It’s a trap: effective methods for monitoring house mouse populations in grain-growing regions of south-eastern Australia
Peter R. Brown A D , Steve Henry
A D , Steve Henry  A , Roger P. Pech
A , Roger P. Pech  B , Jennyffer Cruz
B , Jennyffer Cruz  B , Lyn A. Hinds
B , Lyn A. Hinds  A , Nikki Van de Weyer
A , Nikki Van de Weyer  A , Peter Caley
A , Peter Caley  C and Wendy A. Ruscoe
C and Wendy A. Ruscoe  A
A
A CSIRO Health & Biosecurity, GPO Box 1700, Canberra, ACT 2601, Australia.
B Manaaki Whenua Landcare Research, PO Box 69040, Lincoln 7640, New Zealand.
C CSIRO Data 61, GPO Box 1700, Canberra, ACT 2601, Australia.
D Corresponding author. Email: peter.brown@csiro.au
Wildlife Research 49(4) 347-359 https://doi.org/10.1071/WR21076
Submitted: 18 May 2021 Accepted: 2 October 2021 Published: 14 February 2022
Journal Compilation © CSIRO 2022 Open Access CC BY-NC-ND
Abstract
Context: Wild house mice cause substantial economic damage to grain crops in Australia, particularly during mouse plagues. Populations were monitored to detect changes in abundance, with data from surveys used in models to forecast likely mouse outbreaks. However, it is not always feasible to use live-trapping (the ‘gold standard’) for assessing mouse abundance at a large number of monitoring sites spread across south-eastern Australia. A range of alternative methods was tried to assist the grains industry with strategic decisions to reduce crop damage.
Aims: The aim of this work was to determine which survey methods could provide useful and effective indexes of mouse abundance across a large area.
Methods: Monitoring of mouse populations was conducted at representative grain farms by using (1) live-trapping at long-term ‘benchmark’ sites (n = 2), and (2) mouse chew cards and active burrow counts at ‘rapid-assessment’ sites (n = 44 farms across 5 regions). Monitoring was conducted for 22 monitoring sessions over 7.5 years through low, medium and high mouse abundance conditions.
Key results: Live-trapping provided the most useful, but most resource-intensive, information. There were strong relationships between the index of mouse abundance from live-trapping with mouse chew cards and active burrow counts at a local (explaining 63% and 71% of variation respectively) and regional (explaining 71% and 81% of variation respectively) scales. The same quantitative relationship held between the mouse chew cards and trapping regardless of season and year. However, the relationship between active burrow counts and trapping was best in winter and autumn seasons. There was a strong relationship between mouse abundance from live-trapping and active burrows across 1 ha grids (R2 = 0.88). We determined there were 1.3 ± 0.2 (mean ± s.e.) mice per active burrow.
Conclusions: Live-trapping supplemented with data from chew cards and active burrows remains sufficient to monitor a wide range of sites to show regional trends.
Implications: It is likely that live-trapping will need to be used for the foreseeable future to provide useful parameters such as breeding condition and population abundance that are required for the forecast models. Supplementary monitoring at rapid-assessment sites (using chew cards in all seasons and active burrow counts particularly in autumn and winter), that can be collected easily without the need for animal handling, will provide additional indications of region-specific changes in mouse abundance and activity.
Keywords: active burrow counts, mouse chew cards, mouse plague, Mus musculus, population abundance, survey, trapping.
Introduction
Worldwide, pest rodent species can cause significant damage to crops (Singleton et al. 1999, 2010; Stenseth et al. 2003; John 2014; Buckle and Smith 2015; Swanepoel et al. 2017). Many studies use trapping or other indexes to monitor rodent populations, with some data being used to assess potential damage to crops or to assess disease risk (e.g. Stenseth et al. 2003; Whisson et al. 2005; Singleton et al. 2010; Jones et al. 2017; Swanepoel et al. 2017; Rahelinirina et al. 2021; Wang et al. 2021). Good estimates of pest rodent population size relative to damage thresholds are necessary to guide management decisions, and determine the success of control or management operations (Brown et al. 2007; Kaboodvandpour and Leung 2012). In many cases, it is not known whether a simple index of ‘activity’ is enough, or whether robust and reliable measures of abundance or density are needed (through capture–mark–recapture approaches). Given the widespread, often spatially variable occurrence of pest species and the patchy nature of damage (Mulungu et al. 2005; Jones et al. 2017), it is not possible to rely on single-point assessments of pest occurrence to make decisions about likely management or control actions over wide areas, when changes in pest abundance are not synchronous. It takes significant time and resources to reliably monitor rodent populations in different areas in different seasons and be confident about changes in rodent population abundance. It is typically not feasible to undertake trapping studies everywhere.
Wild house mice (Mus musculus) in Australia periodically undergo outbreaks, or ‘mouse plagues’, and cause substantial damage to grain crops (Mutze 1989; Stenseth et al. 2003; Singleton et al. 2005; Brown et al. 2007). Mouse populations can be highly variable, with densities ranging from <50 mice ha−1 (often ~5 mice ha−1) during low phases, to >1000 mice ha−1 during mouse plagues (Singleton et al. 2001, 2005), a 200-fold change (Korpimäki et al. 2004). The mouse plague that affected Victoria and South Australia in 1993/94 was conservatively estimated to cost A$64.5 million (Caughley et al. 1994), and the estimated annual cost is ~A$20 million (McLeod 2004). There has been no update to these figures despite the significant economic, social and environmental impacts of mouse plagues, and present costs are likely to be significantly higher. Mouse plagues can be small and localised or can occur over large areas (Mutze 1989) and in different regions at different times (Singleton et al. 2005). There are few good estimates of the spatial scale of mouse plagues, but one proxy of extent is the area that has been baited with rodenticides. Most of the management of these outbreaks was by reactive management through application of rodenticides (Brown 2007). In South Australia and Victoria during the 1993/94 mouse plague, 350 000 ha were baited with strychnine (Mutze 1998). In Queensland in 1995, 250 000 ha were baited with strychnine (Fisher 1996) and in New South Wales in 1999, 500 000 ha were baited with zinc phosphide (Singleton et al. 2007). There is no reliable estimate of the area baited after the mouse plague that affected large parts of South Australia, Victoria and New South Wales in 2010/11. The current work is aimed at monitoring mouse populations across five states so as to identify potential areas where mouse populations could cause damage to crops, and to provide advance warning to farmers and the grains industry so that management can be proactive. Trapping conducted at long-term (benchmark) sites is used to run forecast models (Pech et al. 1999; Kenney et al. 2003) to predict the likelihood of damage to grain crops.
Much of the previous work on wild house mice used capture–mark–recapture techniques to estimate population size (e.g. Singleton 1987, 1989). Pitfall traps (Singleton 1987) and Ugglan multiple-capture traps (Jacob et al. 2002) have been tested but were not considered as effective as Longworth live-capture traps. Trapping (using Longworth or Elliott live-capture traps) is the ‘gold standard’ because capture–mark–recapture techniques can be employed. However, monitoring with live-trapping is time consuming, resource intensive, and requires specific training and equipment. Various measures of trap success, adjusted trap success (ATS), minimum number of animals known alive (MNKA) or Petersen density estimates have been used previously for estimating house mouse abundance in Australia (e.g. Singleton 1987, 1989; Twigg et al. 1991; Singleton and Chambers 1996; Brown et al. 1997; Ruscoe et al. 2022). Pocock et al. (2004) cautioned against the use of MNKA because of bias. Conn et al. (2006) explored other approaches to improve population estimates from trapping, and Davis et al. (2003) found that Chao’s modified moment estimator was robust when populations had high levels of heterogeneity and low levels of capture probability, but was unreliable for field populations when trapped for <5 days. Population estimation of wild mice is also hampered by low trappability (Krebs et al. 1994). Alternative monitoring using rapid-assessment techniques such as mouse chew cards and active burrow counts are potentially easier, less resource intensive and require less training, thereby enabling a greater number of sites and areas to be covered. Can these alternative methods provide adequate resolution for management purposes? Furthermore, can observations from one location be applied to a broader region, such as, for example, similar grain farms 10, 100 or 1000 km away? Mouse chew cards have been used by several researchers (Mutze 1998; Brown et al. 2004; Kaboodvandpour et al. 2010), but only one example was found where active burrow counts were used (Mutze 1998). No systematic evaluations or comparisons of these indexes have been made with trapping indexes of abundance.
To cover a broader range of locations, we have implemented alternative approaches to monitor mouse populations by using mouse chew cards and active burrow counts. By using these approaches, it is possible to cover hundreds of sites at a reasonable effort. The aim of this study was to determine how useful a range of monitoring techniques are for assessing changes in the population abundance of mice in agricultural landscapes. In particular, how do indexes of mouse abundance from chew cards and active burrows compare to live-trapping? How much variation is there in mouse abundance/activity across local and regional scales (through low, medium or high population abundance) or across seasons? Furthermore, what is the relationship between density of mice and number of active burrows, i.e. can we estimate the number of mice per burrow?
Materials and methods
Study sites
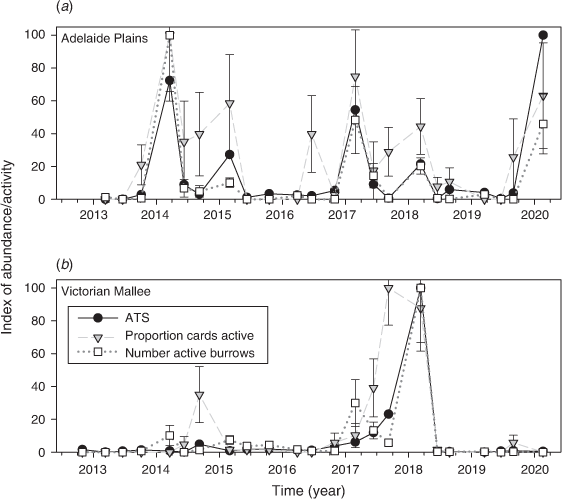
The following two main agricultural regions were used for this study: the Central Mallee of north-western Victoria and the North Adelaide Plains of south-eastern South Australia (Fig. 1). Some additional data were collected from other nearby locations, namely, Yorke Peninsula in South Australia, the Wimmera in Victoria and Coleambally in New South Wales (Fig. 1). All these regions have a Mediterranean climate, with hot summers and predominantly winter rainfall. There were two levels of data collection, including (1) ‘benchmark sites’ with live-trapping, mouse chew cards and active burrow counts (Figs 2, 3a; at these sites mouse populations have been monitored using live-capture traps since the early 1980s; Singleton et al. 2005), and (2) rapid-assessment sites, where mouse chew card and active burrow count data were collected (Fig. 3b). There were two benchmark sites and five regions with sets of rapid-assessment sites, located ~10 km apart along a ~100 km transect (Fig. 1).
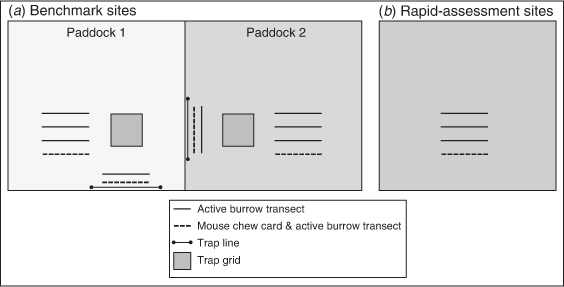
|
The benchmark site at Walpeup, Central Mallee, Victoria (35°06′S, 142°01′E, ~100 m asl), is at a typical 2000 ha grain farm. Mean annual rainfall in the district is ~340 mm (81 years from 1939, annual CV = 32%, April–October growing season mean = 212 mm, CV = 34%, from the Bureau of Meteorology (BOM) Station 076064 at the Walpeup Research Station). The benchmark site at Mallala, North Adelaide Plains, South Australia (34°22′S, 138°35′E, ~70 m asl), is a typical 3000 ha grain farm. Mean annual rainfall in the district is ~440 mm (130 years from 1887, annual CV = 23%, April–October growing season mean = 334 mm, CV = 26%, from the BOM Station 023021 at Roseworthy, ~20 km from Mallala). The topography is flat to mildly undulating, and the soil type is predominantly sandy loams. Growers generally implement a 3-year crop rotation that consists of a winter cereal (wheat, barley or oats) in rotation with canola, peas or other legumes. Minimum tillage practices are predominantly used by growers in these regions. Summer weeds are normally controlled by chemicals rather than by ploughing, resulting in little soil disturbance except at sowing, normally in March, April or May (autumn). Crops are harvested from late October through to December (early summer) and paddocks are left fallow, with retained stubble, until sowing the following year. Additional rapid-assessment monitoring (mouse chew cards and active burrow counts) was conducted on similar sites at Coleambally (n = 4), the Wimmera (n = 11) and Yorke Peninsula (n = 13; Fig. 1). The sites at Coleambally are irrigated if water is available (from the Coleambally Irrigation Area; see Brown et al. 2004 for details).
Monitoring commenced in October 2012 and continued until March 2020. Monitoring on benchmark and rapid-assessment sites was conducted three times each year (22 sampling sessions over 7.5 years), and coincided with key seasons in mouse population dynamics and crop growth. These were (1) April (autumn; end of breeding, peak population phase, and just before sowing of winter crops), (2) June (winter; over-winter mouse survival and likely damage to growing crops) and (3) September (spring; low point of population cycle, commencement of breeding, and likely damage to maturing crops). Mouse populations were not monitored in summer because there were no crops grown at that time.
Live-trapping
Mouse populations were monitored with Longworth live-capture traps (Longworth Scientific, Abingdon, UK; Fig. 2a) at benchmark sites only. Two trapping grids and two trap lines were set at each site and were permanently marked (Fig. 3). Traps were placed 50 m from the edge of a paddock, in a 6 × 6 grid at 10-m intervals (except at Mallala, where an 8 × 8 grid was used from March 2019 for five consecutive nights). A line of 15 traps, at 10-m intervals, was placed along a fenceline adjacent to the grids; undisturbed fencelines provide important habitat for mice in winter (Singleton 1989). Traps were set in grids in a cereal crop and another type of crop (canola or pea/legume, depending on what the farmers chose to grow each year). At benchmark sites, traps were set for three consecutive nights and baited with wheat, giving 306 trap-nights per site per trap session (except at Mallala from March 2019, where there were with 640 trap-nights per site per trap session).
For simplicity, at each benchmark site all mouse captures from the grids and fencelines were combined to provide a single measure of adjusted trap success (ATS), which was derived for each monitoring session. ATS accounts for occupied traps, which is especially important when mouse abundance is high. A frequency–density transformation (Caughley 1977) was applied to the raw trap success, and thus the adjusted trap success can exceed 100, as follows:
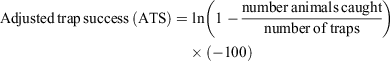
Mouse density per hectare, derived using Petersen estimates from marked individuals, was strongly associated with adjusted trap success (R2 > 0.85), so we use ATS for our analyses. Because animals were not individually identified, it was not possible to estimate population size from more sophisticated models.
Mouse chew cards
Mouse chew cards were set out to estimate mouse activity or abundance by determining the amount of card eaten by mice. Mouse chew cards were 10 × 10 cm standard photocopy paper printed with a 1 cm grid (Fig. 2b). The cards were soaked in canola oil (~95%) with some linseed oil (~5%) to enhance attractiveness to mice. Ten cards were set in a row, spaced every 10 m along a 90 m transect through the crop (Fig. 3). Transects were positioned ~50 m from the edge of the crop and generally ran along the furrow lines of the crop. Start and end points of transects were marked with GPS so that the same transects were monitored in each session. The cards were left overnight, then assessed the following morning for chewing by mice. Two indexes of mouse abundance/activity can be derived from the chew cards: (1) of the total number of cards set, the proportion of cards with any sign of chewing (proportion cards active), and (2) the percentage of individual cards eaten (% cards chewed; i.e. the average per cent of each card chewed of all cards set and retrieved at the site, including zeros). Chewing by mice was unambiguous (Fig. 2b). Some chewing by snails or slugs did occur, but this had a characteristic thinning pattern along the edge of the cards and could be discounted. Some cards (<1%) were disturbed by birds (e.g. Australian ravens, Corvus coronoides) or European foxes (Vulpes vulpes), which was also obvious because of a torn edge, beak or tooth marks, footprints and scent marks (these cards were excluded from analyses).
Active burrow counts
Between one (first year of study) and four (remainder of the study) 100 m long × 1 m wide transects were set at each rapid-assessment site (Fig. 3). Transects were ~20 m apart. Start and end points of transects were marked with GPS so that the same transects were monitored in each session. All potentially active burrows were dusted with corn flour (which does not affect the behaviour of mice). The following morning, active burrows were clearly obvious with disturbed flour and mouse footprints (Fig. 2c). The total number of active burrows scaled to four transects was used in analyses (i.e. number active burrows per 400 m2).
Intensive burrow monitoring
An opportunistic study at the benchmark site at Mallala, North Adelaide Plains, was conducted to estimate the number of mice per active burrow by intensively monitoring active burrows and mouse abundance over four sessions from November 2019 to March 2020 (three sessions in summer and one in autumn). Four 100 × 100 m grids (1 ha) were established, with two grids on each of two typical grain farms (separated by 2 km). All potentially active burrows within each grid were marked as described above and checked for activity the following morning. Live-trapping on 8 × 8 trapping grids (traps placed at 10 m intervals) occurred at the same time as the active burrow counts. Trapping was conducted for five consecutive nights. ATS was calculated as above in addition to Petersen estimates to calculate a density of mice ha−1. Linear regression (using log-transformed data) was used to determine the number of mice per active burrow. Because of the short duration of this trial, we were unable to assess any seasonal differences.
Analyses
All data were log-transformed before analysis to meet the assumptions required for statistical inference. To account for the real zeros in the data, a constant was added to all measures before transforming; ATS + 1, proportion of cards active + 0.1, % cards chewed + 0.1, number active burrows per 400 m2 + 0.1. Linear regressions were performed to explore the relationships among the different indexes of mouse abundance and activity by using R (R Core Team 2020). The additional effects that season and year had on the relationships were assessed by comparing model Akaike information criterion (AIC) values. The proportion of variance explained from regression (R2) was used to determine goodness of fit. The relationship between % cards chewed and proportion cards active was used to determine which of these chew card indexes was more sensitive to changes in mouse activity, with data used from all rapid-assessment sites (Yorke Peninsula, Adelaide Plains, Victorian Mallee, Wimmera and Coleambally). We determined the relationship between ATS and number active burrows, and between ATS and the selected index for chew cards, at (1) the local scale, i.e. the benchmark sites only where all indexes were gathered from the same paddocks, and (2) regional scale where ATS from each benchmark site was compared with data from chew cards and active burrows from the region within which the benchmark site was located (Victorian Mallee and Adelaide Plains). We modelled ATS as a linear function of the index of interest with additive effects of region, season and year. For simplicity, when visualising different types of data (ATS, chew cards and active burrows), we converted all data to a relative index. The index was calculated by deriving the maximum value for each type of data across all monitoring sessions for each region, then dividing each data point by the maximum and multiplying by 100 (thus, the index ranges from 0 to 100). Mean values are presented ± 1 standard error (s.e.).
Results
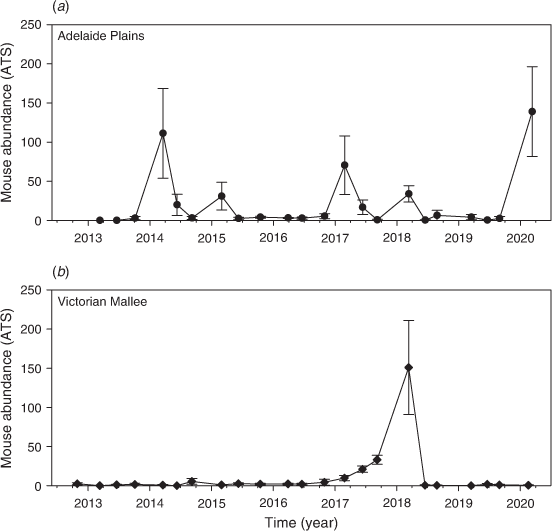
Changes in mouse abundance and activity
Mouse populations were generally low throughout the entire study (Fig. 4), but there were some seasonal fluctuations at Mallala in autumn every year (considered ‘moderate’) and one instance of ‘high’ mouse abundance in May 2018 at Walpeup. Overall, 969 mice were captured from 6610 trap-nights at Mallala (15.9 ATS), and 476 mice were captured from 6688 trap-nights at Walpeup (7.4 ATS).
|
Chew card indices comparison
There was a positive relationship between the proportion cards active and % cards chewed (R2 = 0.58); however, it appeared that the proportion cards active was a more sensitive index (Fig. 5a) because it extended over a greater range at low levels of activity. The relationship was improved when both indexes were log-transformed (R2 = 0.91; Fig. 5b). Additionally, when using data from rapid-assessment sites (Adelaide Plains and Victorian Mallee combined), we found that the proportion cards active (Fig. 5c) explained more variation in ATS than did % cards eaten (R2 = 0.69 vs 0.42; Fig. 5d); therefore, the proportion cards active was used hereon.
Local scale (all data from benchmark sites only)
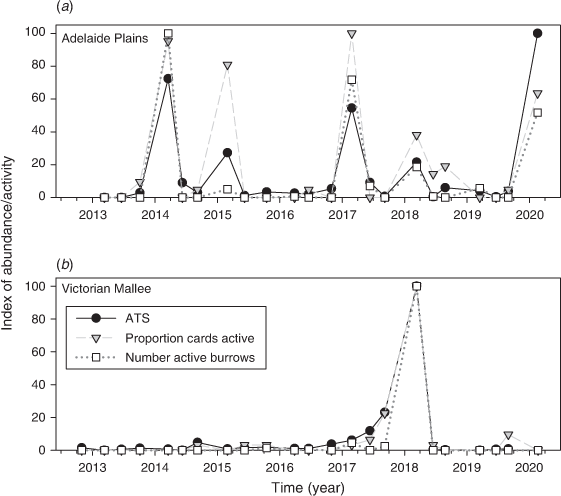
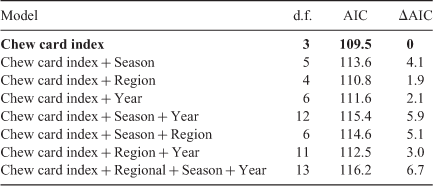
Using the benchmark sites where the chew card and burrow data were collected within 100 m of the trapping grid, we found that the proportion cards active was positively associated with ATS (Fig. 6). Adding region, season and year did not improve the model fit (on the basis of ΔAIC), indicating that the same quantitative relationship held between the proportion cards active and ATS regardless of region, season and year (Table 1). The proportion cards active alone explained 63% of the variation in ATS.
|
|
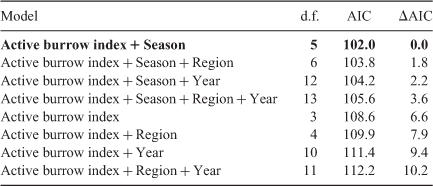
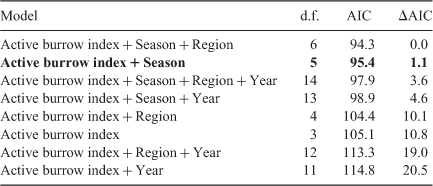
The number burrows active was positively associated with ATS. Adding season vastly improved the model fit (ΔAIC = 6.6) and adding year and region had a marginal effect (ΔAIC = ~2.0; Table 2). The model that included season showed that the relationship between ATS and number active burrows was similar between autumn and winter but that the relationship was different for spring (Appendix 1). The number active burrows and season explained 71% of the variation in ATS.
|
Regional scale
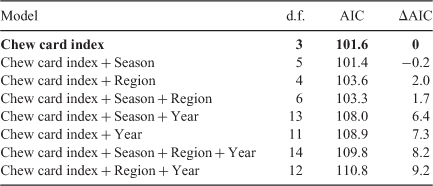
For the Victorian Mallee and the Adelaide Plains, we compared benchmark trapping site data with rapid-assessment data collected along the associated regional transect. We found that the proportion cards active recorded along the 100 km transect was positively associated with the ATS from within the region (Fig. 7). Adding region, season and year did not improve the model fit (Table 3). The proportion cards active explained 71% of the variation in ATS within the region.
|
|
The number active burrows was also positively associated with ATS. Adding season vastly improved the model fit (ΔAIC > 10), whereas adding year and region did not improve the model (ΔAIC < < 2.0; Table 4). The number active burrows and season explained 75% of the variation in ATS within regions and this increased to 85% if winter and autumn data only were used.
|
Intensive burrow monitoring
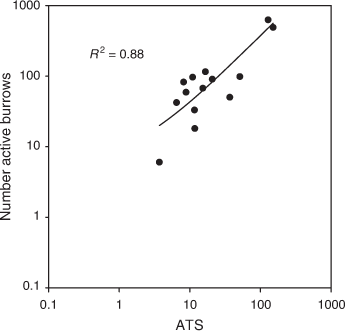
Using the data from all sampling periods, there was a strong positive relationship between ATS and number active burrows from the 1 ha grids in the Adelaide Plains, during a period of low, moderate and high mouse densities (Fig. 8; R2 = 0.88; F1,13 = 86.49; P < 0.001). There were two data points where mouse densities were very high; however, the strong positive relationship held true across all densities. Using Petersen estimates, we calculated that there were ~1.3 ± 0.2 (mean ± s.e.) mice per active burrow.
|
Discussion
Live-trapping has been used at our benchmark sites since the early 1980s (Singleton et al. 2005) and remains our ‘gold-standard’ for localised monitoring of mouse populations. In cereal cropping systems in south-eastern Australia, trapping data (ATS) are used to determine changes in mouse abundance and, together with rainfall, are a key input in population forecast models (Pech et al. 1999; Kenney et al. 2003). Moreover, live-trapping provides opportunities to sample tissues for population genetic studies and also assess breeding status and the overall condition of mice, which are important when interpreting forecast predictions. As stated earlier, live-trapping is labour intensive and requires highly trained staff and appropriate resources, so only a small number of locations (our benchmark sites) can be realistically assessed in detail. It is important to conduct additional monitoring at multiple sites using rapid-assessment techniques such as mouse chew cards and active burrow counts. Results suggest that the rapid-assessment techniques are as good at the regional scale as they are at the local scale and confirm that these rapid-assessment techniques are appropriate for regional-scale monitoring. These findings would be relevant for other outbreaking rodent species where regional-scale monitoring is required (e.g. through Southeast Asia: Brown et al. 2006; Jacob et al. 2010; Jones et al. 2017; Asia: Wang et al. 2021; Europe: Jacob et al. 2014; and Africa Mulungu et al. 2010). We observed some differences in mouse population dynamics between grain farms in two regions, namely, the Adelaide Plains and the Victorian Mallee (~350 km apart). These areas have been considered similar agro-ecological regions (Mallee/Murray and Central North South Australia; Williams et al. 2002), but, historically, mouse abundance and frequency of outbreaks have been different (Singleton et al. 2005). It is therefore important to maintain rapid-assessment monitoring within each region. Consideration should be given to establishment of benchmark sites in other areas also. A new benchmark site has recently been established near Parkes in the Central West of New South Wales, which is representative of the farming system through much of New South Wales (including fodder production and grazing components) with a higher and summer-dominant rainfall distribution (annual mean ~608 mm, 37 years from 1942, annual CV = 32%, April–October growing season mean = 322, CV = 38%, from the BOM Station 65068 at Parkes Airport). On the central Darling Downs of Queensland, some long-term monitoring sites have been monitored since 1974 by using snap traps to detect changes in mouse population abundance (Pople et al. 2013). A similar analysis should be undertaken to investigate the relationship of snap traps with live traps, chew cards and active burrows counts for these Darling Downs sites; however, this was outside the scope of the current analysis.
It is still worth considering other applications of capture–mark–recapture methods to further improve our mouse population abundance estimates. Many approaches and analytical methods require individual identification, such as, for example, using Program MARK (e.g. Kaboodvandpour et al. 2010). We used ear punches to mark animals so that Petersen estimates could be determined. However, there were very few animals that remained resident on our trapping grids over the 3- or 6-month intervals between monitoring, so individual identification would add value only within a trapping session. There is also a need to account for heterogeneity in detection probabilities (Conn et al. 2006), which might be improved only with trapping for a longer period of time (Davis et al. 2003). However, this increases the effort required for estimating population abundance, to determine mouse damage risks to crops, and as input into forecast models. Kaboodvandpour et al. (2010) found that the number of individual animals caught performed better as an index than did trap success, but that study was conducted only as sorghum crops were maturing, and thus the utility for year-round monitoring is unknown.
Mouse populations are highly variable in abundance (Mutze 1989; Stenseth et al. 2003; Singleton et al. 2005; Brown et al. 2007). Our data showed several periods when mouse abundance was ‘moderate’ on the Adelaide Plains and one season of ‘high’ abundance in the Victorian Mallee. Of the 22 monitoring sessions, most were ‘low’, with many observations containing zeros. These zero-inflated data reflect the boom–bust nature of mouse outbreaks. More research is required to link levels of mouse activity to levels of damage so that management thresholds can be established to trigger control actions. Several studies have already established thresholds (Brown et al. 2007; Kaboodvandpour and Leung 2012), but these need to be extended to include alternative monitoring approaches such as mouse chew cards or active burrow counts in different seasons. It is a requirement (label condition) when farmers apply broadacre zinc phosphide baits, that there is an indication of crop damage by mice. Such an approach to identify threshold values for population monitoring or population indices would be relevant for many rodent pest species (as indicated above).
Mouse chew cards provide an easy-to-use indication of mouse ‘activity’ (Mutze 1998; Whisson et al. 2005), as do tracking tunnels used in New Zealand (Ruscoe et al. 2001). We chose to use proportion cards active as a measure of mouse activity rather than % cards chewed because the former was more sensitive to change when activity levels were low, was consistent across seasons and thus may provide key signals of potential damage for farmers in the early stages of mouse plagues. However, as is true for estimates based on active burrow counts, there is no technique that can discern between one mouse’s activity and the activity of several mice when examining an active chew card. There remains a question about how useful chew cards are when plentiful alternative food is available; however, because mice are neophilic (Singleton and Krebs 2007), it may not matter too much how much alternative food is available.
Active burrow counts were useful particularly in autumn, which is the period immediately before sowing crops, and winter. There are potential problems with detecting mouse burrows when plant biomass is high (e.g. crop growing or near harvest (spring), or when high levels of straw are retained post-harvest); so, this technique may not be particularly useful across different seasons. The number of active burrows before sowing could be an important indicator of potential damage at sowing (mice dig up the seeds and eat them), traditionally the time when most economic damage occurs in these wheat-based farming systems (Brown and Singleton 2002; Singleton et al. 2005; Brown et al. 2007). Thus, burrow activity may provide a trigger for management intervention by farmers (predominantly application of zinc phosphide baits, Mutze and Sinclair 2004; Brown et al. 2010).
There remain questions about how many mice are using these burrows and how the burrows survive over time, but we calculated ~1.3 mice per active burrow. Burrow use is likely to be different during breeding and non-breeding periods because of parental care of young (Mutze 1998). Furthermore, mice are likely to use multiple burrows, and one burrow is likely to be used by more than one mouse (Sutherland and Singleton 2003). More research is needed to determine the number of mice inhabiting burrows and whether active burrow counts should be conducted on more than single-visit assessments.
There are advantages and disadvantages of different monitoring methods (Table 5). There are several other types of information that can add value to the existing approaches based on benchmark and rapid-assessment sites. These could include observations made by farmers collated from phone surveys or via smart phone apps such as MouseAlert (https://www.feralscan.org.au/mousealert/), automated monitoring of social media traffic, plus using camera traps, automated passive infrared sensor devices and drone-mounted infrared cameras. There are advantages and disadvantages of these too, although none has been rigorously tested to determine how suitable they might be. Data from Twitter for mentions of mice over a 2-year period were examined, and a positive relationship between mouse abundance and the number of tweets was found for one year, but not the other (Marijke Walvaert and Peter Caley, unpubl. data). It appears likely that a combination of different techniques will be required for the foreseeable future. Given the highly variable spatial and temporal abundance of mice, recommendations for farmers to do their own monitoring before they implement management strategies remain relevant.

|
Until new technologies are developed, data from trapping at a small number of benchmark sites (using ATS), broad-scale monitoring using rapid-assessment techniques (such as chew cards and active burrow counts) and anecdotal reports from farmers and agricultural advisers will be the basis for advice to Australian grain growers about trends in mouse abundance and the likelihood of impending and/or ongoing damage to crops.
Conclusions
Our research confirmed there were strong relationships among several indexes of mouse abundance, and that a range of monitoring techniques can detect changes in population abundance of mice in agricultural landscapes. Adjusted trap success (ATS) was strongly related to chew card data (at a local and regional scale for all seasons) and active burrow counts (at a local and regional scale, but only in autumn/winter). So as to track changes in mouse populations, it will be necessary to maintain trapping at benchmark sites and additional monitoring at rapid-assessment sites (using chew cards in all seasons and active burrow counts particularly in autumn) to provide an indication of changes in mouse abundance at regional scales.
Author contributions
PRB, SH and RPP conceived and designed the project. PRB wrote the manuscript and NVdW and WAR assisted with statistical analyses. All authors read, revised and approved the manuscript.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
PRB, LAH and PC are Associate Editors for Wildlife Research. Despite this relationship, they did not at any stage have editor-level access to this manuscript while in peer review, as is the standard practice when handling manuscripts submitted by an editor of this journal. The authors have no further conflicts of interest to declare.
Declaration of funding
This work was supported by the Grains Research and Development Corporation (GRDC) through projects IAC00002 and CSP1806-017RTX and supported by CSIRO Agriculture and Food and the Invasive Animal Cooperative Research Centre.
Acknowledgements
We sincerely thank farmers for allowing us access to their farms. We thank Bridget Armstrong, Elliott Luck, Freya Robinson, Cody Williams for providing assistance with field work. We thank Ken Young and Leigh Nelson (GRDC) for ongoing support. Andrea Byrom provided significant support to this project from the early phases. All experiments and procedures were approved by the CSIRO Wildlife, Livestock and Laboratory Animal – Animal Ethics Committee, and conform to the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes (Approval No. 2018-33). We thank Kevin Oh, Freya Robinson, and two anonymous reviewers for comments that improved the manuscript.
References
Brown, P. R. (2007). Reducing the impact of feral house mice in agricultural ecosystems. In ‘Pest or Guest: the Zoology of Overabundance’. (Eds D. Lunney, P. Eby, P. Hutchings, and S. Burgin.) pp. 8–15. (Royal Zoological Society of NSW: Sydney, NSW, Australia.)Brown, P. R., and Singleton, G. R. (2002). Impacts of house mice on crops in Australia: costs and damage. In ‘Human Conflicts with Wildlife: Economic Considerations’. (Eds L. Clark, J. Hone, J. A. Shivik, R. A. Watkins, K. C. VerCauteren, and J. K. Yoder.) pp. 48–58. (National Wildlife Research Center: Fort Collins, CO, USA.)
Brown, P. R., Singleton, G. R., Kearns, B., and Griffiths, J. (1997). Evaluation and cost-effectiveness of strychnine for control of populations of wild house mice (Mus domesticus) in Victoria. Wildlife Research 24, 159–172.
| Evaluation and cost-effectiveness of strychnine for control of populations of wild house mice (Mus domesticus) in Victoria.Crossref | GoogleScholarGoogle Scholar |
Brown, P. R., Davies, M. J., Croft, J. D., and Singleton, G. R. (2004). Can farm management practices reduce the impact of house mouse populations on crops in an irrigated farming system? Wildlife Research 31, 597–604.
| Can farm management practices reduce the impact of house mouse populations on crops in an irrigated farming system?Crossref | GoogleScholarGoogle Scholar |
Brown, P. R., Tuan, N. P., Singleton, G. R., Ha, P. T. T., Hoa, P. T., Hue, D. T., Tan, T. Q., Tuat, N. V., Jacob, J., and Muller, W. J. (2006). Ecologically-based management of rodents in the real world: application to a mixed agro-ecosystem in Vietnam. Ecological Applications 16, 2000–2010.
| Ecologically-based management of rodents in the real world: application to a mixed agro-ecosystem in Vietnam.Crossref | GoogleScholarGoogle Scholar | 17069390PubMed |
Brown, P. R., Huth, N. I., Banks, P. B., and Singleton, G. R. (2007). Relationship between abundance of rodents and damage to agricultural crops. Agriculture, Ecosystems & Environment 120, 405–415.
| Relationship between abundance of rodents and damage to agricultural crops.Crossref | GoogleScholarGoogle Scholar |
Brown, P. R., Singleton, G. R., Pech, R. P., Hinds, L. A., and Krebs, C. J. (2010). Rodent outbreaks in Australia: mouse plagues in cereal crops. In ‘Rodent Outbreaks: Ecology and Impacts’. (Eds G. R. Singleton, S. R. Belmain, P. R. Brown, and B. Hardy.) pp. 225–238. (International Rice Research Institute: Los Baños, Philippines.)
Buckle, A. P., and Smith, R. H. (2015). ‘Rodent Pests and their Control.’ 2nd edn. (CAB International: Oxford, UK.)
Caughley, G. (1977). ‘Analysis of Vertebrate Populations.’ (John Wiley and Sons: London, UK.)
Caughley, J., Monamy, V., and Heiden, K. (1994). ‘Impact of the 1993 Mouse Plague.’ (GRDC: Canberra, ACT, Australia)
Conn, P. B., Arthur, A. D., Bailey, L. L., and Singleton, G. R. (2006). Estimating the abundance of mouse populations of known size: promises and pitfalls of new methods. Ecological Applications 16, 829–837.
| Estimating the abundance of mouse populations of known size: promises and pitfalls of new methods.Crossref | GoogleScholarGoogle Scholar | 16711066PubMed |
Davis, S. A., Akison, L. K., Farroway, L. N., Singleton, G. R., and Leslie, K. E. (2003). Abundance estimators and truth: accounting for individual heterogeneity in wild house mice. The Journal of Wildlife Management 67, 634–645.
| Abundance estimators and truth: accounting for individual heterogeneity in wild house mice.Crossref | GoogleScholarGoogle Scholar |
Fisher, G. (1996). Overall coordination. In ‘Report on aerial baiting with strychnine during the 1995 mouse plague in the Dalby–Goondiwindi area, Queensland’. (Ed. V. Eldershaw.) pp. 14–21. (Department of Natural Resources: Qld, Australia.)
Jacob, J., Sudarmaji, , Singleton, G. R., Rahmini, , Herawati, N. A., and Brown, P. R. (2010). Ecologically based management of rodents in lowland irrigated rice fields in Indonesia. Wildlife Research 37, 418–427.
| Ecologically based management of rodents in lowland irrigated rice fields in Indonesia.Crossref | GoogleScholarGoogle Scholar |
Jacob, J., Ylönen, H., and Hodkinson, C. G. (2002). The trappability of house mice with Ugglan traps and Longworth traps. Wildlife Research 29, 101–103.
| The trappability of house mice with Ugglan traps and Longworth traps.Crossref | GoogleScholarGoogle Scholar |
Jacob, J., Manson, P., Barfknecht, R., and Fredricks, T. (2014). Common vole (Microtus arvalis) ecology and management: implications for risk assessment of plant protection products. Pest Management Science 70, 869–878.
| Common vole (Microtus arvalis) ecology and management: implications for risk assessment of plant protection products.Crossref | GoogleScholarGoogle Scholar | 24293354PubMed |
John, A. (2014). Rodent outbreaks and rice pre-harvest losses in Southeast Asia. Food Security 6, 249–260.
| Rodent outbreaks and rice pre-harvest losses in Southeast Asia.Crossref | GoogleScholarGoogle Scholar |
Jones, C. R., Lorica, R. P., Villegas, J. M., Ramal, A. F., Horgan, F. G., Singleton, G. R., and Stuart, A. M. (2017). The stadium effect: rodent damage patterns in rice fields explored using giving-up densities. Integrative Zoology 12, 438–445.
| The stadium effect: rodent damage patterns in rice fields explored using giving-up densities.Crossref | GoogleScholarGoogle Scholar | 27992109PubMed |
Kaboodvandpour, S., and Leung, L. K.-P. (2012). Modelling density thresholds for managing mouse damage to maturing wheat. Crop Protection 42, 134–140.
| Modelling density thresholds for managing mouse damage to maturing wheat.Crossref | GoogleScholarGoogle Scholar |
Kaboodvandpour, S., Free, C., and Leung, L. K. P. (2010). Comparison of population estimators and indices for monitoring house mice in sorghum crops. Integrative Zoology 5, 53–62.
| Comparison of population estimators and indices for monitoring house mice in sorghum crops.Crossref | GoogleScholarGoogle Scholar | 21392322PubMed |
Kenney, A. J., Krebs, C. J., Davis, S. A., Pech, R. P., Mutze, G. J., and Singleton, G. R. (2003). Predicting house mouse outbreaks in the wheat-growing areas of south-eastern Australia. In ‘Rats, Mice and People: Rodent Biology and Management’. (Eds G. R. Singleton, L. A. Hinds, C. J. Krebs, and D. M. Spratt.) pp. 325–328. ACIAR Monograph 96. (ACIAR: Canberra, ACT, Australia.)
Korpimäki, E., Brown, P. R., Jacob, J., and Pech, R. P. (2004). The puzzles of population cycles and outbreaks of small mammals solved? Bioscience 54, 1071–1079.
| The puzzles of population cycles and outbreaks of small mammals solved?Crossref | GoogleScholarGoogle Scholar |
Krebs, C. J., Singleton, G. R., and Kenney, A. J. (1994). Six reasons why feral house mouse populations might have low recapture rates. Wildlife Research 21, 559–567.
| Six reasons why feral house mouse populations might have low recapture rates.Crossref | GoogleScholarGoogle Scholar |
McLeod, R. (2004). ‘Counting the Cost: Impact of Invasive Animals in Australia 2004.’ (Cooperative Research Centre for Pest Animal Control: Canberra, ACT, Australia.)
Mulungu, L. S., Makundi, R. H., Massawe, A. W., Machang’u, R. S., Ngowo, V., and Leirs, H. (2005). Spatial patterns and distribution of damage in maize fields due to Mastomys natalensis in Tanzania. Belgian Journal of Zoology 135, 183–185.
Mulungu, L. S., Ngowo, V. D., Makundi, R. H., Massawe, A. W., and Leirs, H. (2010). Winning the fight against rodent pests: recent developments in Tanzania. The Journal of Biological Sciences 10, 333–340.
| Winning the fight against rodent pests: recent developments in Tanzania.Crossref | GoogleScholarGoogle Scholar |
Mutze, G. J. (1989). Mouse plagues in South Australian cereal-growing areas. I. Occurrence and distribution of plagues. Australian Wildlife Research 16, 677–683.
| Mouse plagues in South Australian cereal-growing areas. I. Occurrence and distribution of plagues.Crossref | GoogleScholarGoogle Scholar |
Mutze, G. J. (1998). The 1993 strychnine baiting program for mouse control in South Australian grain crops. I. Efficacy. Wildlife Research 25, 533–546.
| The 1993 strychnine baiting program for mouse control in South Australian grain crops. I. Efficacy.Crossref | GoogleScholarGoogle Scholar |
Mutze, G. J., and Sinclair, R. (2004). Efficacy of zinc phosphide, strychnine and chlorpyrifos as rodenticides for the control of house mice in South Australian cereal crops. Wildlife Research 31, 249–257.
| Efficacy of zinc phosphide, strychnine and chlorpyrifos as rodenticides for the control of house mice in South Australian cereal crops.Crossref | GoogleScholarGoogle Scholar |
Pech, R. P., Hood, G., Singleton, G. R., Salmon, E., Forrester, R., and Brown, P. R. (1999). Models for predicting plagues of house mice (Mus domesticus) in Australia. In ‘Ecologically-based Management of Rodent Pests’. (Eds G. R. Singleton, L. A. Hinds, H. Leirs, and Z. Zhang.) pp. 81–112. ACIAR Monograph No. 59. (Australian Centre for International Agricultural Research: Canberra, ACT, Australia.)
Pocock, M. J. O., Frantz, A. C., Cowan, D. P., White, P. C. L., and Searle, J. B. (2004). Tapering bias inherent in minimum number alive (MNA) population indices. Journal of Mammalogy 85, 959–962.
| Tapering bias inherent in minimum number alive (MNA) population indices.Crossref | GoogleScholarGoogle Scholar |
Pople, A., Scanlan, J. C., Cremasco, P., and Farrell, J. (2013). Population dynamics of house mice in Queensland grain-growing areas. Wildlife Research 40, 661–674.
| Population dynamics of house mice in Queensland grain-growing areas.Crossref | GoogleScholarGoogle Scholar |
R Core Team (2020). ‘R: a Language and Environment for Statistical Computing.’ (R Foundation for Statistical Computing: Vienna, Austria.) Available at https://www.R-project.org/.
Rahelinirina, S., Scobie, K., Ramasindrazana, B., Andrianaivoarimanana, V., Rasoamalala, F., Randriantseheno, L. N., Rakotoniaina, J. S., Gorge, O., Lambin, X., Valade, E., Telfer, S., and Rajerison, M. (2021). Rodent control to fight plague: field assessment of methods based on rat density reduction. Integrative Zoology 16, 868–885.
| Rodent control to fight plague: field assessment of methods based on rat density reduction.Crossref | GoogleScholarGoogle Scholar | 33694282PubMed |
Ruscoe, W. A., Goldsmith, R., and Choquenot, D. (2001). A comparison of population estimates and the abundance indices for house mice inhabiting beech forests in New Zealand. Wildlife Research 28, 173–178.
| A comparison of population estimates and the abundance indices for house mice inhabiting beech forests in New Zealand.Crossref | GoogleScholarGoogle Scholar |
Ruscoe, W. A., Brown, P. R., Henry, S., van de Weyer, N., Robinson, F., Hinds, L. A., and Singleton, G. R. (2022). Conservation agriculture practices have changed habitat use by rodent pests: implications for management of feral house mice. Journal of Pest Science 95, 493–503.
| Conservation agriculture practices have changed habitat use by rodent pests: implications for management of feral house mice.Crossref | GoogleScholarGoogle Scholar |
Singleton, G. R. (1987). A comparison of the effectiveness of pitfall and Longworth live-trapping techniques in population studies of house mice. Acta Theriologica 32, 245–259.
| A comparison of the effectiveness of pitfall and Longworth live-trapping techniques in population studies of house mice.Crossref | GoogleScholarGoogle Scholar |
Singleton, G. R. (1989). Population dynamics of an outbreak of house mice (Mus domesticus) in the mallee wheatlands of Australia: hypothesis of plague formation. Journal of Zoology 219, 495–515.
| Population dynamics of an outbreak of house mice (Mus domesticus) in the mallee wheatlands of Australia: hypothesis of plague formation.Crossref | GoogleScholarGoogle Scholar |
Singleton, G. R., and Chambers, L. K. (1996). A manipulative field experiment to examine the effect of Capillaria hepatica (Nematoda) on wild mouse populations in southern Australia. International Journal for Parasitology 26, 383–398.
| A manipulative field experiment to examine the effect of Capillaria hepatica (Nematoda) on wild mouse populations in southern Australia.Crossref | GoogleScholarGoogle Scholar | 8773526PubMed |
Singleton, G. R., and Krebs, C. J. (2007). The secret world of wild mice. In ‘The Mouse in Biomedical Research’. 2nd edn. (Eds J. G. Fox, M. T. Davisson, F. W. Quimby, S. W. Barthold, C. E. Newcomer, and A. L. Smith) pp. 25–51. (Academic Press: Burlington, MA, USA.)
Singleton, G. R., Hinds, L. A., Leirs, H., and Zhang, Z. (Eds) (1999). ‘Ecologically-based management of rodent pests.’ ACIAR Monograph No. 59. (Australian Centre for International Agricultural Research: Canberra, ACT, Australia.)
Singleton, G. R., Krebs, C. J., Davis, S. A., Chambers, L. K., and Brown, P. R. (2001). Reproductive changes in fluctuating house mouse populations in southeastern Australia. Proceedings. Biological Sciences 268, 1741–1748.
| Reproductive changes in fluctuating house mouse populations in southeastern Australia.Crossref | GoogleScholarGoogle Scholar |
Singleton, G. R., Brown, P. R., Pech, R. P., Jacob, J., Mutze, G. J., and Krebs, C. J. (2005). One hundred years of eruptions of house mice in Australia: a natural biological curio. Biological Journal of the Linnean Society. Linnean Society of London 84, 617–627.
| One hundred years of eruptions of house mice in Australia: a natural biological curio.Crossref | GoogleScholarGoogle Scholar |
Singleton, G. R., Brown, P. R., Jacob, J., Aplin, K. P., and Sudarmaji, (2007). Unwanted and unintended effects of culling: a case for ecologically-based rodent management. Integrative Zoology 2, 247–259.
| Unwanted and unintended effects of culling: a case for ecologically-based rodent management.Crossref | GoogleScholarGoogle Scholar | 21396042PubMed |
Singleton, G. R., Belmain, S. R., Brown, P. R., Aplin, K. P., and Htwe, N. M. (2010). Impacts of rodent outbreaks on food security in Asia. Wildlife Research 37, 355–359.
| Impacts of rodent outbreaks on food security in Asia.Crossref | GoogleScholarGoogle Scholar |
Stenseth, N. C., Leirs, H., Skonhoft, A., Davis, S. A., Pech, R. P., Andreassen, H. P., Singleton, G. R., Lima, M., Machangu, R. M., Makundi, R. H., Zhang, Z., Brown, P. R., Shi, D., and Wan, X. (2003). Mice, rats and people: the bio-economics of agricultural rodent pests. Frontiers in Ecology and the Environment 1, 367–375.
| Mice, rats and people: the bio-economics of agricultural rodent pests.Crossref | GoogleScholarGoogle Scholar |
Sutherland, D. R., and Singleton, G. R. (2003). Monitoring activity patterns and social interactions of small mammals with an automated event-recording system: wild house mice (Mus domesticus) as a case study. In ‘Rats, Mice and People: Rodent Biology and Management’. (Eds G. R. Singleton, L. A. Hinds, C. J. Krebs, and D. M. Spratt.) pp. 159–164. ACIAR Monograph 96. (ACIAR: Canberra, ACT, Australia.)
Swanepoel, L. H., Swanepoel, C. M., Brown, P. R., Eiseb, S. J., Goodman, S. M., Keith, M., Kirsten, F., Leirs, H., Mahlaba, T. A. M., Makundi, R. H., Melabane, P., von Maltitz, E. F., Massawe, A. W., Monadjem, A., Mulungu, L. S., Singleton, G. R., Taylor, P. J., Soarimalala, V., and Belmain, S. R. (2017). A systematic review of rodent pest research in Afro-Malagasy small-holder farming systems: are we asking the right questions? PLoS One 12, e0174554.
| A systematic review of rodent pest research in Afro-Malagasy small-holder farming systems: are we asking the right questions?Crossref | GoogleScholarGoogle Scholar | 28358899PubMed |
Twigg, L. E., Singleton, G. R., and Kay, B. J. (1991). Evaluation of bromadiolone against house mice (Mus domesticus) populations in irrigated soybean crops. I. Efficacy of control. Wildlife Research 18, 265–274.
| Evaluation of bromadiolone against house mice (Mus domesticus) populations in irrigated soybean crops. I. Efficacy of control.Crossref | GoogleScholarGoogle Scholar |
Wang, D., Anderson, D. P., Li, K., Guo, Y., Yang, Z., and Pech, R. P. (2021). Predicted population dynamics of an indigenous rodent, Apodemus agrarius, in an agricultural system. Crop Protection 147, 105683.
| Predicted population dynamics of an indigenous rodent, Apodemus agrarius, in an agricultural system.Crossref | GoogleScholarGoogle Scholar |
Whisson, D. A., Engeman, R. M., and Collins, K. (2005). Developing relative abundance techniques (RATs) for monitoring rodent populations. Wildlife Research 32, 239–244.
| Developing relative abundance techniques (RATs) for monitoring rodent populations.Crossref | GoogleScholarGoogle Scholar |
Williams, J., Hook, R. A., and Hamblin, A. (2002) ‘Agro-Ecological Regions of Australia: Methodologies for their Derivation and Key Issues in Resource Management.’ (CSIRO Land and Water: Canberra, ACT, Australia.)
Appendix 1. Summary statistics for comparative analysis of ATS by using number active burrows and season (autumn, winter, spring)

|





