Olfactory lures in predator control do not increase predation risk to birds in areas of conservation concern
Page E. Klug A B D , Amy A. Yackel Adams
A B D , Amy A. Yackel Adams  A and Robert N. Reed
A and Robert N. Reed  A C
A C
A U.S. Geological Survey, Fort Collins Science Center, 2150 Centre Avenue, Building C, Fort Collins, CO 80526, USA.
B Present address: U.S. Department of Agriculture, Animal and Plant Health Inspection Service, Wildlife Services, National Wildlife Research Center, North Dakota State University, Department of Biological Sciences, 1340 Bolley Drive, Fargo, ND 58102, USA.
C Present address: U.S. Geological Survey, Pacific Island Ecosystems Research Center, PO Box 44, Building 344, Hawai’i Volcanoes National Park, HI 96718, USA.
D Corresponding author. Email: page.e.klug@usda.gov
Wildlife Research 49(2) 183-192 https://doi.org/10.1071/WR21022
Submitted: 26 January 2021 Accepted: 13 July 2021 Published: 4 November 2021
Journal Compilation © CSIRO 2022 Open Access CC BY
Abstract
Context: Lethal control of predators is often undertaken to protect species of conservation concern. Traps are frequently baited to increase capture efficacy, but baited traps can potentially increase predation risk by attracting predators to protected areas. This is especially important if targeted predators can escape capture due to low trap success. Snake traps using live mouse lures may be beneficial if traps effectively remove snakes in the presence of birds and do not attract additional snakes to the area.
Aims: The present study evaluated whether mouse-lure traps in areas occupied by birds (simulated by deploying bird-lure traps) could influence predation risk from an invasive snake on Guam.
Methods: Snake traps were used, with Japanese quail (Coturnix japonica) as a proxy for predation risk, to assess if an adjacent trap with a mouse (Mus musculus) would attract brown treesnakes (Boiga irregularis) to a focal area and increase contact between an invasive snake and avian prey. Catch per unit effort (CPUE) at stations containing either a bird-lure trap, mouse-lure trap or pair of traps (i.e. one bird-lure and one mouse-lure trap) was evaluated.
Key results: Bird-lure traps paired with mouse-lure traps did not differ in CPUE from isolated bird-lure traps. At paired stations, CPUE of snakes in mouse-lure traps was 2.3× higher than bird-lure traps, suggesting mouse lures were capable of drawing snakes away from avian prey. Bird-lure traps at paired stations experienced a decay in captures over time, whereas CPUE for isolated bird-lure traps increased after 9 weeks and exceeded mouse-lure traps after 7 weeks.
Conclusions: Mouse lures did not increase the risk of snakes being captured in bird-lure traps. Instead, mouse-lure traps may have locally suppressed snakes, whereas stations without mouse-lure traps still had snakes in the focal area, putting avian prey at greater risk. However, snakes caught with bird lures tended to be larger and in better body condition, suggesting preference for avian prey over mammalian prey in larger snakes.
Implications: Strategic placement of olfactory traps within areas of conservation concern may be beneficial for protecting birds of conservation concern from an invasive snake predator.
Keywords: avian conservation, bait preference, chemoreception, islands, introduced species, invasive reptile, predator control, trap attraction.
Introduction
Lethal control of predators to protect prey of conservation concern or for game management has a long history (Imler 1945; Beamesderfer et al. 1996; Reynolds and Tapper 1996), with the control of invasive predators of particular importance in maintaining native species and ecosystems (Wilcove et al. 1998; Lowe et al. 2004). In response to their dramatic effect on native prey populations, numerous control programs exist to remove introduced predators (Salo et al. 2007). The removal of invasive predators often focuses on mammals (Courchamp et al. 2003; Hunter et al. 2018; Starling-Windhof et al. 2011), but introduced bird, reptile, amphibian and fish species have also been the targets of control programs (Govindarajulu et al. 2005; Morris et al. 2011; Reed et al. 2011; Klug et al. 2015b; Klug et al. 2019; Klug et al. 2021).
Although a variety of taxa benefit from predator control, birds are often the intended beneficiaries (Dowding and Murphy 2001). Predator control tactics range from localised control at bird breeding sites to landscape-scale eradication where possible (Veitch and Clout 2002). In many instances the lethal removal of invasive predators has successfully protected imperilled bird species by enhancing hatching success, fledgling success and breeding populations (Côté and Sutherland 1997; Smith et al. 2010). The use of lures to lethally trap predators is common and has resulted in increased avian reproductive success (O’Donnell et al. 1996; Whitehead et al. 2008), but also holds the potential to increase predation risk. Odour cues can travel considerable distances both in water and in the air (Atema 1985; Cardé and Willis 2008), allowing predators to respond to scent from long distances compared with visual cues (Savarie and Clark 2006). For example, lures may attract predators to protected areas and increase predation risk to local prey if control tools are not effective (Côté and Sutherland 1997).
Most predator control studies evaluate the impact of lethal removal on imperilled prey at the population level but do not evaluate if the presence of a baited trap increases predation risk to individual birds. Increased risk may occur by drawing predators into habitat occupied by the prey, or through a temporary increase in the abundance of foraging predators. This possible risk becomes important when dealing with prey species that are clustered or only found in a few geographically restricted locations, which is often the case for small, isolated populations of endangered species. Small populations face an inherent risk of extinction due to chance environmental or demographic events (Pimm et al. 1988; Rosenzweig and Clark 1994), and individual predators may have an outsized impact. Thus the effective control of invasive predators that target these geographically limited populations is important for recovery or reintroduction of imperilled prey (Yackel Adams et al. 2019). This is especially true for situations where a few individuals are recalcitrant to trapping, which could result in detrimental effects on vulnerable prey species (Klug et al. 2021).
Olfaction is used by a diverse range of predators to detect prey and conspecifics (Conover 2007), and is thus an important attraction stimulus in the control of invasive predators. Sex pheromones have been evaluated as trap lures for brown treesnakes (Boiga irregularis; BTS) but show limited usefulness in the field – therefore prey odours may be a more productive approach (Parker et al. 2018). Control techniques capitalising on the foraging behaviour of invasive brown treesnakes are used to suppress snake populations on Guam and prevent spread to outlying islands (Engeman and Vice 2001). For example, trapping using a variety of lures (Rodda et al. 1999; Rodda et al. 2007; Tyrrell et al. 2009) and poisoning through toxic bait (Savarie et al. 2001; Siers et al. 2020) have been optimised by focusing on the chemosensory and visual foraging strategies employed by BTS (Shivik and Clark 1997; Shivik et al. 2000). Control devices using a live or dead mouse (Mus musculus) lure are the most common for capturing BTS. Traps using live mice as attractants (hereafter ‘mouse-lure traps’) can remove most large BTS (≥950 mm snout-to-vent length; SVL) given sufficient spatial and temporal trapping effort (Tyrrell et al. 2009), and dead neonatal mice affixed with a toxicant (acetaminophen) can remove a somewhat greater size range of snakes (≥843 mm) after the ontogenetic shift to endothermic prey (Shivik and Clark 1999; Lardner et al. 2013). In particular, the protection of native bird species on Guam has included the use of mouse lures to protect nesting trees for Mariana crows (Åga; Corvus kubaryi; Aguon et al. 2002), nest boxes occupied by Micronesian starlings (Såli, Aplonis opaca; J. Savidge and T. Seibert, Colorado State University, pers. comm., 2021; Pollock et al. 2019), and caves occupied by Mariana swiftlets (Yǻyaguak; Aerodramus bartschi; Klug et al. 2021). Although extensive testing of lures has shown live mice to be highly effective (Rodda et al. 1999), limited research has been conducted on the potential drawbacks of using mouse odour in areas important to native endangered birds (Yackel Adams et al. 2019).
The goal of the present study was to evaluate whether deploying mouse-lure traps for BTS would decrease or inadvertently increase predation risk when deployed near birds. Given the endangered status of birds on Guam (U.S. Fish and Wildlife Service 1984), we used Japanese quail (Coturnix japonica) as a proxy for predation risk to assess the influence of mouse-lure traps in increasing contact between BTS and birds. We evaluated the number and catch-per-unit-effort (CPUE) of BTS in bird-lure traps and mouse-lure traps when isolated and paired to answer the following questions.
-
Does the presence of mouse lure increase snake capture in bird-lure traps?
-
Are mouse-lure traps effective at removing snakes when bird lure is present?
-
Does mouse lure draw in more snakes than bird lure or do multiple lures draw in more snakes?
If mouse-lure traps are effective at removing BTS in the presence of birds and do not draw more snakes into an area, mouse-lure traps in protected areas may benefit avian conservation. Prey preferences in snakes can be influenced by past feeding experience and by ambient prey odour (Burghardt 1993). Thus, it is possible that BTS found near avifauna, on an otherwise avian-depauperate island, may focus on birds after their ontogenetic shift to endothermic prey, and scent from mouse-lure traps may not effectively attract these snakes. Conversely, ambient odour from inaccessible prey may decrease the preference for that prey (Burghardt 1993). In some scenarios on Guam, birds are less accessible than frogs, geckos, skinks and rodents (including mice in traps), suggesting mouse-lure traps may be effective (e.g. in caves with swiftlets nesting on ceilings). In the case of avian reintroductions in forest habitats, birds will be more accessible than locally abundant herpetofauna (or mice in traps). Given the small bird populations on Guam, BTS may be focused on rodent odour rather than novel avian odours, rendering the traps effective for BTS naïve to avian prey if novel prey is not preferred. Thus, we hypothesised that mouse-lure traps could be an effective predator control method if placed in locations easily accessible to snakes before they encounter birds for the first time, and may be effective on snakes familiar with birds if the accessibility of mouse-lure traps results in a switch to alternative prey (i.e. mice in traps). If BTS are not successfully trapped upon first encounter with mouse-lure traps, snakes may become refractory and shift their focus to the free-ranging birds, requiring an alternative form of predator control (Klug et al. 2021). The present study contributes to the research needed to optimise the deployment of olfactory control devices.
Materials and methods
Study design
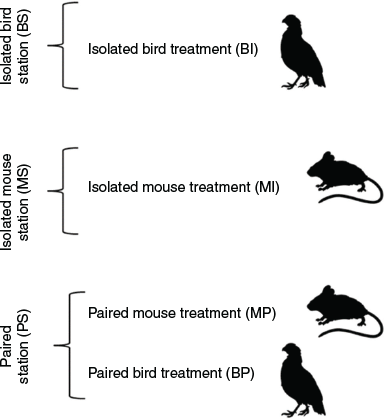
We set up 30 trap stations consisting of three combinations: isolated bird-lure traps (BI); isolated mouse-lure traps (MI); and paired stations (PS). At the paired stations, we included two traps: one bird-lure trap (BP) and one mouse-lure trap (MP); (Fig. 1). All stations were at least 60 m apart and each of the station treatments was replicated 10 times. Traps at paired stations ranged from 1.7 to 6.7 m apart (mean 3.22 ± 0.46), depending on availability of vegetation from which to suspend traps. Both mouse-lure traps and bird-lure traps were adapted from standard modified commercial minnow traps composed of 6-mm galvanised steel mesh, although the bird-lure trap was slightly longer (Rodda et al. 1999; Yackel Adams et al. 2019; Supplementary Material). The traps were operational for 67 trap nights to get a time trend as refuse odour accumulated in and under the trap, and to evaluate trap efficacy as the snake population was suppressed. Traps were checked every 48 to 72 h for a total of 29 trap checks. No fatalities occurred among birds used as bait. A few mice died during the trial and were replaced on the same day as the trap check. Dead mice have been shown to be as effective as live mice as bait (Shivik and Clark 1997; Shivik and Clark 1999), thus no reduction in trap nights occurred with a dead mouse. Trap stations were located on Guam National Wildlife Refuge outside the BTS exclosure fence (Yackel Adams et al. 2019). We used three areas outside of the barrier fence with balanced station treatments at each site. The habitat was mainly homogenous limestone forest except for a section we characterised as disturbed limestone forest dominated by coconut trees (Cocos nucifera L.).
|
Research was approved by the U.S. Geological Survey, Fort Collins Science Center, Institutional Animal Care and Use Committee (FORT IACUC #2013-13). Research permission was provided by U.S. Fish and Wildlife Service (#2013-I-0242) and Guam Division of Aquatic and Wildlife Resources.
BTS characteristics
All trapped snakes were removed from the population per request of refuge personnel and to simulate realistic snake interdiction. We recorded morphological measurements including mass (g) with a Pesola spring scale, SVL and total length (mm) by stretching the snake along a tape measure. We determined sex using snake probes. We calculated body condition index (CI) by dividing the actual mass by expected mass, with expected mass based on a regression of log mass to log SVL using a power equation (y = 0.000475x4.733045) of all snakes captured in the present study. We humanely killed snakes after recording morphological measurements.
Statistical analyses
We calculated the number of trapped snakes weekly for each trap and station. Overall and weekly CPUE for each trap and station were calculated by dividing the number of snakes caught by number of trap nights. We analysed differences among trap types in CPUE and number of snakes using repeated-measures analysis of variance (ANOVA), with time as the repeated measure (t = 10 weeks; 22 July to 27 September 2013). One-way analysis of variance and Tukey’s post hoc tests were used to examine overall CPUE and number of snakes for both trap types and station treatments. To assess the effect of mouse lures on snake capture rates in bird-lure traps, we compared CPUE and number of snakes caught in isolated bird-lure traps with bird-lure traps at paired stations (Objective 1: BI versus BP). We also compared CPUE and number of snakes caught in bird-lure traps with mouse-lure traps at paired stations to understand if mouse-lure traps were effective at removing BTS when paired with bird lure (Objective 2: BP versus MP). In separate ANOVA and Tukey’s post hoc tests, we compared the number and CPUE of snakes caught at paired stations (PS (MP + BP)) with isolated bird-lure stations and isolated mouse-lure stations to understand if mouse lures drew snakes into a focal area (Objective 3: PS versus BS and MS stations). Preliminary analyses revealed that SVL did not differ significantly between sexes (t248 = 1.97, P = 0.12), so we pooled males and females in analyses of CPUE. To investigate possible prey preferences, we used Kruskal–Wallis (KW) and Conover-Inman post hoc tests to evaluate if snake characteristics varied by station type (i.e. BS, MS, PS), trap treatment (i.e. BI, MI, BP, MP), or lure type (i.e. bird, mouse). We conducted a Fisher’s exact test to decipher if sex ratios varied by lure type at paired stations (i.e. BP, MP) or overall lure type (i.e. bird, mouse). Statistical tests were performed in SYSTAT 13 (Systat Software, Chicago, IL, USA). We show means with standard errors where applicable.
Results
CPUE and number of BTS
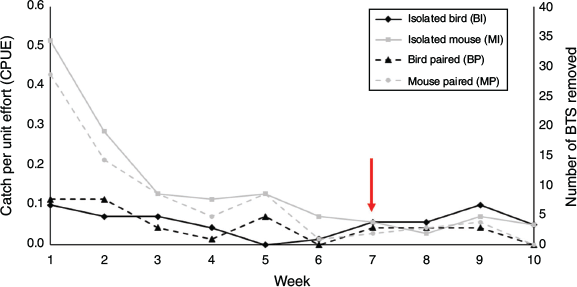
CPUE and number of snakes caught varied among trap types and weeks, with a significant interaction (Table 1, Fig. 2). Mouse-lure traps (at both isolated and paired stations) started out with higher CPUE than bird-lure traps (at both isolated and paired stations), but eventually converged after 7 weeks with isolated bird-lure traps surpassing all other trap-treatment combinations (BI > BP, MP, MI; Fig. 2).
|
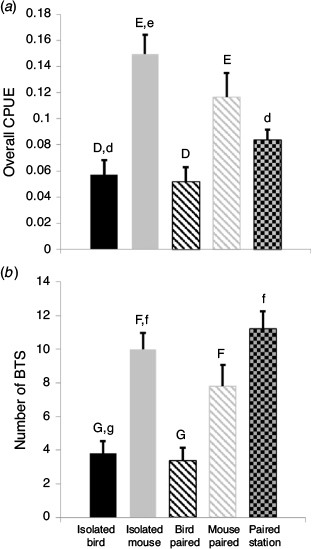
The four trap treatments differed in overall CPUE (ANOVA, F3, 36 = 11.05, P < 0.001) and total number of snakes caught (ANOVA, F3, 36 = 11.34, P < 0.001). Overall, CPUE in bird-lure traps (isolated = 0.0572 ± 0.0110; paired = 0.0515 ± 0.0112) was significantly less than mouse-lure traps (isolated = 0.1497 ± 0.0145; paired = 0.1164 ± 0.0187; Fig. 3a). The average number of snakes in bird-lure traps (isolated = 3.8 ± 0.73; paired = 3.4 ± 0.73) was significantly lower than in mouse-lure traps (isolated = 10.0 ± 0.98; paired = 7.8 ± 1.25; Fig. 3b).
|
At the station level, CPUE (ANOVA, F2, 27 = 17.02, P < 0.001) was higher at isolated mouse-lure stations than either isolated bird-lure stations (MS > BS, Tukey’s post hoc test, P < 0.001) or paired stations with both lure types (MS > PS, Tukey’s post hoc test, P = 0.001; Fig. 3). The number of snakes differed (ANOVA, F2, 27 = 18.11, P < 0.001) with isolated mouse-lure stations similar to paired stations (MS = PS, Tukey’s post hoc test, P = 0.64), but greater than isolated bird-lure stations (MS > BS, Tukey’s post hoc test, P < 0.001; Fig. 3).
BTS characteristics
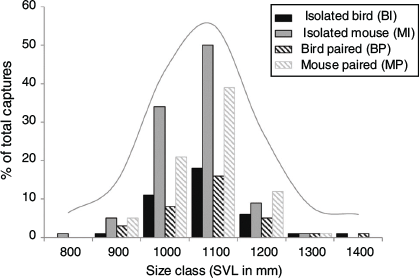
The sex ratio for bird-lure traps was not significantly different than for mouse-lure traps (Fisher’s exact test: P = 0.49), nor was the sex ratio different when comparing bird-lure (BP) and mouse-lure (MP) traps at paired stations (Fisher’s exact test: P = 1.0). Mouse-lure traps caught the smallest and bird-lure traps caught the largest snakes, but size distributions did not differ (Table 2, Fig. 4). The three station treatments (BS, MS, PS) did not differ in SVL (Kruskal–Wallis test, H = 1.36, P = 0.51), mass (H = 1.85, P = 0.40), or body condition (H = 3.97, P = 0.14). The four trap treatments (BI, MI, BP, MP) did not differ significantly in SVL (Kruskal–Wallis test, H = 2.65, P = 0.45) or mass (H = 4.67, P = 0.20; Fig. 5). Body condition was significantly different by treatment (H = 7.92, P = 0.05). Snakes caught in mouse-lure traps at paired stations had significantly lower body condition than those caught with bird-lure traps at paired stations (MP < BP, P = 0.05). Snakes caught in mouse-lure traps at paired stations had significantly lower body condition than those caught at isolated bird-lure traps (MP < BI, P = 0.01; Table 2, Fig. 5). Snakes caught with bird lure had significantly greater body condition (Kruskal–Wallis test, H = 5.39, P = 0.02) and mass (H = 4.55, P = 0.03), but did not differ in SVL (H = 1.65, P = 0.20) when compared with snakes caught with mouse lure.
|

|
Discussion
Deploying traps with mouse lures in areas occupied by birds does not appear to increase predation risk and may alleviate risk to birds by suppressing the snake population. Whether considering CPUE or the total number of snakes caught in bird-lure traps at isolated stations (BI, CPUE = 0.0572 ± 0.0110; n = 38) versus bird-lure traps at paired stations (BP, CPUE = 0.0515 ± 0.0112; n = 34), our results suggest that presence of mouse lures did not increase or decrease the risk of BTS contacting birds (i.e. snakes caught in bird-lure traps; Fig. 3). Bird-lure traps at isolated stations had the same CPUE at 1 and 9 weeks, indicating snake suppression did not occur at isolated bird-lure stations, but that suppression occurred at stations containing mouse-lure traps where CPUE significantly decreased over time (Table 1, Fig. 2). The weekly CPUE for bird-lure traps paired with mouse-lure traps remained low over time and did not consistently surpass mouse-lure traps at isolated or paired stations in the same manner as isolated bird-lure traps (Fig. 2). This indicates that mouse-lure traps were efficient in suppressing BTS populations in the presence of birds. In an operational scenario, BTS would not be removed upon contact with avian prey, only with capture in mouse-lure traps. If we had not removed snakes from bird-lure traps, we assume the CPUE would remain stable at the isolated bird-lure stations around the initial high of 0.100 and become consistently higher than the CPUE at stations containing mouse-lure traps in a manner more pronounced than the current overtake after 7 weeks (Fig. 2). The bird-lure traps may have caught BTS refractory to mouse-lure traps at the paired stations (Yackel Adams et al. 2019). We did not release snakes caught in bird-lure traps, and therefore do not know if these snakes would have eventually been captured by mouse-lure traps.
Mouse-lure traps in conservation areas will not increase risk to birds if the traps have a limited extent of effectiveness and BTS are not being drawn in by rodent odour. Regarding attraction radius, Klug et al. (2015a) found that capture of BTS with mouse-lure traps peaks for previously trapped snakes within 12 m and declines rapidly after 20 m. However, natural movements and dispersal of BTS may cause snakes from longer distances to occasionally be caught (Tobin et al. 1999). It does not appear that extra prey activity and odour attracted more snakes, given the total number of BTS caught at paired stations (n = 112) was similar to isolated mouse-lure stations (n = 100; Table 1, Fig. 3). The number of snakes caught in isolated bird-lure traps was higher than the number caught in isolated mouse-lure traps after 7 weeks (Fig. 2). This suggests bird-lure traps were less efficient than mouse-lure traps at suppressing BTS (i.e. same number of snakes removed but it takes longer at stations without mouse-lure traps; Fig. 4).
Yackel Adams et al. (2019) found that BTS entering bird-lure traps tended to be larger and in better body condition. Although marginally significant in our study, BTS with increased body condition and size may have been more attracted to birds in an environment that naturally had abundant small lizards along with less common small mammals. We used Japanese quail (mass = 150–180 g) and laboratory mice (mass = 20–40 g) as lures, indicating that smaller mouse lures were effective in the presence of much larger bird lures. If BTS visually inspected live lures, they may have preferentially selected prey items in relation to their body size, although BTS are known to attempt to consume prey too large for their gape size. BTS use a combination of visual and chemical prey cues in foraging (Chiszar et al. 1988; Smith et al. 1988). Other olfactory predators are first drawn in by scent and upon approach use vision to assess the lure (Tourani et al. 2020). Because snakes swallow their prey whole, prey size may be evaluated before ingestion (Radcliffe et al. 1980; Shine et al. 1998; Glaudas et al. 2019; King 2002), with prey size shown to influence post-strike behaviour of rattlesnakes and garter snakes (Radcliffe et al. 1980). The discrepancy in size may also be due to the bird-lure traps being slightly longer than mouse-lure traps, which would give advantage to longer snakes in finding trap entrances. While the size distribution and SVL of trapped BTS did not differ, trapping the largest individuals with bird lure indicated some snakes may be refractory to a mouse lure. Those larger snakes were of a size class that could more easily access vulnerable birds in hard-to-reach locations (e.g. endangered Mariana swiftlets on cave ceilings; Klug et al. 2021).
The deployment of mouse-lure traps in focal areas appears to suppress snake populations. Indeed, prior research in a 5-ha enclosed area has shown that all snakes of trappable size can be caught using mouse-lure traps given sufficient trapping (traps spaced every 16 m) over 53 days (Tyrrell et al. 2009). However, Yackel Adams et al. (2019) suggested that trapping efforts not bounded by a snake barrier removed only 20% of the BTS that had contacted a bird lure. Although placing mouse-lure traps within protected areas may suppress resident BTS (Fig. 2), a subset of snakes will likely be (1) refractory to the traps (i.e. size bias (Rodda et al. 2007; Tyrrell et al. 2009), (2) exhibit avian prey preference (Nafus et al. 2021) or (3) have difficulty finding a trap entrance (Yackel Adams et al. 2019). If BTS are not successfully trapped upon first encounter with mouse-lure traps, snakes may become refractory to the traps and shift their focus to free-ranging birds; this suggests that visual surveys may be required to augment trapping efforts in conservation areas. The benefit of traps deployed in protected areas is the continual ability to catch resident BTS, whereas visual surveys have higher labour costs and occur in only a fraction of the time snakes are active (Klug et al. 2021).
Another important step in evaluating the value of mouse-lure traps in conservation areas is identifying whether snakes will prefer to pursue a mouse lure over nearby avian prey. At paired stations the overall CPUE and number of snakes caught in mouse-lure traps (MP, n = 78) was higher than in bird-lure traps (BP, n = 34) suggesting that mouse lures were capable of drawing snakes away from novel avian prey and that a smaller mouse lure (20–40 g) overpowered a larger bird lure (150–180 g; Fig. 3). Although bird odour does attract BTS (Fritts et al. 1989), testing has shown that traps with live mice are more effective than traps baited with live quail or soiled bedding (Rodda et al. 1999), potentially because mice are active at night when BTS are foraging. Yackel Adams et al. (2019) also found that mouse-lure traps had a higher overall CPUE than bird-lure traps on Guam when deployed together in the field.
In past studies of prey preference in juvenile BTS, repeated prey encounters did not increase snake preference for a prey item. However, snakes showed ontogenic changes in prey preference with growth (Lardner et al. 2009). This suggests that BTS accustomed to foraging for birds may be susceptible to mouse-lure traps within areas important to endangered birds. Yackel Adams et al. (2019) found that some BTS were only willing to enter a trap with a bird lure but not numerous adjacent traps with mouse lure, providing some evidence for dietary preference. Prey preferences in adult BTS were explored for repeatability and in the context of previous experience (Nafus et al. 2021), which is especially important in natural conditions with endangered, free-ranging prey (e.g. BTS found in swiftlet caves). Snakes in the present study had limited access to free-ranging birds, so it is not known if BTS familiar with avian prey will be as susceptible to mouse-lure traps; this question would require a study within areas populated by birds. Whether BTS can be drawn away from a free-ranging prey source where previous experience has proven rewarding (e.g. A. bartschi caves or A. opaca nest boxes) remains an open question (Pollock et al. 2019; Klug et al. 2021).
BTS contact rates with traps can be 15× greater than the number of successfully captured snakes (Yackel Adams et al. 2019). When predators repeatedly fail at securing prey it may cause reduced interest (Garvey et al. 2017), resulting in olfactory habituation from unrewarded prey cues (Rankin et al. 2009). Additionally, BTS may switch to prey that are easier to access than the mouse inside a trap, and evidence exists that other olfactory foragers divide attention among different prey types to maximise energy intake (Dukas and Ellner 1993; Dukas and Kamil 2001). Prey population stability in environments with diverse prey is linked to frequency-dependent predation where predators switch prey types at varying densities (Murdoch 1969), although some studies have shown that invasive predators prefer native prey and do not switch at lower prey densities (Cuthbert et al. 2018). In considering prey found naturally on the landscape, rodents will leave an odour trail on traversed substrates as opposed to bird nests and roosts that are stationary point sources (Stark et al. 2002). Although we do not know how the intersection of prey type and odour deposition influences BTS foraging behaviour, increased odour or faeces accumulation is known to attract snakes to nesting cavities (Berkunsky et al. 2011) or to roosts (Threlfall et al. 2013). Effectively reducing predation on endangered prey may require deployment of control tools to manipulate the odour landscape, in efforts to encourage BTS to focus on prey odours emanating from control devices and not free-ranging prey.
Additional research is needed to understand the response of BTS in various scenarios where control devices simulate the presence of prey on the landscape. Limited work has been done to understand the response of BTS to odour cues of unfamiliar prey. Responsiveness to novel prey, as shown in red foxes (Vulpes vulpes), is an important predictor of invasiveness (Bytheway et al. 2016) and could inform the spatial deployment of control tools where introduced predators are already established. Although prey preferences of BTS have been evaluated (Qualls and Hackman 2004; Lardner et al. 2009), the use of novel prey odours in control devices deserves further exploration in field settings. Bird odour could be used sparingly as a novel lure to attract snakes to control devices, especially larger BTS shown to be refractory to mouse lure (Yackel Adams et al. 2019). Alternatively, managers could deploy bird odour across the landscape to camouflage endangered birds through olfactory swamping or odour priming (Ruxton 2009; Price and Banks 2012, 2017; Latham et al. 2019; Norbury et al. 2020).
Additional research on the sensory ability of BTS and trap attraction radius can inform trap spacing and trap density to enhance cost effectiveness (Engeman and Linnell 2004; Klug et al. 2015a). However, excessive use of an inaccessible mouse lure could lead to predator demotivation for mouse-lure traps (Price and Banks 2012). The extent of effectiveness for lure traps can inform how olfactory stimuli are distributed in time and space (Latham et al. 2019). On Guam this could include the deployment of traps, but also the distribution of bird odours (e.g. swiftlet guano, old starling nests or artificial bird odours) to confuse BTS before avian nesting seasons (Cleland et al. 2009; Campagna et al. 2012; Wright et al. 2017). When creating artificial bird lures, it is important to consider how odour may vary with species, age, sex and season (Campagna et al. 2012; Peacor 2006). This is especially important given BTS respond to lures differently by season (Shivik et al. 2000), and little is known about how BTS may generalise avian odours. Environmental conditions along with cue strength and age may also influence the efficacy of various lures in complex field situations (Bullard et al. 1983; Wright et al. 2017; Buesching et al. 2002). We encourage future research to evaluate the spatial and temporal properties of odour for both target and non-target prey cues on foraging snakes to promote protection of native prey.
Data availability
Data analysed in the present study are available as a USGS data release (Klug and Yackel Adams 2021).
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
Funding was provided by the U.S. Navy (Commander, Navy Installation Command, NAVFAC Marianas MIPR# N61755-13-MP-001GS) and U.S. Geological Survey Invasive Species Program.
Acknowledgements
We thank B. Lardner, S. Siers, J. Savidge, P. Barnhart, M. Campbell, M. Cook, K. Donmoyer, T. Hinkle, M. Hogan, E. Holldorf, J. Kaseman, R. Lechalk, C. Robinson, M. Spencer, and M. Viernes for assistance. L. Bonewell and M. Mazurek provided project management. A special thanks to the staff at the Guam National Wildlife Refuge for allowing the study to be conducted on refuge land. K. Schneider generously provided her artwork. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
References
Aguon, C. F., Campbell, E. W., and Morton, J. M. (2002). Efficacy of electrical barriers used to protect Mariana crow nests. Wildlife Society Bulletin 30, 703–708.Atema, J. (1985). Chemoreception in the sea: adaptations of chemoreceptors and behaviour to aquatic stimulus conditions. Symposia of the Society for Experimental Biology 39, 386–423.
Beamesderfer, R. C., Ward, D. L., and Nigro, A. A. (1996). Evaluation of the biological basis for a predator control program on northern squawfish (Ptychocheilus oregonensis) in the Columbia and Snake rivers. Canadian Journal of Fisheries and Aquatic Sciences 53, 2898–2908.
| Evaluation of the biological basis for a predator control program on northern squawfish (Ptychocheilus oregonensis) in the Columbia and Snake rivers.Crossref | GoogleScholarGoogle Scholar |
Berkunsky, I., Kacoliris, F. P., Faegre, S. I., Ruggera, R. A., Carrera, J., and Aramburu, R. M. (2011). Nest predation by arboreal snakes on cavity nesting birds in dry Chaco woodlands. Ornitologia Neotropical 22, 459–464.
Buesching, C., Waterhouse, J., and Macdonald, D. (2002). Gas-chromatographic analyses of the subcaudal gland secretion of the European badger (Meles meles) Part I: chemical differences related to individual parameters. Journal of Chemical Ecology 28, 41–56.
| Gas-chromatographic analyses of the subcaudal gland secretion of the European badger (Meles meles) Part I: chemical differences related to individual parameters.Crossref | GoogleScholarGoogle Scholar | 11868678PubMed |
Bullard, R., Turkowski, F., and Kilburn, S. (1983). Responses of free-ranging coyotes to lures and their modifications. Journal of Chemical Ecology 9, 877–888.
| Responses of free-ranging coyotes to lures and their modifications.Crossref | GoogleScholarGoogle Scholar | 24407760PubMed |
Burghardt, G. M. (1993). The comparative imperative: genetics and ontogeny of chemoreceptive prey responses in natricine snakes. Brain, Behavior and Evolution 41, 138–146.
| The comparative imperative: genetics and ontogeny of chemoreceptive prey responses in natricine snakes.Crossref | GoogleScholarGoogle Scholar | 8477338PubMed |
Bytheway, J. P., Price, C. J., and Banks, P. B. (2016). Deadly intentions: naïve introduced foxes show rapid attraction to odour cues of an unfamiliar native prey. Scientific Reports 6, 30078.
| Deadly intentions: naïve introduced foxes show rapid attraction to odour cues of an unfamiliar native prey.Crossref | GoogleScholarGoogle Scholar | 27416966PubMed |
Campagna, S., Mardon, J., Celerier, A., and Bonadonna, F. (2012). Potential semiochemical molecules from birds: a practical and comprehensive compilation of the last 20 years studies. Chemical Senses 37, 3–25.
| Potential semiochemical molecules from birds: a practical and comprehensive compilation of the last 20 years studies.Crossref | GoogleScholarGoogle Scholar | 21798850PubMed |
Cardé, R. T., and Willis, M. A. (2008). Navigational strategies used by insects to find distant, wind-borne sources of odor. Journal of Chemical Ecology 34, 854–866.
| Navigational strategies used by insects to find distant, wind-borne sources of odor.Crossref | GoogleScholarGoogle Scholar | 18581182PubMed |
Chiszar, D., Kandler, K., and Smith, H. M. (1988). Stimulus control of predatory attack in the brown tree snake (Boiga irregularis) 1. Effects of visual cues arising from prey. The Snake 20, 151–155.
Cleland, T. A., Narla, V. A., and Boudadi, K. (2009). Multiple learning parameters differentially regulate olfactory generalization. Behavioral Neuroscience 123, 26–35.
| Multiple learning parameters differentially regulate olfactory generalization.Crossref | GoogleScholarGoogle Scholar | 19170427PubMed |
Conover, M. R. (2007). ‘Predator–prey Dynamics: the Role of Olfaction.’ (CRC Press: Boca Raton, FL USA.)
Côté, I. M., and Sutherland, W. J. (1997). The effectiveness of removing predators to protect bird populations. Conservation Biology 11, 395–405.
| The effectiveness of removing predators to protect bird populations.Crossref | GoogleScholarGoogle Scholar |
Courchamp, F., Chapuis, J. L., and Pascal, M. (2003). Mammal invaders on islands: impact, control and control impact. Biological Reviews 78, 347–383.
| Mammal invaders on islands: impact, control and control impact.Crossref | GoogleScholarGoogle Scholar | 14558589PubMed |
Cuthbert, R. N., Dickey, J. W., McMorrow, C., Laverty, C., and Dick, J. T. (2018). Resistance is futile: lack of predator switching and a preference for native prey predict the success of an invasive prey species. Royal Society Open Science 5, 180339.
| Resistance is futile: lack of predator switching and a preference for native prey predict the success of an invasive prey species.Crossref | GoogleScholarGoogle Scholar | 30225019PubMed |
Dowding, J. E., and Murphy, E. C. (2001). The impact of predation by introduced mammals on endemic shorebirds in New Zealand: a conservation perspective. Biological Conservation 99, 47–64.
| The impact of predation by introduced mammals on endemic shorebirds in New Zealand: a conservation perspective.Crossref | GoogleScholarGoogle Scholar |
Dukas, R., and Ellner, S. (1993). Information processing and prey detection. Ecology 74, 1337–1346.
| Information processing and prey detection.Crossref | GoogleScholarGoogle Scholar |
Dukas, R., and Kamil, A. C. (2001). Limited attention: the constraint underlying search image. Behavioral Ecology 12, 192–199.
| Limited attention: the constraint underlying search image.Crossref | GoogleScholarGoogle Scholar |
Engeman, R. M., and Linnell, M. A. (2004). The effect of trap spacing on the capture of brown tree snakes on Guam. International Biodeterioration & Biodegradation 54, 265–267.
| The effect of trap spacing on the capture of brown tree snakes on Guam.Crossref | GoogleScholarGoogle Scholar |
Engeman, R. M., and Vice, D. S. (2001). Objectives and integrated approaches for the control of brown tree snakes. Integrated Pest Management Reviews 6, 59–76.
| Objectives and integrated approaches for the control of brown tree snakes.Crossref | GoogleScholarGoogle Scholar |
Fritts, T. H., Scott, N. J., and Smith, B. E. (1989). Trapping Boiga irregularis on Guam using bird odors. Journal of Herpetology 23, 189–192.
| Trapping Boiga irregularis on Guam using bird odors.Crossref | GoogleScholarGoogle Scholar |
Garvey, P. M., Glen, A. S., Clout, M. N., Wyse, S. V., Nichols, M., and Pech, R. P. (2017). Exploiting interspecific olfactory communication to monitor predators. Ecological Applications 27, 389–402.
| Exploiting interspecific olfactory communication to monitor predators.Crossref | GoogleScholarGoogle Scholar | 27983773PubMed |
Glaudas, X., Glennon, K. L., Martins, M., Luiselli, L., Fearn, S., Trembath, D. F., Jelić, D., and Alexander, G. J. (2019). Foraging mode, relative prey size and diet breadth: a phylogenetically explicit analysis of snake feeding ecology. Journal of Animal Ecology 88, 757–767.
| Foraging mode, relative prey size and diet breadth: a phylogenetically explicit analysis of snake feeding ecology.Crossref | GoogleScholarGoogle Scholar |
Govindarajulu, P., Altwegg, R., and Anholt, B. R. (2005). Matrix model investigation of invasive species control: bullfrogs on Vancouver Island. Ecological Applications 15, 2161–2170.
| Matrix model investigation of invasive species control: bullfrogs on Vancouver Island.Crossref | GoogleScholarGoogle Scholar |
Hunter, D. O., Lagisz, M., Leo, V., Nakagawa, S., and Letnic, M. (2018). Not all predators are equal: a continent‐scale analysis of the effects of predator control on Australian mammals. Mammal Review 48, 108–122.
| Not all predators are equal: a continent‐scale analysis of the effects of predator control on Australian mammals.Crossref | GoogleScholarGoogle Scholar |
Imler, R. H. (1945). Bullsnakes and their control on a Nebraska wildlife refuge. The Journal of Wildlife Management 9, 265–273.
| Bullsnakes and their control on a Nebraska wildlife refuge.Crossref | GoogleScholarGoogle Scholar |
King, R. (2002). Predicted and observed maximum prey size-snake size allometry. Functional Ecology 16, 766–772.
| Predicted and observed maximum prey size-snake size allometry.Crossref | GoogleScholarGoogle Scholar |
Klug, P.E., and Yackel Adams A.A. (2021). Brown treesnake capture and morphometric data using live mouse- and bird-lure traps on Guam, 2013. Data release. U.S. Geological Survey, Fort Collins Science Center, Fort Collins, CO, USA
Klug, P., Yackel Adams, A., Stricker, C., and Reed, R. (2015a). Protection of caves important to the endangered Mariana swiftlet (Aerodramus bartschi) through effective deployment of control tools based on brown treesnake (Boiga irregularis) behavior. Unpublished Final Report to Naval Base Guam. U.S. Geological Survey, Fort Collins Science Center, Fort Collins, CO, USA.
Klug, P. E., Reed, R. N., Mazzotti, F. J., McEachern, M. A., Vinci, J. J., Craven, K. K., and Yackel Adams, A. A. (2015b). The influence of disturbed habitat on the spatial ecology of Argentine black and white tegu (Tupinambis merianae), a recent invader in the Everglades ecosystem (Florida, USA). Biological Invasions 17, 1785–1797.
| The influence of disturbed habitat on the spatial ecology of Argentine black and white tegu (Tupinambis merianae), a recent invader in the Everglades ecosystem (Florida, USA).Crossref | GoogleScholarGoogle Scholar |
Klug, P. E., Bukoski, W. P., Shiels, A. B., Kleuver, B. M., and Siers, S. R. (2019). Critical review of potential control tools for reducing damage by the invasive rose-ringed parakeet (Psittacula krameri) on the Hawaiian Islands. Unpublished Final Report QA-2836. U.S. Department of Agriculture, Animal and Plant Health Inspection Service, Wildlife Services, National Wildlife Research Center, Fort Collins, CO, USA.
Klug, P. E., Yackel Adams, A. A., Siers, S. R., Brindock, K. M., Mosher, S. M., Mazurek, M. J., Pitt, W. C., and Reed, R. N. (2021). Locally abundant, endangered Mariana swiftlets impact the abundance, behavior, and body condition of an invasive predator. Oecologia 195, 1083–1097.
| Locally abundant, endangered Mariana swiftlets impact the abundance, behavior, and body condition of an invasive predator.Crossref | GoogleScholarGoogle Scholar | 33683442PubMed |
Lardner, B., Savidge, J. A., Rodda, G. H., and Reed, R. N. (2009). Prey preferences and prey acceptance in juvenile brown treesnakes (Boiga irregularis). Herpetological Conservation and Biology 4, 313–323.
Lardner, B., Yackel Adams, A. A., Savidge, J. A., Rodda, G. H., Reed, R. N., and Clark, C. S. (2013). Effectiveness of bait tubes for brown treesnake (Boiga irregularis) control on Guam. Wildlife Society Bulletin 37, 664–673.
Latham, M. C., Anderson, D. P., Norbury, G., Price, C. J., Banks, P. B., and Latham, A. D. M. (2019). Modeling habituation of introduced predators to unrewarding bird odors for conservation of ground‐nesting shorebirds. Ecological Applications 29, e01814.
| Modeling habituation of introduced predators to unrewarding bird odors for conservation of ground‐nesting shorebirds.Crossref | GoogleScholarGoogle Scholar | 30312506PubMed |
Lowe, S., Browne, M., Boudjelas, S., and De Poorter, M. (2004). 100 of the world’s worst invasive alien species: a selection from the global invasive species database. Invasive Species Specialist Group, Species Survival Commission, World Conservation Union, Gland, Switzerland.
Morris, J. A., Shertzer, K. W., and Rice, J. A. (2011). A stage-based matrix population model of invasive lionfish with implications for control. Biological Invasions 13, 7–12.
| A stage-based matrix population model of invasive lionfish with implications for control.Crossref | GoogleScholarGoogle Scholar |
Murdoch, W. W. (1969). Switching in general predators: experiments on predator specificity and stability of prey populations. Ecological Monographs 39, 335–354.
| Switching in general predators: experiments on predator specificity and stability of prey populations.Crossref | GoogleScholarGoogle Scholar |
Nafus, M. G., Xiong, P. X., Paxton, E. H., Yackel Adams, A. A., and Goetz, S. M. (2021). Foraging behavior in a generalist snake (brown treesnake, Boiga irregularis) with implications for avian reintroduction and recovery. Applied Animal Behaviour Science 243, 105450.
| Foraging behavior in a generalist snake (brown treesnake, Boiga irregularis) with implications for avian reintroduction and recovery.Crossref | GoogleScholarGoogle Scholar |
Norbury, G. L., Latham, M. C., Brown, S. J., Latham, A. D. M., Brownstein, G. E., Ricardo, H. C., McArthur, N. J., Price, C. J., and Banks, P. B. (2020). Exploiting olfactory habituation with unrewarding prey cues to reduce unwanted predation. Vertebrate Pest Conference 29, 25.
O’Donnell, C. F., Dilks, P. J., and Elliott, G. P. (1996). Control of a stoat (Mustela erminea) population irruption to enhance mohua (yellowhead) (Mohoua ochrocephala) breeding success in New Zealand. New Zealand Journal of Zoology 23, 279–286.
| Control of a stoat (Mustela erminea) population irruption to enhance mohua (yellowhead) (Mohoua ochrocephala) breeding success in New Zealand.Crossref | GoogleScholarGoogle Scholar |
Parker, M. R., Patel, S. M., Zachry, J. E., and Kimball, B. A. (2018). Feminization of male brown treesnake methyl ketone expression via steroid hormone manipulation. Journal of Chemical Ecology 44, 189–197.
| Feminization of male brown treesnake methyl ketone expression via steroid hormone manipulation.Crossref | GoogleScholarGoogle Scholar | 29508108PubMed |
Peacor, S. D. (2006). Behavioural response of bullfrog tadpoles to chemical cues of predation risk are affected by cue age and water source. Hydrobiologia 573, 39–44.
| Behavioural response of bullfrog tadpoles to chemical cues of predation risk are affected by cue age and water source.Crossref | GoogleScholarGoogle Scholar |
Pimm, S. L., Jones, H. L., and Diamond, J. (1988). On the risk of extinction. American Naturalist 132, 757–785.
| On the risk of extinction.Crossref | GoogleScholarGoogle Scholar |
Pollock, H. S., Savidge, J. A., Kastner, M., Seibert, T. F., and Jones, T. M. (2019). Pervasive impacts of invasive brown treesnakes drive low fledgling survival in endangered Micronesian starlings (Aplonis opaca) on Guam. The Condor 121, duz014.
| Pervasive impacts of invasive brown treesnakes drive low fledgling survival in endangered Micronesian starlings (Aplonis opaca) on Guam.Crossref | GoogleScholarGoogle Scholar |
Price, C. J., and Banks, P. B. (2012). Exploiting olfactory learning in alien rats to protect birds’ eggs. Proceedings of the National Academy of Sciences of the United States of America 109, 19304–19309.
| Exploiting olfactory learning in alien rats to protect birds’ eggs.Crossref | GoogleScholarGoogle Scholar | 23071301PubMed |
Price, C. J., and Banks, P. B. (2017). Food quality and conspicuousness shape improvements in olfactory discrimination by mice. Proceedings of the Royal Society B: Biological Sciences 284, 20162629.
| Food quality and conspicuousness shape improvements in olfactory discrimination by mice.Crossref | GoogleScholarGoogle Scholar | 28123093PubMed |
Qualls, F. J., and Hackman, J. D. (2004). Ontogenetic shift in food preferences of captive juvenile brown treesnakes (Boiga irregularis): at what size do BTS first eat dead neonatal mice? Micronesica 37, 179.
Radcliffe, C. W., Chiszar, D., and O’Connell, B. (1980). Effects of prey size on poststrike behavior in rattlesnakes (Crotalus durissus, C. enyo, and C. viridis). Bulletin of the Psychonomic Society 16, 449–450.
| Effects of prey size on poststrike behavior in rattlesnakes (Crotalus durissus, C. enyo, and C. viridis).Crossref | GoogleScholarGoogle Scholar |
Rankin, C. H., Abrams, T., Barry, R. J., Bhatnagar, S., Clayton, D. F., Colombo, J., Coppola, G., Geyer, M. A., Glanzman, D. L., and Marsland, S. (2009). Habituation revisited: an updated and revised description of the behavioral characteristics of habituation. Neurobiology of Learning and Memory 92, 135–138.
| Habituation revisited: an updated and revised description of the behavioral characteristics of habituation.Crossref | GoogleScholarGoogle Scholar | 18854219PubMed |
Reed, R. N., Hart, K. M., Rodda, G. H., Mazzotti, F. J., Snow, R. W., Cherkiss, M. S., Rozar, R., and Goetz, S. (2011). A field test of attractant traps for invasive Burmese Pythons (Python molurus bivittatus) in southern Florida. Wildlife Research 38, 114–121.
| A field test of attractant traps for invasive Burmese Pythons (Python molurus bivittatus) in southern Florida.Crossref | GoogleScholarGoogle Scholar |
Reynolds, J., and Tapper, S. (1996). Control of mammalian predators in game management and conservation. Mammal Review 26, 127–155.
| Control of mammalian predators in game management and conservation.Crossref | GoogleScholarGoogle Scholar |
Rodda, G. H., Fritts, T. H., Clark, C. S., Gotte, S. W., and Chiszar, D. (1999). A state-of-the-art trap for the brown treesnake. In ‘Problem Snake Management: the Habu and the Brown Treesnake’. (Eds G. H. Rodda, Y. Sawai, D. Chiszar and H. Tanaka.) pp. 268–305. (Cornell University Press: Ithaca, NY, USA.)
Rodda, G. H., Savidge, J. A., Tyrrell, C. L., Christy, M. T., and Ellingson, A. R. (2007). Size bias in visual searching and trapping of brown treesnakes on Guam. The Journal of Wildlife Management 71, 656–661.
| Size bias in visual searching and trapping of brown treesnakes on Guam.Crossref | GoogleScholarGoogle Scholar |
Rosenzweig, M. L., and Clark, C. W. (1994). Island extinction rates from regular censuses. Conservation Biology 8, 491–494.
| Island extinction rates from regular censuses.Crossref | GoogleScholarGoogle Scholar |
Ruxton, G. D. (2009). Non-visual crypsis: a review of the empirical evidence for camouflage to senses other than vision. Philosophical Transactions of the Royal Society B: Biological Sciences 364, 549–557.
| Non-visual crypsis: a review of the empirical evidence for camouflage to senses other than vision.Crossref | GoogleScholarGoogle Scholar |
Salo, P., Korpimäki, E., Banks, P. B., Nordström, M., and Dickman, C. R. (2007). Alien predators are more dangerous than native predators to prey populations. Proceedings of the Royal Society B: Biological Sciences 274, 1237–1243.
| Alien predators are more dangerous than native predators to prey populations.Crossref | GoogleScholarGoogle Scholar | 17360286PubMed |
Savarie, P. J., and Clark, L. (2006). Evaluation of bait matrices and chemical lure attractants for brown tree snakes. Vertebrate Pest Conference 22, 483–488.
Savarie, P. J., Shivik, J. A., White, G. C., Hurley, J. C., and Clark, L. (2001). Use of acetaminophen for large scale control of brown treesnakes. The Journal of Wildlife Management 65, 356–365.
| Use of acetaminophen for large scale control of brown treesnakes.Crossref | GoogleScholarGoogle Scholar |
Shine, R., Harlow, P., and Keogh, J. (1998). The influence of sex and body size on food habits of a giant tropical snake, Python reticulatus. Functional Ecology 12, 248–258.
| The influence of sex and body size on food habits of a giant tropical snake, Python reticulatus.Crossref | GoogleScholarGoogle Scholar |
Shivik, J. A., and Clark, L. (1997). Carrion seeking in brown tree snakes: importance of olfactory and visual cues. The Journal of Experimental Zoology 279, 549–553.
| Carrion seeking in brown tree snakes: importance of olfactory and visual cues.Crossref | GoogleScholarGoogle Scholar |
Shivik, J. A., and Clark, L. (1999). Ontogenetic shifts in carrion attractiveness to brown tree snakes (Boiga irregularis). Journal of Herpetology 33, 334–336.
| Ontogenetic shifts in carrion attractiveness to brown tree snakes (Boiga irregularis).Crossref | GoogleScholarGoogle Scholar |
Shivik, J. A., Wright, W. G., and Clark, L. (2000). Seasonal variability in brown tree snake (Boiga irregularis) response to lures. Canadian Journal of Zoology 78, 79–84.
| Seasonal variability in brown tree snake (Boiga irregularis) response to lures.Crossref | GoogleScholarGoogle Scholar |
Siers, S. R., Shiels, A. B., and Barnhart, P. D. (2020). Invasive snake activity before and after automated aerial baiting. The Journal of Wildlife Management 84, 256–267.
| Invasive snake activity before and after automated aerial baiting.Crossref | GoogleScholarGoogle Scholar |
Smith, H. M., Kandler, K., Lee, R., and Chiszar, D. (1988). Stimulus control of predatory attack in the brown tree snake (Boiga irregularis). 2. Use of chemical cues during foraging. Amphibia-Reptilia 9, 77–88.
| Stimulus control of predatory attack in the brown tree snake (Boiga irregularis). 2. Use of chemical cues during foraging.Crossref | GoogleScholarGoogle Scholar |
Smith, R. K., Pullin, A. S., Stewart, G. B., and Sutherland, W. J. (2010). Effectiveness of predator removal for enhancing bird populations. Conservation Biology 24, 820–829.
| Effectiveness of predator removal for enhancing bird populations.Crossref | GoogleScholarGoogle Scholar | 20067492PubMed |
Stark, C. P., Chiszar, D., Stiles, K., and Smith, H. M. (2002). A laboratory situation for studying the effects of chemical and visual cues on prey trailing in brown treesnakes (Boiga irregularis). Journal of Herpetology 36, 57–62.
| A laboratory situation for studying the effects of chemical and visual cues on prey trailing in brown treesnakes (Boiga irregularis).Crossref | GoogleScholarGoogle Scholar |
Starling-Windhof, A., Massaro, M., and Briskie, J. V. (2011). Differential effects of exotic predator-control on nest success of native and introduced birds in New Zealand. Biological Invasions 13, 1021–1028.
| Differential effects of exotic predator-control on nest success of native and introduced birds in New Zealand.Crossref | GoogleScholarGoogle Scholar |
Threlfall, C., Law, B., and Banks, P. B. (2013). Odour cues influence predation risk at artificial bat roosts in urban bushland. Biology Letters 9, 20121144.
| Odour cues influence predation risk at artificial bat roosts in urban bushland.Crossref | GoogleScholarGoogle Scholar | 23637390PubMed |
Tobin, M. E., Sugihara, R. T., Pochop, P. A., and Linnell, M. A. (1999). Nightly and seasonal movements of Boiga irregularis on Guam. Journal of Herpetology 33, 281–291.
| Nightly and seasonal movements of Boiga irregularis on Guam.Crossref | GoogleScholarGoogle Scholar |
Tourani, M., Brøste, E., Bakken, S., Odden, J., and Bischof, R. (2020). Sooner, closer, or longer: detectability of mesocarnivores at camera traps. Journal of Zoology 312, 259–270.
| Sooner, closer, or longer: detectability of mesocarnivores at camera traps.Crossref | GoogleScholarGoogle Scholar |
Tyrrell, C. L., Christy, M. T., Rodda, G. H., Yackel Adams, A. A., Ellingson, A. R., Savidge, J. A., Dean-Bradley, K., and Bischof, R. (2009). Evaluation of trap capture in a geographically closed population of brown treesnakes on Guam. Journal of Applied Ecology 46, 128–135.
| Evaluation of trap capture in a geographically closed population of brown treesnakes on Guam.Crossref | GoogleScholarGoogle Scholar |
U.S. Fish and Wildlife Service (1984). Endangered and threatened wildlife and plants; determination of Endangered status for seven birds and two bats of Guam and the Northern Mariana Islands. Final rule. Federal Register 49, 33881–33885.
Veitch, C., and Clout, M. (Eds.) (2002). ‘Turning the Tide: the Eradication of Invasive Species.’ Invasive Species Specialist Group, Species Survival Commission, World Conservation Union, Gland, Switzerland.
Whitehead, A. L., Edge, K.-A., Smart, A. F., Hill, G. S., and Willans, M. J. (2008). Large scale predator control improves the productivity of a rare New Zealand riverine duck. Biological Conservation 141, 2784–2794.
| Large scale predator control improves the productivity of a rare New Zealand riverine duck.Crossref | GoogleScholarGoogle Scholar |
Wilcove, D. S., Rothstein, D., Dubow, J., Phillips, A., and Losos, E. (1998). Quantifying threats to imperiled species in the United States: assessing the relative importance of habitat destruction, alien species, pollution, overexploitation, and disease. Bioscience 48, 607–615.
| Quantifying threats to imperiled species in the United States: assessing the relative importance of habitat destruction, alien species, pollution, overexploitation, and disease.Crossref | GoogleScholarGoogle Scholar |
Wright, H. F., Wilkinson, A., Croxton, R. S., Graham, D. K., Harding, R. C., Hodkinson, H. L., Keep, B., Cracknell, N. R., and Zulch, H. E. (2017). Animals can assign novel odours to a known category. Scientific Reports 7, 9019.
| Animals can assign novel odours to a known category.Crossref | GoogleScholarGoogle Scholar | 28827742PubMed |
Yackel Adams, A. A., Nafus, M. G., Klug, P. E., Lardner, B., Mazurek, M. J., Savidge, J. A., and Reed, R. N. (2019). Contact rates with nesting birds before and after invasive snake removal: estimating the effects of trap-based control. NeoBiota 49, 1–17.
| Contact rates with nesting birds before and after invasive snake removal: estimating the effects of trap-based control.Crossref | GoogleScholarGoogle Scholar |




