australia, from coastal forest and heathlands to high-elevation treeless plains (Moriarty 2004; Davis et al. 2016). ‘The reduction in biodiversity of native vegetation by sambar deer’ has been listed as a key threatening process under Victoria’s Flora and Fauna Guarantee Act 1988, including impacts on Alpine Sphagnum Bogs and Associated Fens (hereafter referred to as high-elevation peatlands), a nationally endangered ecological community (Australian Government, Environment Protection and Biodiversity Conservation Act 1999). The impacts of sambar deer result from behaviours such as herbivory, antler thrashing, rubbing of trees, wallowing and trampling (Bennett and Coulson 2010; Bilney 2013; Davis et al. 2016).
During the last 20 years, ground-based shooting (reviewed in Bengsen et al. 2020) has been used to reduce the density and impacts of introduced deer in Australia, including sambar deer (Bennett et al. 2015). Implementation of effective ground-based shooting, however, is hampered by a lack of information about the daily and seasonal activities of sambar deer in their non-native range [but see Forsyth et al. 2009; Davies et al. 2020]. This information is needed to determine at what time(s) of the day and in which month(s) ground-based shooting is likely to be most effective. Ground-based shooting can, in turn, influence the activity of surviving deer spatially and temporally, with deer shifting their activity from diurnal to nocturnal (Ikeda et al. 2019) and selecting densely-vegetated habitats (Benhaiem et al. 2008; Laguna et al. 2021).
In their native range, which spans from sea level in the Philippines to >3500 m in the Himalayas (Green 1987; Whitehead 1993), sambar deer activity is strongly positively associated with wet forest gullies adjacent to flat grasslands (Simcharoen et al. 2014; Yen et al. 2019). In low-elevation catchments in south-eastern Australia, aspect, distance to water and elevation were important predictors of the relative abundance of sambar deer (Forsyth et al. 2009). Sambar deer are considered non-migratory in their native range, but in mountainous regions they move to higher elevations during the warmer months (Green 1987; Yen et al. 2019). Sambar deer are crepuscular, typically resting in dense forest during the day and moving into open areas to feed at dusk and then returning to cover at dawn (Kawanishi and Sunquist 2004; Matsubayashi et al. 2007; Brodie and Brockelman 2009). The seasonal and daily activity of sambar deer in Australia have been little investigated, although Davies et al. (2020) reported a crepuscular daily activity pattern in Baw Baw National Park (Victoria) during spring and early summer. In contrast to deer species indigenous to temperate regions [e.g. fallow deer (Dama dama) and red deer (Cervus elaphus)], tropical species such as sambar deer have low reproductive seasonality. Although sambar deer males with hard antlers, and females with newly-born calves, can be observed throughout the year, in Australia, a peak of rutting activity and birthing has been observed during winter (Bentley 1998; Harrison 2010; Asher 2011; Watter et al. 2020). Most wallowing is thought to be done by adult male sambar deer when rutting (Chalmers 2018), but otherwise little is known of the wallowing activity of sambar deer in Australia.
The objective of this study was to answer five key questions about sambar deer activity in and around high-elevation peatlands in south-eastern Australia: (1) does the strong seasonality of the high-elevation environment influence the monthly activity of sambar deer? (2) does sambar deer activity increase with increasing tree cover and increasing proximity to watercourses? (3) does sambar deer activity peak at dusk and dawn? (4) are there sex–age class differences in wallowing activity by sambar deer? and (5) does ground-based shooting reduce sambar deer activity?
Material and methods
Study areas
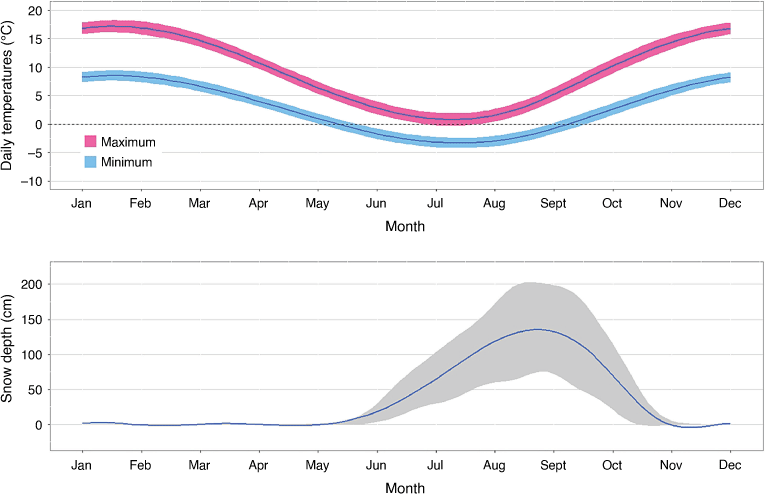
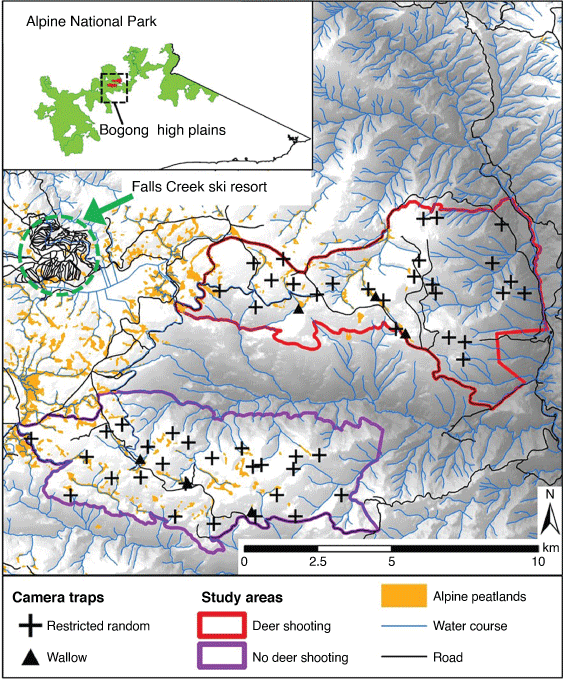
We conducted our study at two high-elevation areas on the Bogong High Plains (BHP), within Alpine National Park, Victoria, south-eastern Australia (36.87°S, 147.28°E; Fig. 1). These two areas were a subset of eight areas included in a management program organised by Parks Victoria assessing the effects of ground-based shooting on the impacts of sambar deer on high-elevation peatlands. The original design proposed that all eight study areas have camera traps (Davis et al. 2015a, 2015b), but for financial reasons they were only placed in two areas. The treatment (ground-based shooting) was allocated to one of the two areas by a coin toss; the other is hereafter referred to as the non-treatment area. The mean elevations of the treatment (4422 ha) and non-treatment (4201 ha) areas were 1417 and 1561 m, respectively. Both areas receive a mean of 1302 mm of rainfall annually, with low monthly variation (range: 92.7–139.3 mm). Mean monthly minimum and maximum temperatures range from 8.3 to 16.9°C in February to −3.2 and 0.9°C in July (Australian Bureau of Meteorology, Falls Creek weather station, http://www.bom.gov.au). Snow typically accumulates on the ground from June to October, with a maximum mean monthly snow depth of 136 cm (2014–2019, Daily Snow Depth Records Falls Creek, Department of Environment, Land, Water & Planning, https://discover.data.vic.gov.au).
|
The study areas consist of mostly flat to undulating terrain with a mosaic of subalpine woodland dominated by snow gums (Eucalyptus pauciflora) and treeless plain including alpine sphagnum bogs and associated fens (dominated by Sphagnum subsecundum and S. cristatum). The steeper slopes along water-formed gullies are mostly covered by montane wet forests (dominated by alpine ash E. delegatensis) with dense understorey (Conn 1993). In addition to their high biodiversity values, these plant communities regulate and filter water flows to major river systems that benefit agriculture, hydroelectricity and drinking water supply. Other medium- or large-herbivores present in the study area, in addition to sambar deer, are the native swamp wallaby (Wallabia bicolor) and bare-nosed wombat (Vombatus ursinus). The management program was designed to be outside of the main distribution of feral horses (Equus caballus; Cairns and Robertson 2014), but some horses were present in both study areas.
Camera trapping
In each study area, 25 camera traps were continuously deployed between April 2015 and April 2019. Two camera trap models were used for the first month of monitoring: the Reconyx PC900 and the Reconyx HC500 (Reconyx Inc., Holmen, Wisconsin, USA). From May 2015 to April 2019, all camera traps (except for one model PC900) were replaced by the Reconyx HC600 model. All camera traps were visited every 6 months to replace the batteries and Secure Digital (SD) cards. Camera locations were selected using restricted random sampling. Each study area was divided into 160-ha hexagonal cells, within which a random point was generated as the camera trap location (Fig. 1). To maximise detection, camera traps were located at the nearest wildlife trail to the random sampling coordinates (within 50 m radius). The average distance between cameras was 617 m (range: 120–1338 m) and 850 m (range: 103–1982 m) in the treatment and non-treatment areas, respectively, and 6560 m (range: 4811–9913 m) between the two areas. To standardise detection probabilities, all cameras were mounted 130–150 cm above ground on a tree facing south (to avoid direct sunlight) and with the detection sensor aimed at a linear distance of 6 m from the camera. Vegetation was trimmed within the detection zone (i.e. 6 m in front of each camera trap and 40° either side of the central line of sight) to prevent false triggers from moving grass and branches. Camera traps were not baited or lured. To maximise the detection and accurate identification of deer, cameras were set to ‘high sensitivity’, ‘three images per movement’ and ‘rapidfire’ with no delay between trigger events.
In addition, three camera traps (Reconyx HC600) were subjectively located within each study area to continuously monitor active wallows in high-elevation peatlands between April 2015 and April 2019 (Fig. 1). These cameras were mounted on a metal stake 200 cm above ground and 200 cm from the edge of the wallow, facing the centre of the wallow. The camera settings were the same as those described above. The objective of these cameras was to quantify wallowing activity by sambar deer (see Question 4), as none of the other 50 cameras described above overlooked wallows.
Image processing
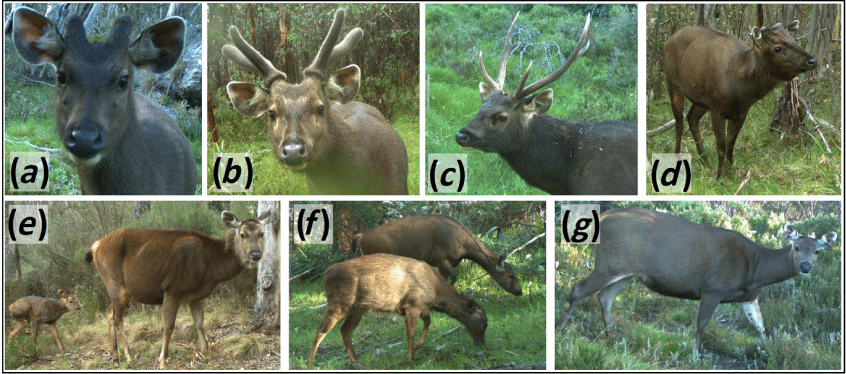
Data were obtained by downloading images from the SD cards onto a computer and manually assigning a defined set of metadata tags to each image, where relevant, using the software ExifPro v2.1 (Kowalski and Kowalski 2013). Images were grouped into independent detection events separated by ≥10 min and the same metadata were assigned to all images within one detection event, with the timestamp of the first image used for all images in that event. The same two experienced observers tagged all images. These detection events were considered a representative sample of the true sambar deer activity distribution in the study areas (Linkie and Ridout 2011). The metadata included the species detected and the number of individuals detected during each event. For sambar deer, individuals were additionally assigned to two sex–age classes: males and females-juveniles (including calves, Fig. 2). All individuals with visible antlers or pedicles were tagged as males. Individuals were classified as females based on their body size and the absence of antlers or external genitalia. The presence of a calf or a juvenile close to the animal was also used to assign adult antlerless individuals as females. All individuals with small body size, calves and yearlings without spike antlers, were considered as juveniles with no sex assigned. For the cameras monitoring the wallows, the antler stage of all male sambar deer was further described as hard antlers, velvet antlers or no antlers (pedicles visible). Due to the low variability in colour and texture of sambar deer coats, it was not possible to confidently identify individuals.
Given that sambar deer activity is likely to be driven by sunlight, and that sunrise and sunset varied through the year in our study areas, we scaled the timestamps of each detection event into sun time (i.e. sunrise and sunset, every day, were represented by 6:00 and 18:00, respectively) using the function sunTime in the R-package overlap version 0.3.3 (Ridout and Linkie 2009).
Habitat variables
Snow depth was recorded daily at Falls Creek ski resort, <5 km from the two study areas (Fig. 1). These measurements started in June and stopped at the end of the ski season in early October, when snow was often still present on the ground. We assumed an absence of snow between November and May by fixing the snow depth to zero. We then modelled the mean monthly snow depth (cm) across the 4 years of the study (2015–2019) using a General Additive Mixed Model (GAMM) with a Tweedie family to handle the zero-inflated and continuously positive data. To account for the circular nature of the months (i.e. December and January are adjacent), we modelled month as a cyclic cubic spline. We included year as a random effect. We used the same approach to model the monthly minimum and maximum temperatures (degrees Celsius) during our 4-year study with Gaussian family GAMMs (Fig. 3).
We described the topography of the two study areas using four variables measured over a grid of 100 m × 100 m: elevation, slope, aspect and terrain ruggedness. We classified the vegetation structure as the proportions of woody vegetation cover and herbaceous cover for each grid cell. We used the geographic coordinates of each camera to extract covariate values for the matching grid cell. For each camera, we measured the distances to the nearest watercourse, road and peatland. The data sources for these 12 variables are given in Supplementary Table S1. We tested for collinearity within all covariates and kept only one variable from each group of dependent variables (Pearson’s correlation coefficient |r| > 0.7; Tables 1, S2).

|
Spatio-temporal activity
We measured sambar deer activity as the number of independent detections for both sex–age classes (males and females-juveniles) on each camera trap during each calendar month (1–12) and year (1–5). We fitted a generalised linear mixed model (GLMM) in a Bayesian framework to estimate the effects of the habitat variables (Table 1) on sambar deer activity with a negative binomial family and a random effect for month:
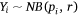
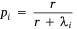
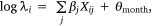
and
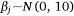
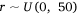
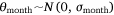
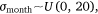
where Yi represents the negative binomial density function of sambar deer activity during 1 month on one camera trap, with pi the success parameter, r the dispersion parameter, βjXij the linear predictors, and θmonth the random effect of month.
The effect of snow depth (cm) on sambar deer detections was modelled as linear predictors with sex–age-specific intercepts and slopes. The effect of year (2015–2019) on sambar deer detections was modelled as linear predictors with intercepts and slopes for each study area. Slope (0–90°), distances (m) to water, road and peatland, and woody vegetation cover (1–100), were modelled as linear predictors. Aspect is a circular variable (0–360°) and hence it was modelled using a sine function for easterly aspect and a cosine function for the northerly aspect. We added a random effect of month to account for multiple measurements during the same month across the years of the study.
All predictor variables, except for aspect, were centred on their mean to facilitate the mixing of the Monte Carlo Markov chains (MCMC). We ran three chains of 150 000 iterations each, with 5000 adaptation runs and 5000 burn-in runs. We assessed the mixing of the MCMC chains visually and with the Gelman–Rubin diagnostic (R; Gelman and Rubin 1992).
The snow depth predictions were made using the R-package mgcv v1.8-31 (Wood 2011; R Core Team 2020). Spatial analyses were performed using the R-packages raster version 3.1-5, rgdal version 1.5-10 and rgeos version 0.5-3 (Bivand and Rundel 2020; Bivand et al. 2020; Hijmans 2020). We evaluated the Pearson correlation among covariates using the package Hmisc version 4.4-0 (Harrel 2020). The Bayesian regression analyses were performed using the packages runjags version 2.0.4-6 (Denwood 2016) and coda version 0.19-3 (Plummer et al. 2006).
Diel activity
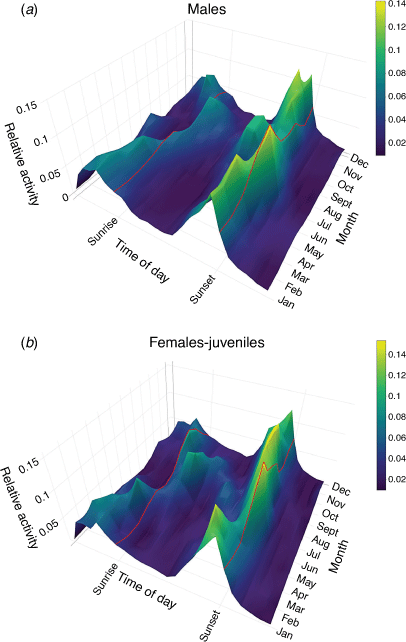
Annual and daily activity patterns are circular and likely to be non-linear. Generalised additive models have been used to describe the diel cycles of deer (Bischof et al. 2014). To obtain a sample size sufficient to meaningfully estimate seasonal trends, we pooled detection events by month. For each camera, for each month, we then constructed the response variable as the proportion of detections for every hour of the day (0–23, scaled as sun time 6:00 being sunrise and 18:00 sunset) resulting in a sum of 1 for each camera-month. We retained camera-months with at least three detection events. To account for the circular nature of deer activity, we used a cyclic cubic spline for both hour and month, and included their interaction term in the model using a tensor product interaction. We allowed both terms to have sex–age-specific smoothing functions. We also included camera within each area and within each year as a nested random effect. The model was fitted with a quasibinomial distribution (to account for overdispersion), with a penalty added to the null space of each smooth term, using the R-package mgcv version 1.8-31.
Wallowing activity
Sambar deer detected at a wallow were not always wallowing. We discarded detections of deer that were walking by the wallow, only grazing outside the wallow or drinking from the wallow. A wallowing event was defined as an individual observed lying down in the wallow, rolling from side to side and rubbing its head or antlers on the adjacent vegetation (Fig. 4).

|
We first characterised the proportion of images with wallowing each month in the treatment and non-treatment areas using the previously described sex–age classes and the different antler stages. We then compared the circadian distribution of wallowing activity in both areas using non-parametric kernel density estimates calculated with the R-package overlap version 0.3.3 (Ridout and Linkie 2009). We compared the mean and variance of the two distributions using a Mardia–Watson–Wheeler test of homogeneity (Zar 1996) in the R-package circular version 0.4-93 (Agostinelli and Lund 2017).
Results
Data scope
Between April 2015 and April 2019, the 50 camera traps recorded 444 516 images. The theft of five cameras resulted in a 6-month gap in data for each camera location before the camera was replaced. Sambar deer were detected on 8218 independent events (39% in the treatment area with ground-based shooting and 61% in the non-treatment area with no ground-based shooting) for a total of 10 227 deer (mean and maximum group sizes = 1.24 and 8). The detections consisted of 62% adult males, 29% adult females and 9% unsexed juveniles and calves. Sex–age class could not be determined for 1465 detections (14%), and these were excluded from analyses. No deer species other than sambar were detected. A total of 54 feral horses were detected on 24 independent events (maximum group size = 9; 75% of detections in the treatment area). A total of 454 wild dogs/dingoes (Canis familiaris) were detected on 322 independent events (maximum group size = 5; 65% of detections in the treatment area). A total of 75 humans were detected on 30 independent events (maximum group size = 10; 35% of detections in the treatment area). Of those, only one deer shooter (involved in the management program) was detected, once on one camera in the treatment area.
Spatio-temporal activity
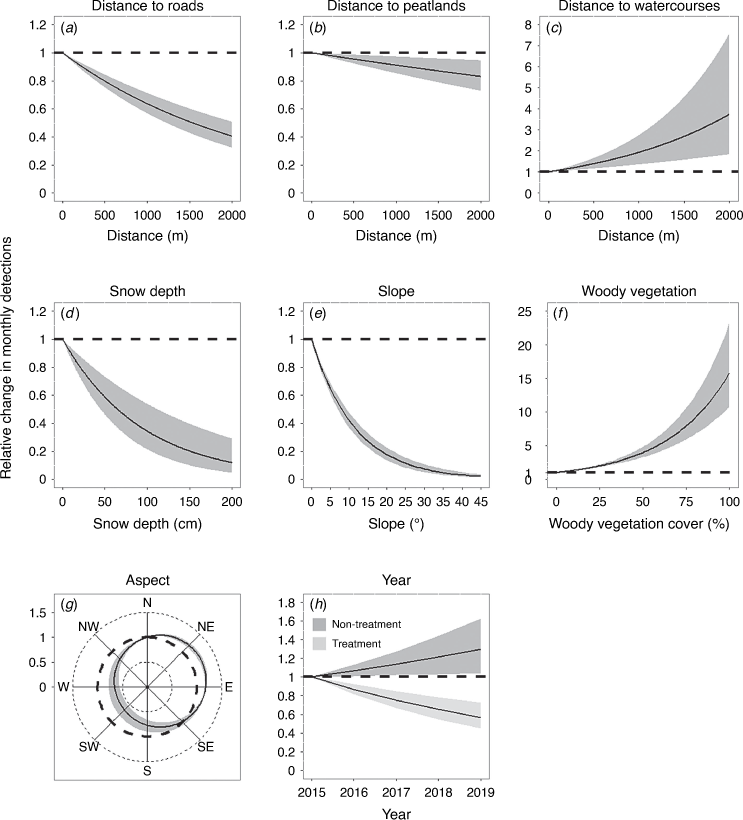
All covariates included in our model were significantly associated with sambar deer activity (i.e. none of the 95% credible intervals included zero; Table S3), although effect sizes varied greatly (Fig. 5). Sambar deer detections were 1.6 times more likely to be due to males than females-juveniles. As predicted, sambar deer activity in the study area was strongly influenced by seasonal snow accumulation. The number of detections of both males and females-juveniles decreased by approximately 80% when snow depth reached its mean monthly maximum (136 cm). Sambar deer avoided the steepest slopes and selected easterly and northerly aspects. As expected, sambar deer were more active in areas with dense woody vegetation, and were more active closer to roads and peatlands, but away from watercourses (Fig. 5). At the beginning of the study, sambar deer detections were 30% lower in the treatment area (i.e. subject to ground-based shooting) than in the non-treatment area (Table S3). During the 4-year study (across five calendar years) there was a 14% annual decrease in sambar deer activity in the treatment area, but a 7% annual increase in the non-treatment area.
Diel activity
We recorded detections of sambar deer on 1153 camera-days (721 for males and 432 for females-juveniles), representing 23% of the total survey effort. The GAMM revealed a significant interaction between the hour of the day and month for both sex–age classes (males: EDF = 77.52, P = <0.01; females-juveniles: EDF = 82.79, P = <0.01; variance explained = 12.4%; see Table S4 for full model output). Sambar deer activity was mainly crepuscular, with most activity occurring just before sunset and, to a lesser extent, around sunrise (Fig. 6). The diel cycle differed in the winter months (July–September), with activity peaking during daylight, later in the morning for females-juveniles, and earlier in the afternoon for males. This shift in peak activity period coincided with the presence of snow in the study areas (Fig. 3).
|
Wallowing activity
Of the six cameras deployed on wallows in April 2015, one camera was stolen in May 2015 and not replaced (so no images were available for that wallow), two cameras recorded images until November 2016 when one was stolen and the other was removed. Three cameras recorded activity until the end of the study in April 2019. Between April 2015 and April 2019, we recorded 101 222 images during 5576 camera trap nights at five wallows. A total of 1049 independent detections were recorded for a total of 2047 sambar deer, making sambar deer the most common large mammal species detected at the wallows. Feral horses (n = 27 detections) were detected during the last 4 months of the study at a single wallow while grazing, drinking and wallowing. Humans were detected riding horses on seven occasions in and around two wallows. Other species detected at the wallows were bare-nosed wombat (n = 7 detections on two cameras, always in darkness), swamp wallaby (n = 7 detections on one camera always during daylight), red fox (Vulpes vulpes, n = 55 detections), wild dog/dingo (Canis familiaris, n = 16 detections) and feral cat (Felis catus, n = 8 detections). The European hare (Lepus europaeus) was commonly detected at wallows in winter when snow was present. None of these taxa were detected in a wallow; rather, they were walking outside, or drinking from, the wallow.
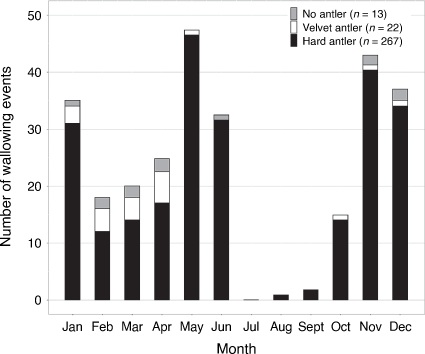
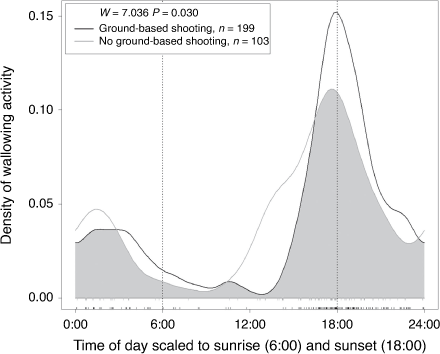
A total of 302 out of 1049 detections (29%) showed deer wallowing (i.e. lying in the mud, rolling on their sides or rubbing their head; Fig. 4). All wallowing by sambar deer was by males, with only one juvenile that could not be sexed. Wallowing by sambar deer on Bogong High Plains occurred in two seasonal peaks, one between April and June, and the second between November and January (Fig. 7). Wallowing almost never occurred between July and September, when there was deep snow cover and low sambar deer activity. There was no difference in wallowing activity during the day between male sambar deer in hard antler (n = 267) and combined velvet or no antlers (n = 22 and n = 13, respectively; Mardia–Watson–Wheeler test: W = 2.359, P = 0.307). Wallowing activity was higher in the treatment area (Table 2), but robust comparison between the two areas is limited by the small number of cameras that monitored wallows. Yet, the daily pattern of wallowing activity was significantly different in the treatment area compared to the non-treatment area. Wallowing peaked around sunset at both areas, but was more widely distributed throughout the afternoon at the non-treatment area (Mardia–Watson–Wheeler test: W = 7.036, P = 0.030; Fig. 8).
|

|
|
Discussion
The seasonal and daily activity of sambar deer, including wallowing, has not previously been investigated in high-elevation environments with strong seasonality, such as occur in Alpine National Park in south-eastern Australia. In contrast to previous studies of this species in tropical and low-elevation temperate environments, our 4-year study revealed a strong decrease in sambar deer activity in our two study areas during July–September, when snow was present on the ground. As expected, sambar deer activity was greatest within dense woody vegetation but was also higher close to roads and high-elevation peatlands and away from watercourses. Sambar deer showed strong crepuscular activity, with peak activity near sunset. During winter there was a shift in daily activity towards more diurnal activity after sunrise or before sunset. Only male sambar deer were observed to wallow, with a seasonal peak immediately before snow was present. Wallowing peaked near sunset, a pattern that was accentuated in the treatment area subject to management operations using ground-based shooting.
Ground-based shooting is commonly used to reduce the impacts of introduced deer in Australia. We found that sambar deer activity decreased in the treatment area relative to the non-treatment area. Detection rates of deer on camera traps were positively correlated with deer densities estimated using spatial mark–resight models for white-tailed deer (Odocoileus virginianus) across 20 sites in the United States (Parsons et al. 2017) and for sambar deer across fives sites in eastern Australia (Bengsen et al. 2022). We therefore believe that sambar deer activity on camera traps is a reliable index of sambar deer relative abundance in our study areas. In the non-treatment area, the observed increase in sambar deer activity is consistent with a long-term increase in sambar deer abundance in eastern Victoria, as indexed by hunter’s catch-per-unit-effort (Forsyth et al. 2018; Moloney et al. 2022). Since recreational hunting was prohibited in both study areas, the most likely cause of reduced sambar deer activity in the treatment area was the ground-based shooting implemented there but not in the non-treatment area. During the management operations, 164 sambar deer were removed from the treatment area by ground-based shooting (Parks Victoria, unpubl. data), which could also have led to surviving deer avoiding that area. Human hunting activities can alter the perceived risk for prey, creating a ‘landscape of fear’ (Laundre et al. 2010), which can result in changes in activity or avoidance of risky areas (Benhaiem et al. 2008; Cromsigt et al. 2013; Le Saout et al. 2014). Analysis of the sambar deer catch and effort data recorded by the ground-based shooters, as well as before-after monitoring of deer impacts, will be reported in a subsequent paper and will help understand the effectiveness of ground-based shooting as a management tool for reducing sambar deer impacts in high-elevation areas of south-eastern Australia.
Our results support the general findings that sambar deer prefer dense woody vegetation cover as protection against predators (including humans) and shelter against wind and cold temperatures (Yamada et al. 2003; Gormley et al. 2011; Sotorra et al. 2021). The lower activity of sambar deer close to watercourses in our study may reflect the high availability of surface water (e.g. natural pools, wallows) within the high-elevation peatlands. The higher activity of sambar deer along roads suggest that those linear structures may act as movement corridors across the landscape as was observed in Baw Baw National Park (Davies et al. 2020), an area with a similar road network (i.e. mostly unsealed) and pattern of use by traffic (i.e. seasonal road closures in winter).
Sambar deer were largely absent from our two study areas during July–September, when snow was accumulating on the ground (maximum mean monthly snow depth = 136 cm). The accumulation of snow increases the energetic cost of moving and reduces access to food (White et al. 2009). Red deer in the Italian Alps showed vertical movements towards lower elevations associated with seasonal snow accumulation of 20–60 cm (Luccarini et al. 2006). In China, native sambar deer living in a mixed coniferous and deciduous forest reserve (elevation range 1600–3200 m) showed a reduced detection rate on camera traps and a stronger use of sheltered areas during the colder months of the year (Zhang et al. 2017). The sambar deer that utilised our study areas in spring, summer and autumn presumably moved to lower elevations during winter, as anecdotally reported in Bentley (1998). GPS-collared sambar deer in Taiwan showed strong seasonal movement patterns, moving from high to low elevations during the cold season and back to high elevations during the hot–wet season (Yen et al. 2019). Attaching GPS collars to sambar deer present in the high-elevation peatlands of Alpine National Park during summer–autumn would reveal where they move to during winter.
As expected, sambar deer activity in our two study areas was crepuscular, a pattern largely observed for ungulates balancing the risk of human contact during the day (Gaynor et al. 2018; Pal et al. 2020) and the risk of predation during the night (Kie 1999; Sih et al. 2000). In China, sambar deer shifted their activity from multiple daily peaks (low human activity) to one major activity peak at dusk (high human activity), suggesting flexibility in their diel activity (Zhang et al. 2017). A similar influence of human presence on sambar deer activity was observed in Bandipur, in southern India, where activity during the day only occurred in the least disturbed areas (Johnsingh 1983). As our study was conducted in a national park, human activities shown to influence deer activity elsewhere [e.g. livestock grazing (Stewart et al. 2002) and off-road vehicles (Wisdom et al. 2004)] were limited with the exception of the ground-based shooting conducted in the treatment area. Yet, when moving away from the study areas during the winter months, sambar deer likely experienced interactions with humans closer to adjacent farmland and areas open to recreational hunting, which could influence their activity once they returned to our study areas. In south-eastern Australia, sambar deer are eaten by wild dogs/dingoes, and there was one detection of wild dogs eating a calf by one camera in our study, but the extent to which this is scavenging (Forsyth et al. 2014) or predation is unclear (Forsyth et al. 2019). Wild dogs/dingoes are unlikely to kill a healthy adult sambar deer, but they can kill calves (Bentley 1998). In Borneo, the presence of a predator, Diardi’s clouded leopard (Neofelis diardi), was associated with a shift in sambar deer activity from nocturnal to crepuscular (Ross et al. 2013). Although unlikely to have a limiting effect on sambar deer abundance, the presence of wild dogs/dingoes in our study areas have contributed to the crepuscular nature of sambar deer activity.
This is the first study to quantify the wallowing activity of sambar deer, a behaviour impacting the endangered high-elevation peatlands. Our study confirmed that sambar deer was the main species wallowing in high-elevation peatlands of the Bogong High Plains. Although our study areas were selected to be outside the most-heavily occupied parts of the feral horse distribution in Alpine National Park, feral horses were also observed wallowing, adding to the pressure on high-elevation peatlands. Additionally, domestic horses were detected by the camera traps while being ridden in and around existing wallows, adding to the trampling and grazing impacts on high-elevation peatlands. In our study, only male sambar deer wallowed, and usually when they were in hard antler. These findings are consistent with male sambar deer wallowing when rutting (Semiadi et al. 1994; Bentley 1998; Harrison 2010; Dahlan and Dawend 2013; Chalmers 2018). Sambar deer wallowing was concentrated just before sunset, matching the general crepuscular activity pattern of this species in the study areas. In addition to a reduced abundance of sambar deer in the treatment area, the ground-based shooting may also have reduced the propensity of surviving sambar deer to use the high-elevation peatlands for wallowing during the afternoon (sensu Cromsigt et al. 2013; Ikeda et al. 2019). A broader survey of peatlands across the whole Victorian Alps between 2004 and 2009 suggested that wallows were more frequent in peatlands close to forested areas (Tolsma 2009). Although limited by the small sample size, our results suggest that not all wallows are visited equally by sambar deer. Understanding what physical characteristics and environmental context are associated with the wallowing activity could improve the cost-effectiveness of ground-based shooting by targeting the most visited wallows.
Management implications
Effective management of an invasive species and its impacts requires understanding the ecology of the target species. The results of our study have important implications for the management of sambar deer and their impacts on the high-elevation peatlands in south-eastern Australia. First, in our study areas sambar deer were the main non-native species active in high-elevation peatlands, and are therefore the species causing the greatest disturbance to these endangered communities, through trampling, antler thrashing, grazing and browsing and also through wallowing by males. Hence, it is reasonable to focus management activities on sambar deer to reduce these impacts. The abundance and impacts of feral horses should be monitored because they can significantly impact high-elevation peatlands (Robertson et al. 2019). Second, the decrease in sambar deer activity in the treatment area supports the potential of ground-based shooting as a tool to manage this species in high-elevation peatlands in south-eastern Australia. Third, sambar deer made seasonal use of high-elevation peatlands, being present and wallowing from October to June (i.e. spring–autumn). The departure of sambar deer from these high-elevation areas coincided with the accumulation of snow. There seems little point in attempting to shoot sambar deer in and around high-elevation peatlands during July–September, when they are absent. Fourth, sambar deer activity (including wallowing) was concentrated around sunset. We therefore predict that ground-based shooters would most likely encounter and shoot sambar deer around sunset rather than at other times (Little et al. 2014).
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
The Victorian Alpine Peatland Protection Program is an initiative jointly funded through Parks Victoria, the West Gippsland Catchment Management Authority, the Australian Government’s National Landcare Programme (via North East, West Gippsland and East Gippsland Catchment Management Authorities) and the Victorian Government through the Alps Intensive Management Program. The analysis of these data, and the writing of the manuscript, were funded by the Centre for Invasive Species Solutions project ‘Cost-effective management of wild deer’ (PO1-L-001).
Supplementary material
Supplementary material is available online.
Acknowledgements
All ground-based shooting data used in this study were collected during a management program organised by Parks Victoria. We thank the Parks Victoria staff, in particular Anthony Thomas, Amanda Deller and Wendy Grant, and contractors who deployed and serviced the cameras. Rena Gaborov and Anthony Thomas tagged images. We thank all deer shooters that participated in the ground-shooting program. Comments by Patrick L. Taggart and two anonymous reviewers greatly improved this manuscript.
References
Agostinelli C, Lund U (2017) R package ‘circular’: Circular statistics. version 0.4-93. Available at https://CRAN.R-project.org/package=circular [verified 22 September 2021].Allen, RB, Forsyth, DM, Allen, RKJ, Affeld, K, and MacKenzie, DI (2015). Solar radiation determines site occupancy of coexisting tropical and temperate deer species introduced to New Zealand forests. PLoS One 10, e0128924.
| Solar radiation determines site occupancy of coexisting tropical and temperate deer species introduced to New Zealand forests.Crossref | GoogleScholarGoogle Scholar | 26061426PubMed |
Asher, GW (2011). Reproductive cycles of deer. Animal Reproduction Science 124, 170–175.
| Reproductive cycles of deer.Crossref | GoogleScholarGoogle Scholar | 20884138PubMed |
Bengsen, AJ, Forsyth, DM, Harris, S, Latham, ADM, McLeod, SR, and Pople, A (2020). A systematic review of ground-based shooting to control overabundant mammal populations. Wildlife Research 47, 197–207.
| A systematic review of ground-based shooting to control overabundant mammal populations.Crossref | GoogleScholarGoogle Scholar |
Bengsen, AJ, Forsyth, DM, Ramsey, DSL, Amos, M, Brennan, M, Pople, AR, Comte, S, and Crittle, T (2022). Estimating deer density and abundance using spatial mark-resight models with camera trap data. Journal of Mammalogy , gyac016.
| Estimating deer density and abundance using spatial mark-resight models with camera trap data.Crossref | GoogleScholarGoogle Scholar |
Benhaiem, S, Delon, M, Lourtet, B, Cargnelutti, B, Aulagnier, S, Hewison, AJM, Morellet, N, and Verheyden, H (2008). Hunting increases vigilance levels in roe deer and modifies feeding site selection. Animal Behaviour 76, 611–618.
| Hunting increases vigilance levels in roe deer and modifies feeding site selection.Crossref | GoogleScholarGoogle Scholar |
Bennett, A, and Coulson, G (2010). The impacts of sambar cervus unicolor on the threatened shiny nematolepis Nematolepis wilsonii. Pacific Conservation Biology 16, 251–260.
| The impacts of sambar cervus unicolor on the threatened shiny nematolepis Nematolepis wilsonii.Crossref | GoogleScholarGoogle Scholar |
Bennett, A, Haydon, S, Stevens, M, and Coulson, G (2015). Culling reduces fecal pellet deposition by introduced sambar (Rusa unicolor) in a protected water catchment. Wildlife Society Bulletin 39, 268–275.
| Culling reduces fecal pellet deposition by introduced sambar (Rusa unicolor) in a protected water catchment.Crossref | GoogleScholarGoogle Scholar |
Bentley A (1998) ‘An introduction to the deer of Australia, with special reference to Victoria.’ (The Australian Deer Research Foundation Ltd: Melbourne)
Bilney, RJ (2013). Antler rubbing of yellow-wood by sambar in east Gippsland, Victoria. The Victorian Naturalist 130, 68–74.
Bischof, R, Ali, H, Kabir, M, Hameed, S, and Nawaz, MA (2014). Being the underdog: an elusive small carnivore uses space with prey and time without enemies. Journal of Zoology 293, 40–48.
| Being the underdog: an elusive small carnivore uses space with prey and time without enemies.Crossref | GoogleScholarGoogle Scholar |
Bivand R, Rundel C (2020) Rgeos: Interface to geometry engine - open source (‘geos’). R package version 0.5-3. Available at https://CRAN.R-project.org/package=rgeos [verified 22 September 2021]
Bivand R, Keitt T, Rowlingson B (2020) Rgdal: Bindings for the ‘geospatial’ data abstraction library. R package version 1.5-10. Available at https://CRAN.R-project.org/package=rgdal [verified 22 September 2021]
Brodie, JF, and Brockelman, WY (2009). Bed site selection of red muntjac (Muntiacus muntjak) and sambar (Rusa unicolor) in a tropical seasonal forest. Ecological Research 24, 1251–1256.
| Bed site selection of red muntjac (Muntiacus muntjak) and sambar (Rusa unicolor) in a tropical seasonal forest.Crossref | GoogleScholarGoogle Scholar |
Cairns S, Robertson G (2014) ‘Feral horses in the Australian alps national parks: The design and analysis of surveys conducted in April–may 2014.’ (Australian Alps Liaison Committee)
Chalmers PRS (2018) ‘New Zealand’s sambar and rusa deer.’ (Philip R. S. Chalmers: Whakatane)
Coe, PK, Clark, DA, Nielson, RM, Gregory, SC, Cupples, JB, Hedrick, MJ, Johnson, BK, and Jackson, DH (2018). Multiscale models of habitat use by mule deer in winter. The Journal of Wildlife Management 82, 1285–1299.
| Multiscale models of habitat use by mule deer in winter.Crossref | GoogleScholarGoogle Scholar |
Conn BJ (1993) Natural regions and vegetation of Victoria. In ‘Flora of Victoria’. Vol. 1. pp. 79–158. (Inkata Press)
Côté, SD, Rooney, TP, Tremblay, J-P, Dussault, C, and Waller, DM (2004). Ecological impacts of deer overabundance. Annual Review of Ecology, Evolution, and Systematics 35, 113–147.
| Ecological impacts of deer overabundance.Crossref | GoogleScholarGoogle Scholar |
Cromsigt, JPGM, Kuijper, DPJ, Adam, M, Beschta, RL, Churski, M, Eycott, A, Kerley, GIH, Mysterud, A, Schmidt, K, and West, K (2013). Hunting for fear: Innovating management of human–wildlife conflicts. Journal of Applied Ecology 50, 544–549.
| Hunting for fear: Innovating management of human–wildlife conflicts.Crossref | GoogleScholarGoogle Scholar |
Dahlan, I, and Dawend, J (2013). Growth and reproductive performance of sambar deer in Sabal forest reserve of Sarawak, Malaysia. Tropical Animal Health and Production 45, 1469–1476.
| Growth and reproductive performance of sambar deer in Sabal forest reserve of Sarawak, Malaysia.Crossref | GoogleScholarGoogle Scholar | 23475732PubMed |
Davies, C, Wright, W, Hogan, FE, and Davies, H (2020). Detectability and activity patterns of sambar deer (Rusa unicolor) in Baw Baw National Park, Victoria. Australian Mammalogy 42, 312–320.
| Detectability and activity patterns of sambar deer (Rusa unicolor) in Baw Baw National Park, Victoria.Crossref | GoogleScholarGoogle Scholar |
Davis NE, Bennett A, Forsyth DM (2015a) ‘Monitoring changes in deer density/abundance and habitat use associated with the Parks Victoria deer control trial in the Alpine National Park: Field survey manual.’ Report prepared for Parks Victoria, Melbourne. p. 51.
Davis NE, Bennett A, Forsyth DM (2015b) ‘Monitoring changes in deer density/abundance and habitat use associated with the Parks Victoria deer control trial in the Alpine National Park: Survey design and rationale.’ Report prepared for Parks Victoria, Melbourne. p. 35.
Davis, NE, Bennett, A, Forsyth, DM, Bowman, DMJS, Lefroy, EC, Wood, SW, Woolnough, AP, West, P, Hampton, JO, and Johnson, CN (2016). A systematic review of the impacts and management of introduced deer (family Cervidae) in Australia. Wildlife Research 43, 515–532.
| A systematic review of the impacts and management of introduced deer (family Cervidae) in Australia.Crossref | GoogleScholarGoogle Scholar |
Denwood, MJ (2016). Runjags: An r package providing interface utilities, model templates, parallel computing methods and additional distributions for mcmc models in jags. Journal of Statistical Software 71, 1–25.
| Runjags: An r package providing interface utilities, model templates, parallel computing methods and additional distributions for mcmc models in jags.Crossref | GoogleScholarGoogle Scholar |
Diagne, C, Leroy, B, Vaissière, A-C, Gozlan, RE, Roiz, D, Jarić, I, Salles, J-M, Bradshaw, CJA, and Courchamp, F (2021). High and rising economic costs of biological invasions worldwide. Nature 592, 571–576.
| High and rising economic costs of biological invasions worldwide.Crossref | GoogleScholarGoogle Scholar | 33790468PubMed |
Didham, RK, Tylianakis, JM, Hutchison, MA, Ewers, RM, and Gemmell, NJ (2005). Are invasive species the drivers of ecological change? Trends in Ecology and Evolution 20, 470–474.
| Are invasive species the drivers of ecological change?Crossref | GoogleScholarGoogle Scholar | 16701420PubMed |
Dolman, PM, and Wäber, K (2008). Ecosystem and competition impacts of introduced deer. Wildlife Research 35, 202–214.
| Ecosystem and competition impacts of introduced deer.Crossref | GoogleScholarGoogle Scholar |
Forman, RTT, and Deblinger, RD (2000). The ecological road-effect zone of a Massachusetts (U.S.A.) suburban highway. Conservation Biology 14, 36–46.
| The ecological road-effect zone of a Massachusetts (U.S.A.) suburban highway.Crossref | GoogleScholarGoogle Scholar |
Forsyth, DM, McLeod, SR, Scroggie, MP, and White, MD (2009). Modelling the abundance of wildlife using field surveys and GIS: Non-native sambar deer (Cervus unicolor) in the Yarra Ranges, south-eastern Australia. Wildlife Research 36, 231–241.
| Modelling the abundance of wildlife using field surveys and GIS: Non-native sambar deer (Cervus unicolor) in the Yarra Ranges, south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Forsyth, DM, Woodford, L, Moloney, PD, Hampton, JO, Woolnough, AP, and Tucker, M (2014). How does a carnivore guild utilise a substantial but unpredictable anthropogenic food source? Scavenging on hunter-shot ungulate carcasses by wild dogs/dingoes, red foxes and feral cats in south-eastern Australia revealed by camera traps. PLoS One 9, e97937.
| How does a carnivore guild utilise a substantial but unpredictable anthropogenic food source? Scavenging on hunter-shot ungulate carcasses by wild dogs/dingoes, red foxes and feral cats in south-eastern Australia revealed by camera traps.Crossref | GoogleScholarGoogle Scholar | 24918425PubMed |
Forsyth, DM, Caley, P, Davis, NE, Latham, ADM, Woolnough, AP, Woodford, LP, Stamation, KA, Moloney, PD, and Pascoe, C (2018). Functional responses of an apex predator and a mesopredator to an invading ungulate: dingoes, red foxes and sambar deer in south-east Australia. Austral Ecology 43, 375–384.
| Functional responses of an apex predator and a mesopredator to an invading ungulate: dingoes, red foxes and sambar deer in south-east Australia.Crossref | GoogleScholarGoogle Scholar |
Forsyth, DM, Latham, ADM, Davis, NE, Caley, P, Letnic, M, Moloney, PD, Woodford, LP, and Woolnough, AP (2019). Interactions between dingoes and introduced wild ungulates: concepts, evidence and knowledge gaps. Australian Mammalogy 41, 12–26.
| Interactions between dingoes and introduced wild ungulates: concepts, evidence and knowledge gaps.Crossref | GoogleScholarGoogle Scholar |
Gaynor, KM, Hojnowski, CE, Carter, NH, and Brashares, JS (2018). The influence of human disturbance on wildlife nocturnality. Science 360, 1232–1235.
| The influence of human disturbance on wildlife nocturnality.Crossref | GoogleScholarGoogle Scholar | 29903973PubMed |
Gelman, A, and Rubin, DB (1992). Inference from iterative simulation using multiple sequences. Statistical Science 7, 457–511.
| Inference from iterative simulation using multiple sequences.Crossref | GoogleScholarGoogle Scholar |
Gormley, AM, Forsyth, DM, Griffioen, P, Lindeman, M, Ramsey, DSL, Scroggie, MP, and Woodford, L (2011). Using presence-only and presence–absence data to estimate the current and potential distributions of established invasive species. Journal of Applied Ecology 48, 25–34.
| Using presence-only and presence–absence data to estimate the current and potential distributions of established invasive species.Crossref | GoogleScholarGoogle Scholar | 21339812PubMed |
Green, MJB (1987). Ecological separation in Himalayan ungulates. Journal of Zoology 1, 693–719.
| Ecological separation in Himalayan ungulates.Crossref | GoogleScholarGoogle Scholar |
Harrel Jr FE (2020) Hmisc: Harrell miscellaneous. R package version 4.4-0. Available at https://CRAN.R-project.org/packageHmisc [verified 22 September 2021]
Harrison, M (2010) ‘Sambar the magnificent deer.’ (The Australian Deer Research Foundation Ltd: Melbourne)
Hijmans RJ (2020) Raster: Geographic data analysis and modeling. R package version 3.1-5. Available at https://CRAN.R-project.org/package=raster [verified 22 September 2021]
Ikeda, T, Takahashi, H, Igota, H, Matsuura, Y, Azumaya, M, Yoshida, T, and Kaji, K (2019). Effects of culling intensity on diel and seasonal activity patterns of sika deer (Cervus nippon). Scientific Reports 9, 17205.
| Effects of culling intensity on diel and seasonal activity patterns of sika deer (Cervus nippon).Crossref | GoogleScholarGoogle Scholar | 31748671PubMed |
Jackson SM, Groves C (2015) ‘Taxonomy of Australian Mammals.’ (CSIRO Publishing: Melbourne, Vic., Australia)
Johnsingh, AJT (1983). Large mammalian prey and predators in Bandipur India. The Journal of the Bombay Natural History Society 80, 1–57.
Kawanishi, K, and Sunquist, ME (2004). Conservation status of tigers in a primary rainforest of peninsular Malaysia. Biological Conservation 120, 329–344.
| Conservation status of tigers in a primary rainforest of peninsular Malaysia.Crossref | GoogleScholarGoogle Scholar |
Kie, JG (1999). Optimal foraging and risk of predation: Effects on behavior and social structure in ungulates. Journal of Mammalogy 80, 1114–1129.
| Optimal foraging and risk of predation: Effects on behavior and social structure in ungulates.Crossref | GoogleScholarGoogle Scholar |
Kowalski M, Kowalski M (2013) Exifpro photo browser v 2.1.0. Available at https://github.com/mikekov/ExifPro [verified 22 September 2021]
Laguna, E, Carpio, AJ, Vicente, J, Barasona, JA, Triguero-Ocaña, R, Jiménez-Ruiz, S, Gómez-Manzaneque, Á, and Acevedo, P (2021). The spatial ecology of red deer under different land use and management scenarios: protected areas, mixed farms and fenced hunting estates. Science of The Total Environment 786, 147124.
| The spatial ecology of red deer under different land use and management scenarios: protected areas, mixed farms and fenced hunting estates.Crossref | GoogleScholarGoogle Scholar | 33965822PubMed |
Laundre, JW, Hernandez, L, and Ripple, WJ (2010). The landscape of fear: Ecological implications of being afraid. The Open Ecology Journal 3, 1–7.
| The landscape of fear: Ecological implications of being afraid.Crossref | GoogleScholarGoogle Scholar |
Le Saout, S, Padié, S, Chamaillé-Jammes, S, Chollet, S, Côté, S, Morellet, N, Pattison, J, Harris, E, and Martin, J-L (2014). Short-term effects of hunting on naïve black-tailed deer (Odocoileus hemionus sitkensis): Behavioural response and consequences on vegetation growth. Canadian Journal of Zoology 92, 915–925.
| Short-term effects of hunting on naïve black-tailed deer (Odocoileus hemionus sitkensis): Behavioural response and consequences on vegetation growth.Crossref | GoogleScholarGoogle Scholar |
Linkie, M, and Ridout, MS (2011). Assessing tiger–prey interactions in Sumatran rainforests. Journal of Zoology 284, 224–229.
| Assessing tiger–prey interactions in Sumatran rainforests.Crossref | GoogleScholarGoogle Scholar |
Little, AR, Demarais, S, Gee, KL, Webb, SL, Riffell, SK, Gaskamp, JA, and Belant, JL (2014). Does human predation risk affect harvest susceptibility of white-tailed deer during hunting season? Wildlife Society Bulletin 38, 797–805.
| Does human predation risk affect harvest susceptibility of white-tailed deer during hunting season?Crossref | GoogleScholarGoogle Scholar |
Long JL (2003) ‘Introduced mammals of the world: their history, distribution and influence.’ (CSIRO Publishing: Melbourne, Victoria, Australia, and CABI Publishing: Wallingford, UK)
Luccarini, S, Mauri, L, Ciuti, S, Lamberti, P, and Apollonio, M (2006). Red deer (‘Cervus elaphus’) spatial use in the Italian alps: home range patterns, seasonal migrations, and effect of snow and winter feeding. Ethology Ecology & Evolution 18, 127–145.
| Red deer (‘Cervus elaphus’) spatial use in the Italian alps: home range patterns, seasonal migrations, and effect of snow and winter feeding.Crossref | GoogleScholarGoogle Scholar |
Matsubayashi, H, Lagan, P, Sukor, JRA, and Kitayama, K (2007). Seasonal and daily use of natural licks by sambar deer (Cervus unicolor) in a Bornean tropical rain forest. Tropics 17, 81–86.
| Seasonal and daily use of natural licks by sambar deer (Cervus unicolor) in a Bornean tropical rain forest.Crossref | GoogleScholarGoogle Scholar |
Menichetti, L, Touzot, L, Elofsson, K, Hyvönen, R, Kätterer, T, and Kjellander, P (2019). Interactions between a population of fallow deer (Dama dama), humans and crops in a managed composite temperate landscape in southern Sweden: conflict or opportunity? PLoS One 14, e0215594.
| Interactions between a population of fallow deer (Dama dama), humans and crops in a managed composite temperate landscape in southern Sweden: conflict or opportunity?Crossref | GoogleScholarGoogle Scholar | 31013322PubMed |
Moloney, PD, Gormley, AM, Toop, SD, Flesch, JS, Forsyth, DM, Ramsey, DSL, and Hampton, JO (2022). Bayesian modelling reveals differences in long-term trends in the harvest of native and introduced species by recreational hunters in Australia. Wildlife Research. , .
| Bayesian modelling reveals differences in long-term trends in the harvest of native and introduced species by recreational hunters in Australia.Crossref | GoogleScholarGoogle Scholar |
Moriarty, A (2004). The liberation, distribution, abundance and management of wild deer in Australia. Wildlife Research 31, 291–299.
| The liberation, distribution, abundance and management of wild deer in Australia.Crossref | GoogleScholarGoogle Scholar |
Pal, R, Thakur, S, Arya, S, Bhattacharya, T, and Sathyakumar, S (2020). Mammals of the Bhagirathi basin, western Himalaya: understanding distribution along spatial gradients of habitats and disturbances. Oryx 55, 657–667.
| Mammals of the Bhagirathi basin, western Himalaya: understanding distribution along spatial gradients of habitats and disturbances.Crossref | GoogleScholarGoogle Scholar |
Parsons, AW, Forrester, T, McShea, WJ, Baker-Whatton, MC, Millspaugh, JJ, and Kays, R (2017). Do occupancy or detection rates from camera traps reflect deer density? Journal of Mammalogy 98, 1547–1557.
| Do occupancy or detection rates from camera traps reflect deer density?Crossref | GoogleScholarGoogle Scholar |
Pfeiffer, MB, Iglay, RB, Seamans, TW, Blackwell, BF, and DeVault, TL (2020). Deciphering interactions between white-tailed deer and approaching vehicles. Transportation Research Part D: Transport and Environment 79, 102251.
| Deciphering interactions between white-tailed deer and approaching vehicles.Crossref | GoogleScholarGoogle Scholar |
Plummer, M, Best, N, Cowles, K, and Vines, K (2006). Coda: Convergence diagnosis and output analysis for MCMC. R News 6, 7–11.
R Core Team (2020) ‘R: a language and environment for statistical computing.’ (R Foundation for Statistical Computing: Vienna, Austria)
Ridout, MS, and Linkie, M (2009). Estimating overlap of daily activity patterns from camera trap data. Journal of Agricultural, Biological, and Environmental Statistics 14, 322–337.
| Estimating overlap of daily activity patterns from camera trap data.Crossref | GoogleScholarGoogle Scholar |
Robertson, G, Wright, J, Brown, D, Yuen, K, and Tongway, D (2019). An assessment of feral horse impacts on treeless drainage lines in the Australian Alps. Ecological Management & Restoration 20, 21–30.
| An assessment of feral horse impacts on treeless drainage lines in the Australian Alps.Crossref | GoogleScholarGoogle Scholar |
Ross, J, Hearn, AJ, Johnson, PJ, and Macdonald, DW (2013). Activity patterns and temporal avoidance by prey in response to Sunda clouded leopard predation risk. Journal of Zoology 290, 96–106.
| Activity patterns and temporal avoidance by prey in response to Sunda clouded leopard predation risk.Crossref | GoogleScholarGoogle Scholar |
Semiadi, G, Muir, PD, and Barry, TN (1994). General biology of sambar deer (Cervus unicolor) in captivity. New Zealand Journal of Agricultural Research 37, 79–85.
| General biology of sambar deer (Cervus unicolor) in captivity.Crossref | GoogleScholarGoogle Scholar |
Sih, A, Ziemba, R, and Harding, KC (2000). New insights on how temporal variation in predation risk shapes prey behavior. Trends in Ecology and Evolution 15, 3–4.
| New insights on how temporal variation in predation risk shapes prey behavior.Crossref | GoogleScholarGoogle Scholar |
Simberloff, D, Parker, IM, and Windle, PN (2005). Introduced species policy, management, and future research needs. Frontiers in Ecology and the Environment 3, 12–20.
| Introduced species policy, management, and future research needs.Crossref | GoogleScholarGoogle Scholar |
Simcharoen, A, Savini, T, Gale, G, Roche, E, Chimchome, V, and Smith, JL (2014). Ecological factors that influence sambar (Rusa unicolor) distribution and abundance in western Thailand: implications for tiger conservation. Raffles Bulletin of Zoology 62, 100–106.
Sotorra, S, Blair, D, Blanchard, W, and Lindenmayer, D (2021). Modelling the factors influencing Sambar Deer (Rusa unicolor) occurrence in the wet eucalypt forests of south-eastern Australia. Australian Zoologist 41, 241–253.
| Modelling the factors influencing Sambar Deer (Rusa unicolor) occurrence in the wet eucalypt forests of south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Stewart, KM, Bowyer, RT, Kie, JG, Cimon, NJ, and Johnson, BK (2002). Temporospatial distributions of elk, mule deer, and cattle: Resource partitioning and competitive displacement. Journal of Mammalogy 83, 229–244.
| Temporospatial distributions of elk, mule deer, and cattle: Resource partitioning and competitive displacement.Crossref | GoogleScholarGoogle Scholar |
Tolsma A (2009) An assessment of mossbeds across the Victorian Alps, 2004–2009. Report to Parks Victoria. Arthur Rylah Institute for Environmental Research, Department of Sustainability and Environment, Melbourne. Copy of the report was provided by the author on 11 February 2022.
Wardle, DA, Barker, GM, Yeates, GW, Bonner, KI, and Ghani, A (2001). Introduced browsing mammals in New Zealand natural forests: Aboveground and belowground consequences. Ecological Monographs 71, 587–614.
| Introduced browsing mammals in New Zealand natural forests: Aboveground and belowground consequences.Crossref | GoogleScholarGoogle Scholar |
Watter, K, Thomas, E, White, N, Finch, N, and Murray, PJ (2020). Reproductive seasonality and rate of increase of wild sambar deer (Rusa unicolor) in a new environment, Victoria, Australia. Animal Reproduction Science 223, 106630.
| Reproductive seasonality and rate of increase of wild sambar deer (Rusa unicolor) in a new environment, Victoria, Australia.Crossref | GoogleScholarGoogle Scholar | 33166829PubMed |
White, KS, Pendleton, GW, and Hood, E (2009). Effects of snow on sitka black-tailed deer browse availability and nutritional carrying capacity in southeastern Alaska. Journal of Wildlife Management 73, 481–487.
| Effects of snow on sitka black-tailed deer browse availability and nutritional carrying capacity in southeastern Alaska.Crossref | GoogleScholarGoogle Scholar |
Whitehead GK (1993) ‘The whitehead encyclopedia of deer.’ (Swan Hill Press: Shrewsbury, UK)
Wisdom MJ, Ager AA, Preisler HK, Cimon NJ, Johnson BK (2004) Effects of off-road recreation on mule deer and elk. In ‘Transactions of the 69th North American Wildlife and Natural Resources Conference’. pp. 531–550.
Wood, SN (2011). Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 73, 3–36.
| Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models.Crossref | GoogleScholarGoogle Scholar |
Yamada, K, Elith, J, McCarthy, M, and Zerger, A (2003). Eliciting and integrating expert knowledge for wildlife habitat modelling. Ecological Modelling 165, 251–264.
| Eliciting and integrating expert knowledge for wildlife habitat modelling.Crossref | GoogleScholarGoogle Scholar |
Yen, S-C, Wang, Y, Yu, P-H, Kuan, Y-P, Liao, Y-C, Chen, K-H, and Weng, G-J (2019). Seasonal space use and habitat selection of sambar in Taiwan. The Journal of Wildlife Management 83, 22–31.
| Seasonal space use and habitat selection of sambar in Taiwan.Crossref | GoogleScholarGoogle Scholar |
Zar JH (1996) ‘Biostatistical analysis’. 3rd edn. (Prentice Hall: New Jersey)
Zhang, J, Hull, V, Ouyang, Z, Li, R, Connor, T, Yang, H, Zhang, Z, Silet, B, Zhang, H, and Liu, J (2017). Divergent responses of sympatric species to livestock encroachment at fine spatiotemporal scales. Biological Conservation 209, 119–129.
| Divergent responses of sympatric species to livestock encroachment at fine spatiotemporal scales.Crossref | GoogleScholarGoogle Scholar |
1 Mammal taxonomy follows Jackson and Groves (2015).